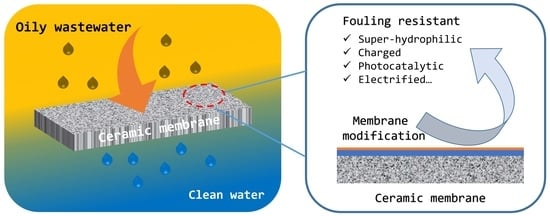
State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application
Abstract
1. Introduction
2. Modification Methods of Ceramic Membranes
2.1. Sol-Gel
2.2. Dip-Coating
2.3. Surface Grafting
2.4. Blending or Doping
2.5. Hydrothermal Method
2.6. Chemical Vapour Deposition
2.7. Atomic Layer Deposition
2.8. Comparison of the Different Ceramic Membrane Modification Methods
| Modification Method | Layer Thickness | Membrane Type | Layer Material | Temperature (°C) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Sol-gel | 50 nm–4 µm | UF, NF | γ-Al2O3, ZrO2, TiO2, TiO2–ZrO2 | 350–600 | - Narrow pore size distribution - Relatively controllable composition - Easy scale up | - Defect formation - Thick layers - Particle agglomeration | [60,61,103,104,105,106,107] |
| Dip-coating | 100 nm–100 µm | MF, UF, NF | Most inorganic materials | 800–1000 | - High flexibility - Excellent homogeneity | - Susceptible to defect - Multiple coating and baking steps - Thick layers | [64,85] |
| Surface grafting | ~ | MF, UF, NF | Polymeric monomers | Room temperature | - Controllable introduction of graft chains - Simple and cheap - Long-term chemical stability | - Need initiation - Mainly for hydrophobisation of ceramic membrane | [70] |
| Doping/blending | ~ | MF, UF | Most inorganic materials | Same temperature as the membrane | - Simplicity - Cost effective - Reproducibility | - Large pore size | [50,72] |
| Hydrothermal synthesis | 1–10 µm | MF, RO | Zeolite, TiO2, γ-Al2O3, Fe2O3 | 80–230 | - Low synthesis temperature - low cost | - Limited materials - Long synthesis time - Thick layer | [81,83,84,108] |
| CVD | 4 nm–10 µm | MF, UF, NF | Organic and inorganic materials | 400–1000 | - High coating uniformity - Few defects and scalable | - High deposition temperature - high cost | [85] |
| ALD | Monolayer to few nanometers | MF, UF, NF | Organic, inorganic and metallic materials | Room temperature to 300 | - Conformal and uniform layers - Pin-hole free films - Ultrathin layers - Low deposition temperature | - Low throughput - high cost - Scale up limitation | [85,107,109] |
3. Performance of Antifouling Ceramic Membranes for Oily Wastewater Treatment
3.1. Active Antifouling Ceramic Membranes
3.1.1. Hydrophilic Ceramic Membrane
3.1.2. Superhydrophilic Ceramic Membrane
3.1.3. Surface Charged Ceramic Membrane
3.1.4. Hydrophilic and Surface Charged Ceramic Membrane
3.1.5. Challenges of Active Antifouling Ceramic Membranes
3.2. Passive Antifouling Ceramic Membranes
3.2.1. Photocatalytic Ceramic Membrane
3.2.2. Piezoelectric Ceramic Membrane
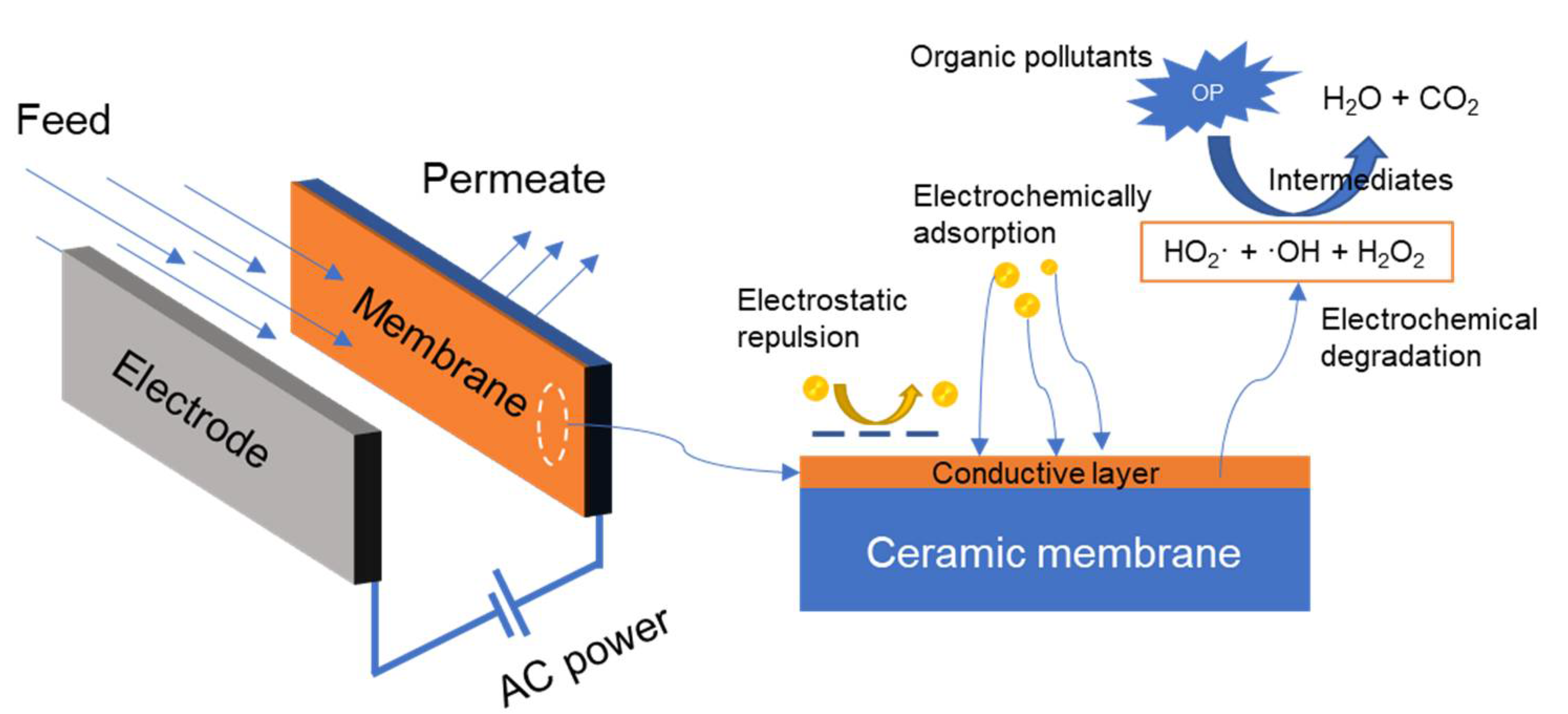
3.2.3. Electrochemically Enhanced Ceramic Membrane
3.3. Comparison of the Performance of Antifouling Ceramic Membranes for Oily Wastewater Treatment
4. Concluding Remarks
- Although sol-gel and dip-coating are the most frequently used methods for ceramic membranes modification, the emerging modification method ALD has shown great potential on the control of layer thickness and pore size distribution of the ceramic membranes;
- Nano-sized metal oxides have mostly been used as material for ceramic membrane modification for oily wastewater treatment. Among them, TiO2, ZrO2, and Fe2O3 are considered the most promising ones to improve ceramic membrane performance, increasing surface hydrophilicity and/or surface charge of membranes. In addition, the GO modified ceramic membranes can improve permeate flux, oil rejection, and fouling resistance at the same time. However, the scalability of these anti-fouling ceramic membranes is still challenging;
- To control the fouling problems by oil droplets, also passive antifouling ceramic membranes have been developed, including photocatalytic, piezoelectric, and electrochemically enhanced modified ceramic membranes. However, the performance of these membranes is only tested in a controlled environment for a short time, making it difficult to upscale, and the appropriate materials used to prepare these membranes are expensive.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Willet, J.; Wetser, K.; Vreeburg, J.; Rijnaarts, H.H.M. Review of methods to assess sustainability of industrial water use. Water Resour. Ind. 2019, 21, 100110. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, L. Efficiency Evaluation and Policy Analysis of Industrial Wastewater Control in China. Energies 2017, 10, 1201. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ang, H.-M.; Wang, S. Removal of emulsified food and mineral oils from wastewater using surfactant modified barley straw. Bioresour. Technol. 2009, 100, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, L.; Chen, J.; Yang, F.; Tang, C.Y.; Guiver, M.D.; Dong, Y. Spinel-based ceramic membranes coupling solid sludge recycling with oily wastewater treatment. Water Res. 2020, 169, 115180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, M.L.; Dong, Y.C.; Tang, C.Y.Y.; Huang, A.S.; Li, L.L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Zhu, L.; Dong, Y.C.; Li, L.L.; Liu, J. Waste-to-Resource Strategy to Fabricate Highly Porous Whisker-Structured Mullite Ceramic Membrane for Simulated Oil-in-Water Emulsion Wastewater Treatment. ACS Sustain. Chem. Eng. 2016, 4, 2098–2106. [Google Scholar] [CrossRef]
- Adham, S.; Hussain, A.; Minier-Matar, J.; Janson, A.; Sharma, R. Membrane applications and opportunities for water management in the oil & gas industry. Desalination 2018, 440, 2–17. [Google Scholar]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Duraisamy, R.T.; Heydari, A.; Henni, A. State of the Art Treatment of Produced Water. Water Treat. 2013, 199–222. [Google Scholar] [CrossRef]
- Coca, J.; Gutierrez, G.; Benito, J.M. Treatment of oily wastewater. In Water Purification and Management; CocaPrados, J., Gutierrez Cervello, G., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–55. [Google Scholar]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil-water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Lin, Y.M.; Song, C.; Rutledge, G.C. Functionalization of Electrospun Membranes with Polyelectrolytes for Separation of Oil-In-Water Emulsions. Adv. Mater. Interfaces 2019, 6, 1901285. [Google Scholar] [CrossRef]
- Zhu, X.B.; Dudchenko, A.; Gu, X.T.; Jassby, D. Surfactant-stabilized oil separation from water using ultrafiltration and nanofiltration. J. Membr. Sci. 2017, 529, 159–169. [Google Scholar] [CrossRef]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Motta Cabrera, S.; Winnubst, L.; Richter, H.; Voigt, I.; Nijmeijer, A. Industrial Application of Ceramic Nanofiltration Membranes for Water Treatment in Oil Sands Mines. Sep. Purif. Technol. 2020, 256, 117821. [Google Scholar] [CrossRef]
- Zou, D.; Xu, J.; Chen, X.; Drioli, E.; Qiu, M.; Fan, Y. A novel thermal spraying technique to fabricate fly ash/alumina composite membranes for oily emulsion and spent tin wastewater treatment. Sep. Purif. Technol. 2019, 219, 127–136. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Zou, D.; Chen, X.; Drioli, E.; Qiu, M.; Fan, Y. Facile Mixing Process To Fabricate Fly-Ash-Enhanced Alumina-Based Membrane Supports for Industrial Microfiltration Applications. Ind. Eng. Chem. Res. 2019, 58, 8712–8723. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Dudek, M.; Qin, J.; Øye, G.; Østerhus, S.W. A multivariate study of backpulsing for membrane fouling mitigation in produced water treatment. J. Environ. Chem. Eng. 2020, 2020, 104839. [Google Scholar] [CrossRef]
- Cakl, J.; Bauer, I.; Doleček, P.; Mikulášek, P. Effects of backflushing conditions on permeate flux in membrane crossflow microfiltration of oil emulsion. Desalination 2000, 127, 189–198. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Scherrenberg, S.M.; Rietveld, L.C.; Heijman, S.J.G. Quantifying defects in ceramic tight ultra- and nanofiltration membranes and investigating their robustness. Sep. Purif. Technol. 2019, 219, 159–168. [Google Scholar] [CrossRef]
- Tummons, E.; Han, Q.; Tanudjaja, H.J.; Hejase, C.A.; Chew, J.W.; Tarabara, V.V. Membrane fouling by emulsified oil: A review. Sep. Purif. Technol. 2020, 248, 116919. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Dashti, A.; Riasat Harami, H.; Hajilari, N. Inamuddin Fouling-resistant membranes for water reuse. Environ. Chem. Lett. 2018, 16, 715–763. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Anantharaman, A.; Chun, Y.; Hua, T.; Chew, J.W.; Wang, R. Pre-deposited dynamic membrane filtration—A review. Water Res. 2020, 173, 115558. [Google Scholar] [CrossRef]
- Lu, D.W.; Cheng, W.; Zhang, T.; Lu, X.L.; Liu, Q.L.; Jiang, J.; Ma, J. Hydrophilic Fe2O3 dynamic membrane mitigating fouling of support ceramic membrane in ultrafiltration of oil/water emulsion. Sep. Purif. Technol. 2016, 165, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, Y.; Wong, F.-S.; Fane, A.G.; Xu, N. Formation of dynamic membranes for oily water separation by crossflow filtration. Sep. Purif. Technol. 2005, 44, 212–220. [Google Scholar] [CrossRef]
- Gu, Q.; Chiang Albert Ng, T.; Bao, Y.; Yong Ng, H.; Ching Tan, S.; Wang, J. Developing Better Ceramic Membranes for Water and Wastewater Treatment: Where Microstructure Integrates with Chemistry and Functionalities. Chem. Eng. J. 2021, 428, 130456. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef] [PubMed]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Zhou, J.-E.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]
- Chang, Q.B.; Zhou, J.E.; Wang, Y.Q.; Liang, J.; Zhang, X.Z.; Cerneaux, S.; Wang, X.; Zhu, Z.W.; Dong, Y.C. Application of ceramic microfiltration membrane modified by nano-TiO2 coating in separation of a stable oil-in-water emulsion. J. Membr. Sci. 2014, 456, 128–133. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Chen, M.; Shang, R.; Sberna, P.M.; Luiten-Olieman, M.W.J.; Rietveld, L.C.; Heijman, S.G.J. Highly permeable silicon carbide-alumina ultrafiltration membranes for oil-in-water filtration produced with low-pressure chemical vapor deposition. Sep. Purif. Technol. 2020, 253, 117496. [Google Scholar] [CrossRef]
- Golshenas, A.; Sadeghian, Z.; Ashrafizadeh, S.N. Performance evaluation of a ceramic-based photocatalytic membrane reactor for treatment of oily wastewater. J. Water Process. Eng. 2020, 36, 101186. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; An, B.; Choi, H. Characterization of natural organic matter treated by iron oxide nanoparticle incorporated ceramic membrane-ozonation process. Water Res. 2012, 46, 5861–5870. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Chen, G.H. Magneli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J. Membr. Sci. 2016, 498, 302–314. [Google Scholar] [CrossRef]
- Mao, H.; Bu, J.; Da, X.; Chen, X.; Qiu, M.; Verweij, H.; Fan, Y. High-performance self-cleaning piezoelectric membrane integrated with in-situ ultrasound for wastewater treatment. J. Eur. Ceram. Soc. 2020, 40, 3632–3641. [Google Scholar] [CrossRef]
- Mao, H.; Bu, J.; Qiu, M.; Ding, D.; Chen, X.; Verweij, H.; Fan, Y. PZT/Ti composite piezoceramic membranes for liquid filtration: Fabrication and self-cleaning properties. J. Membr. Sci. 2019, 581, 28–37. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.-E.; Wang, Y.; Wang, J.; Meng, G. Hydrophilic modification of Al2O3 microfiltration membrane with nano-sized γ-Al2O3 coating. Desalination 2010, 262, 110–114. [Google Scholar] [CrossRef]
- Martin, C.J.; Cherry, T.M. The nature of the antagonism between toxins and antitoxins. Proc. R. Soc. Lond. 1898, 63, 420–432. [Google Scholar] [CrossRef]
- De Lange, R.; Hekkink, J.; Keizer, K.; Burggraaf, A. Formation and characterization of supported microporous ceramic membranes prepared by sol-gel modification techniques. J. Membr. Sci. 1995, 99, 57–75. [Google Scholar] [CrossRef]
- Xia, C.; Zha, S.; Yang, W.; Peng, R.; Peng, D.; Meng, G. Preparation of yttria stabilized zirconia membranes on porous substrates by a dip-coating process. Solid State Ion. 2000, 133, 287–294. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, Y.; Xu, N. Effect of the surface properties on filtration performance of Al2O3–TiO2 composite membrane. Sep. Purif. Technol. 2009, 66, 306–312. [Google Scholar] [CrossRef]
- Wei, C.C.; Li, K. Preparation and Characterization of a Robust and Hydrophobic Ceramic Membrane via an Improved Surface Grafting Technique. Ind. Eng. Chem. Res. 2009, 48, 3446–3452. [Google Scholar] [CrossRef]
- Suresh, K.; Srinu, T.; Ghoshal, A.K.; Pugazhenthi, G. Preparation and characterization of TiO2 and gamma-Al2O3 composite membranes for the separation of oil-in-water emulsions. RSC Adv. 2016, 6, 4877–4888. [Google Scholar] [CrossRef]
- Ha, H.Y.; Nam, S.W.; Lim, T.H.; Oh, I.-H.; Hong, S.-A. Properties of the TiO2 membranes prepared by CVD of titanium tetraisopropoxide. J. Membr. Sci. 1996, 111, 81–92. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Fan, Y.; Xing, W.; Wang, Y. Modification of ceramic membranes for pore structure tailoring: The atomic layer deposition route. J. Membr. Sci. 2012, 397, 17–23. [Google Scholar] [CrossRef]
- Lu, Y.; Ganguli, R.; Drewien, C.A.; Anderson, M.T.; Brinker, C.J.; Gong, W.; Guo, Y.; Soyez, H.; Dunn, B.; Huang, M.H. Continuous formation of supported cubic and hexagonal mesoporous films by sol–gel dip-coating. Nature 1997, 389, 364. [Google Scholar] [CrossRef]
- Nair, B.N.; Yamaguchi, T.; Okubo, T.; Suematsu, H.; Keizer, K.; Nakao, S.-I. Sol-gel synthesis of molecular sieving silica membranes. J. Membr. Sci. 1997, 135, 237–243. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Amin, S.K.; Abdallah, H.; Roushdy, M.; El-Sherbiny, S. An overview of production and development of ceramic membranes. Int. J. Appl. Eng. Res. 2016, 11, 7708–7721. [Google Scholar]
- Guizard, C. Sol-gel chemistry and its application to porous membrane processing. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 227–258. [Google Scholar]
- Cai, Y.; Wang, Y.; Chen, X.; Qiu, M.; Fan, Y. Modified colloidal sol–gel process for fabrication of titania nanofiltration membranes with organic additives. J. Membr. Sci. 2015, 476, 432–441. [Google Scholar] [CrossRef]
- Bayat, A.; Mahdavi, H.R.; Kazemimoghaddam, M.; Mohammadi, T. Preparation and characterization of γ-alumina ceramic ultrafiltration membranes for pretreatment of oily wastewater. Desalin. Water Treat. 2016, 57, 24322–24332. [Google Scholar] [CrossRef]
- Bonekamp, B.C. Chapter 6 Preparation of asymmetric ceramic membrane supports by dip-coating. In Membrane Science and Technology; Burggraaf, A.J., Cot, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 141–225. [Google Scholar]
- Barati, N.; Husein, M.M.; Azaiez, J. Modifying ceramic membranes with in situ grown iron oxide nanoparticles and their use for oily water treatment. J. Membr. Sci. 2020, 617, 118641. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Xu, N.; Shi, J. Preparation and application in oil–water separation of ZrO2/α-Al2O3 MF membrane. J. Membr. Sci. 1998, 142, 235–243. [Google Scholar] [CrossRef]
- Faibish, R.S.; Cohen, Y. Fouling-resistant ceramic-supported polymer membranes for ultrafiltration of oil-in-water microemulsions. J. Membr. Sci. 2001, 185, 129–143. [Google Scholar] [CrossRef]
- Krajewski, S.R.; Kujawski, W.; Bukowska, M.; Picard, C.; Larbot, A. Application of fluoroalkylsilanes (FAS) grafted ceramic membranes in membrane distillation process of NaCl solutions. J. Membr. Sci. 2006, 281, 253–259. [Google Scholar] [CrossRef]
- Faibish, R.S.; Cohen, Y. Fouling and rejection behavior of ceramic and polymer-modified ceramic membranes for ultrafiltration of oil-in-water emulsions and microemulsions. Colloid Surf. A 2001, 191, 27–40. [Google Scholar] [CrossRef]
- Atallah, C.; Mortazavi, S.; Tremblay, A.Y.; Doiron, A. Surface-Modified Multi-lumen Tubular Membranes for SAGD-Produced Water Treatment. Energy Fuels 2019, 33, 5766–5776. [Google Scholar] [CrossRef]
- Jou, J.-D.; Yoshida, W.; Cohen, Y. A novel ceramic-supported polymer membrane for pervaporation of dilute volatile organic compounds. J. Membr. Sci. 1999, 162, 269–284. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Hendren, Z.D.; Brant, J.; Wiesner, M.R. Surface modification of nanostructured ceramic membranes for direct contact membrane distillation. J. Membr. Sci. 2009, 331, 1–10. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Effect of TiO2 addition on the fabrication of ceramic membrane supports: A study on the separation of oil droplets and bovine serum albumin (BSA) from its solution. Desalination 2011, 279, 104–114. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Majid, M.A.; Ooi, B.S. Functionalized PSf/SiO2 nanocomposite membrane for oil-in-water emulsion separation. Desalination 2011, 268, 266–269. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Kim, E.-S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394–395, 37–48. [Google Scholar] [CrossRef]
- Liu, R.; Raman, A.K.Y.; Shaik, I.; Aichele, C.; Kim, S.-J. Inorganic microfiltration membranes incorporated with hydrophilic silica nanoparticles for oil-in-water emulsion separation. J. Water Process. Eng. 2018, 26, 124–130. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Huang, A.; Yang, W. Hydrothermal synthesis of uniform and dense NaA zeolite membrane in the electric field. Microporous Mesoporous Mater. 2007, 102, 58–69. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Microwave-assisted hydrothermal synthesis of hydroxy-sodalite zeolite membrane. Microporous Mesoporous Mater. 2004, 75, 173–181. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chew, T.L.; Zhu, P.W.; Mohamed, A.R.; Chai, S.-P. Synthesis and performance of microporous inorganic membranes for CO2 separation: A review. J. Porous Mater. 2013, 20, 1457–1475. [Google Scholar] [CrossRef]
- Senapati, S.; Maiti, P. 9-Emerging bio-applications of two-dimensional nanoheterostructure materials. In 2D Nanoscale Heterostructured Materials; Jit, S., Das, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–255. [Google Scholar]
- Paiman, S.H.; Rahman, M.A.; Uchikoshi, T.; Nordin, N.A.H.M.; Alias, N.H.; Abdullah, N.; Abas, K.H.; Othman, M.H.D.; Jaafar, J.; Ismail, A.F. In situ growth of α-Fe2O3 on Al2O3/YSZ hollow fiber membrane for oily wastewater. Sep. Purif. Technol. 2020, 236, 116250. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; McPherson, B.; Lee, R. Removal of organics from produced water by reverse osmosis using MFI-type zeolite membranes. J. Membr. Sci. 2008, 325, 357–361. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; McBride, S.A.; Warsinger, D.M.; Dizge, N.; Hasan, S.W.; Arafat, H.A. Thin film deposition techniques for polymeric membranes—A review. J. Membr. Sci. 2020, 610, 118258. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Silica membranes for hydrogen separation prepared by chemical vapor deposition (CVD). Sep. Purif. Technol. 2013, 111, 20–42. [Google Scholar] [CrossRef]
- Pedersen, H. Time as the Fourth Dimension: Opening up New Possibilities in Chemical Vapor Deposition. Chem. Mater. 2016, 28, 691–699. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.-L.; Yamada, H.; Saito, T.; Kai, T.; Murakami, K.; Nakashima, M.; Ohshita, J.; Akamatsu, K.; Nakao, S.-I. Development of hydrogen-selective triphenylmethoxysilane-derived silica membranes with tailored pore size by chemical vapor deposition. J. Membr. Sci. 2016, 499, 28–35. [Google Scholar] [CrossRef]
- Meng, G.; Ma, G.; Ma, Q.; Peng, R.; Liu, X. Ceramic membrane fuel cells based on solid proton electrolytes. Solid State Ion. 2007, 178, 697–703. [Google Scholar] [CrossRef]
- Nomura, M.; Seshimo, M.; Aida, H.; Nakatani, K.; Gopalakrishnan, S.; Sugawara, T.; Ishikawa, T.; Kawamura, M.; Nakao, S.-I. Preparation of a catalyst composite silica membrane reactor for steam reforming reaction by using a counterdiffusion CVD method. Ind. Eng. Chem. Res. 2006, 45, 3950–3954. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Z.W.; Chen, L.S.; Jiang, Y.J.; Xu, C.Y.; Wu, Z.H.; Wang, Y.Y.; Peng, C.S. Porous superhydrophobic and superoleophilic surfaces prepared by template assisted chemical vapor deposition. Surf. Coat. Technol. 2017, 315, 385–390. [Google Scholar] [CrossRef]
- Chen, X.W.; Hong, L.; Xu, Y.F.; Ong, Z.W. Ceramic Pore Channels with Inducted Carbon Nanotubes for Removing Oil from Water. ACS Appl. Mater. Interfaces 2012, 4, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Kukli, K.; Salmi, E.; Jõgiaas, T.; Zabels, R.; Schuisky, M.; Westlinder, J.; Mizohata, K.; Ritala, M.; Leskelä, M. Atomic layer deposition of aluminum oxide on modified steel substrates. Surf. Coat. Technol. 2016, 304, 1–8. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, L.; Liao, X.; Wang, Y. Precise pore size tuning and surface modifications of polymeric membranes using the atomic layer deposition technique. J. Membr. Sci. 2011, 385–386, 1–9. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Goulas, A.; Tang, C.Y.; de Frias Serra, X.; Rietveld, L.C.; Heijman, S.G.J. Atmospheric pressure atomic layer deposition for tight ceramic nanofiltration membranes: Synthesis and application in water purification. J. Membr. Sci. 2017, 528, 163–170. [Google Scholar] [CrossRef]
- Yang, H.-C.; Xie, Y.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.K.R.S.; Elam, J.W.; Darling, S.B. Crude-Oil-Repellent Membranes by Atomic Layer Deposition: Oxide Interface Engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Sun, Y.; Li, Y.; Qin, W.; Wu, X. Mechanically Robust Fish-Scale Microstructured TiO2-Coated Stainless Steel Mesh by Atomic Layer Deposition for Oil–Water Separation. Ind. Eng. Chem. Res. 2020, 59, 21088–21096. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Thibault, Y.; McEvoy, J.G.; Mortazavi, S.; Smith, D.; Doiron, A. Characterization of fouling processes in ceramic membranes used for the recovery and recycle of oil sands produced water. J. Membr. Sci. 2017, 540, 307–320. [Google Scholar] [CrossRef]
- Li, R.; Li, N.; Hou, J.; Yu, Y.; Liang, L.; Yan, B.; Chen, G. Aquatic environment remediation by atomic layer deposition-based multi-functional materials: A review. J. Hazard. Mater. 2021, 402, 123513. [Google Scholar] [CrossRef]
- Vacassy, R.; Guizard, C.; Thoraval, V.; Cot, L. Synthesis and characterization of microporous zirconia powders: Application in nanofilters and nanofiltration characteristics. J. Membr. Sci. 1997, 132, 109–118. [Google Scholar] [CrossRef]
- Das, N.; Maiti, H.S. Ceramic membrane by tape casting and sol–gel coating for microfiltration and ultrafiltration application. J. Phys. Chem. Solids 2009, 70, 1395–1400. [Google Scholar] [CrossRef]
- Tsuru, T.; Hironaka, D.; Yoshioka, T.; Asaeda, M. Titania membranes for liquid phase separation: Effect of surface charge on flux. Sep. Purif. Technol. 2001, 25, 307–314. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 270–297. [Google Scholar]
- Lee, J.; Kim, I.S.; Hwang, M.-H.; Chae, K.-J. Atomic layer deposition and electrospinning as membrane surface engineering methods for water treatment: A short review. Environ. Sci. Water Res. Technol. 2020, 6, 1765–1785. [Google Scholar] [CrossRef]
- Cui, J.Y.; Zhang, X.F.; Liu, H.O.; Liu, S.Q.; Yeung, K.L. Preparation and application of zeolite/ceramic microfiltration membranes for treatment of oil contaminated water. J. Membr. Sci. 2008, 325, 420–426. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Kim, S.S.; Bechelany, M. Atomic layer deposition (ALD) on inorganic or polymeric membranes. J. Appl. Phys. 2019, 126, 041101. [Google Scholar] [CrossRef]
- Murić, A.; Petrinić, I.; Christensen, M.L. Comparison of ceramic and polymeric ultrafiltration membranes for treating wastewater from metalworking industry. Chem. Eng. J. 2014, 255, 403–410. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, J.; Ding, L.; Jaffrin, M.Y. A Review on Flux Decline Control Strategies in Pressure-Driven Membrane Processes. Ind. Eng. Chem. Res. 2015, 54, 2843–2861. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, T.; Ma, J. Ceramic membrane fouling during ultrafiltration of oil/water emulsions: Roles played by stabilization surfactants of oil droplets. Environ. Sci. Technol. 2015, 49, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Vidic, R.D. Application of microfiltration for the treatment of Marcellus Shale flowback water: Influence of floc breakage on membrane fouling. J. Membr. Sci. 2016, 510, 348–354. [Google Scholar] [CrossRef]
- Trinh, T.A.; Han, Q.; Ma, Y.; Chew, J.W. Microfiltration of oil emulsions stabilized by different surfactants. J. Membr. Sci. 2019, 579, 199–209. [Google Scholar] [CrossRef]
- Zhu, X.; Loo, H.-E.; Bai, R. A novel membrane showing both hydrophilic and oleophobic surface properties and its non-fouling performances for potential water treatment applications. J. Membr. Sci. 2013, 436, 47–56. [Google Scholar] [CrossRef]
- Marzouk, S.S.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of TiO2/SiO2 ceramic membranes via dip coating for the treatment of produced water. Chemosphere 2021, 273, 129684. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhang, T.; Gutierrez, L.; Ma, J.; Croué, J.-P. Influence of Surface Properties of Filtration-Layer Metal Oxide on Ceramic Membrane Fouling during Ultrafiltration of Oil/Water Emulsion. Environ. Sci. Technol. 2016, 50, 4668–4674. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; Bukenhoudt, A.; Jacobs, M.; McKay, G.; Atieh, M.A. Novel hybrid ceramic/carbon membrane for oil removal. J. Membr. Sci. 2018, 559, 42–53. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Zhou, J.; Wang, Y.; Liang, J.; Zhang, X.; Chang, Q.; Song, L. The improved oil/water separation performance of graphene oxide modified Al2O3 microfiltration membrane. J. Membr. Sci. 2015, 476, 200–204. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Chen, T.; Duan, M.; Fang, S.W. Fabrication of novel superhydrophilic and underwater superoleophobic hierarchically structured ceramic membrane and its separation performance of oily wastewater. Ceram. Int. 2016, 42, 8604–8612. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- de Leon, A.; Advincula, R.C. Reversible superhydrophilicity and superhydrophobicity on a lotus-leaf pattern. ACS Appl. Mater. Interfaces 2014, 6, 22666–22672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially Wettable Membranes for Oil–Water Separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Wu, W.; Huang, R.; Qi, W.; Su, R.; He, Z. Bioinspired Peptide-Coated Superhydrophilic Poly(vinylidene fluoride) Membrane for Oil/Water Emulsion Separation. Langmuir 2018, 34, 6621–6627. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; He, Y.; Pan, Y.; Di, H.H.; Zeng, G.Y.; Zhang, L.; Zhang, C.L. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, G.; Gao, S.; Jin, H.; Zhu, Y.; Zhang, F.; Jin, J. Cupric Phosphate Nanosheets-Wrapped Inorganic Membranes with Superhydrophilic and Outstanding Anticrude Oil-Fouling Property for Oil/Water Separation. ACS Nano 2018, 12, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jin, Q.; Zong, D.; Yu, J.; Ding, B. Biomimetic Multilayer Nanofibrous Membranes with Elaborated Superwettability for Effective Purification of Emulsified Oily Wastewater. ACS Appl. Mater. Interfaces 2018, 10, 16183–16192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, G.; Zhi, S.; Xu, K.; Zhu, L.; Li, W.; Zeng, Z.; Xue, Q. Superhydrophilicity and underwater superoleophobicity TiO2/Al2O3 composite membrane with ultra low oil adhesion for highly efficient oil-in-water emulsions separation. Appl. Surf. Sci. 2018, 458, 157–165. [Google Scholar] [CrossRef]
- Maguire-Boyle, S.J.; Huseman, J.E.; Ainscough, T.J.; Oatley-Radcliffe, D.L.; Alabdulkarem, A.A.; Al-Mojil, S.F.; Barron, A.R. Superhydrophilic Functionalization of Microfiltration Ceramic Membranes Enables Separation of Hydrocarbons from Frac and Produced Water. Sci. Rep. 2017, 7, 12267. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Trinh, T.A.; Kalyan, M.N.; Ho, J.S.; Chew, J.W. In-situ monitoring of oil emulsion fouling in ultrafiltration via electrical impedance spectroscopy (EIS): Influence of surfactant. J. Membr. Sci. 2020, 616, 118527. [Google Scholar] [CrossRef]
- Lobo, A.; Cambiella, Á.; Benito, J.M.; Pazos, C.; Coca, J. Ultrafiltration of oil-in-water emulsions with ceramic membranes: Influence of pH and crossflow velocity. J. Membr. Sci. 2006, 278, 328–334. [Google Scholar] [CrossRef]
- Atallah, C.; Tremblay, A.Y.; Mortazavi, S. Silane surface modified ceramic membranes for the treatment and recycling of SAGD produced water. J. Petrol. Sci. Eng. 2017, 157, 349–358. [Google Scholar] [CrossRef]
- Skibinski, B.; Müller, P.; Uhl, W. Rejection of submicron sized particles from swimming pool water by a monolithic SiC microfiltration membrane: Relevance of steric and electrostatic interactions. J. Membr. Sci. 2016, 499, 92–104. [Google Scholar] [CrossRef]
- Abadikhah, H.; Zou, C.-N.; Hao, Y.-Z.; Wang, J.-W.; Lin, L.; Khan, S.A.; Xu, X.; Chen, C.-S.; Agathopoulos, S. Application of asymmetric Si3N4 hollow fiber membrane for cross-flow microfiltration of oily waste water. J. Eur. Ceram. Soc. 2018, 38, 4384–4394. [Google Scholar] [CrossRef]
- Keramati, H.; Saidi, M.H.; Zabetian, M. Stabilization of the Suspension of Zirconia Microparticle Using the Nanoparticle Halos Mechanism: Zeta Potential Effect. J. Dispers. Sci. Technol. 2016, 37, 6–13. [Google Scholar] [CrossRef]
- Aghaeinejad-Meybodi, A.; Ghasemzadeh, K. Chapter 8—Silica Membrane Application for Desalination Process. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–216. [Google Scholar]
- Al-Harbi, O.A.; Khan, M.M.; Ozgur, C. Improving the performance of silica-based crossflow membranes by surface crystallization for treatment of oily wastewater. J. Aust. Ceram. Soc. 2017, 53, 883–894. [Google Scholar] [CrossRef]
- Xu, M.; Xu, C.; Rakesh, K.P.; Cui, Y.; Yin, J.; Chen, C.; Wang, S.; Chen, B.; Zhu, L. Hydrophilic SiC hollow fiber membranes for low fouling separation of oil-in-water emulsions with high flux. RSC Adv. 2020, 10, 4832–4839. [Google Scholar] [CrossRef]
- Jiao, K.; Yu, X.; Yuan, Z.; Zhang, Y.; Liu, J. Enhanced filtration performance of Al2O3-SiC porous ceramic composite tube depending on microstructure and surface properties. Desalin. Water Treat. 2019, 150, 99–104. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Zhang, L.; Lyu, Z.; Zhang, Z.; Ng, H.Y.; Wang, J. Interfacial diffusion assisted chemical deposition (ID-CD) for confined surface modification of alumina microfiltration membranes toward high-flux and anti-fouling. Sep. Purif. Technol. 2020, 235, 116177. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Huang, A.; Soesanto, J.F.; Luo, Y.-L.; Hsu, T.-Y.; Chen, C.-H.; Hwang, K.-J.; Ho, C.-D.; Tung, K.-L. 3D printing design of turbulence promoters in a cross-flow microfiltration system for fine particles removal. J. Membr. Sci. 2019, 573, 647–656. [Google Scholar] [CrossRef]
- Lyu, Z.; Ng, T.C.A.; Tran-Duc, T.; Lim, G.J.H.; Gu, Q.; Zhang, L.; Zhang, Z.; Ding, J.; Phan-Thien, N.; Wang, J.; et al. 3D-printed surface-patterned ceramic membrane with enhanced performance in crossflow filtration. J. Membr. Sci. 2020, 606, 118138. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Molinari, R.; Grande, C.; Drioli, E.; Palmisano, L.; Schiavello, M. Photocatalytic membrane reactors for degradation of organic pollutants in water. Catal. Today 2001, 67, 273–279. [Google Scholar] [CrossRef]
- Kumar, K.; Chowdhury, A. Use of Novel Nanostructured Photocatalysts for the Environmental Sustainability of Wastewater Treatments. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Oxford, UK, 2020; pp. 949–964. [Google Scholar]
- Sakka, S. Chapter 11.1.2—Sol–Gel Process and Applications. In Handbook of Advanced Ceramics, 2nd ed.; Somiya, S., Ed.; Academic Press: Oxford, UK, 2013; pp. 883–910. [Google Scholar]
- Azmi, N.F.A.N.; Abdullah, N.; Pauzi, M.Z.M.; Rahman, M.A.; Abas, K.H.; Aziz, A.A.; Othman, M.H.D.; Jaafar, J.; Ismail, A.F. Highly permeable photo-catalytic mesoporous aluminum oxide membrane for oil emulsion separation. J. Aust. Ceram. Soc. 2019, 55, 323–335. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- Starr, B.J.; Tarabara, V.V.; Herrera-Robledo, M.; Zhou, M.; Roualdès, S.; Ayral, A. Coating porous membranes with a photocatalyst: Comparison of LbL self-assembly and plasma-enhanced CVD techniques. J. Membr. Sci. 2016, 514, 340–349. [Google Scholar] [CrossRef]
- Nyamutswa, L.T.; Zhu, B.; Collins, S.F.; Navaratna, D.; Duke, M.C. Light conducting photocatalytic membrane for chemical-free fouling control in water treatment. J. Membr. Sci. 2020, 604, 118018. [Google Scholar] [CrossRef]
- Mao, H.; Qiu, M.; Bu, J.; Chen, X.; Verweij, H.; Fan, Y. Self-Cleaning Piezoelectric Membrane for Oil-in-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 18093–18103. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Liu, Y.; Quan, X.; Yu, H.; Chen, S. Enhanced permeability, selectivity, and antifouling ability of CNTs/Al2O3 membrane under electrochemical assistance. Environ. Sci. Technol. 2015, 49, 2293–2300. [Google Scholar] [CrossRef]
- Mantel, T.; Jacki, E.; Ernst, M. Electrosorptive removal of organic water constituents by positively charged electrically conductive UF membranes. Water Res. 2021, 201, 117318. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An Electrocatalytic Membrane Reactor with Self-Cleaning Function for Industrial Wastewater Treatment. Angew. Chem. Int. Ed. 2011, 50, 2148–2150. [Google Scholar] [CrossRef]
- Yang, B.; Geng, P.; Chen, G. One-dimensional structured IrO2 nanorods modified membrane for electrochemical anti-fouling in filtration of oily wastewater. Sep. Purif. Technol. 2015, 156, 931–941. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, L.; Qiao, Y.; Tang, C.; Jung, C.; Yoon, Y.; Li, S.; Yu, M. Ultrafiltration Membranes with Structure—Optimized Graphene—Oxide Coatings for Antifouling Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1400433. [Google Scholar] [CrossRef]
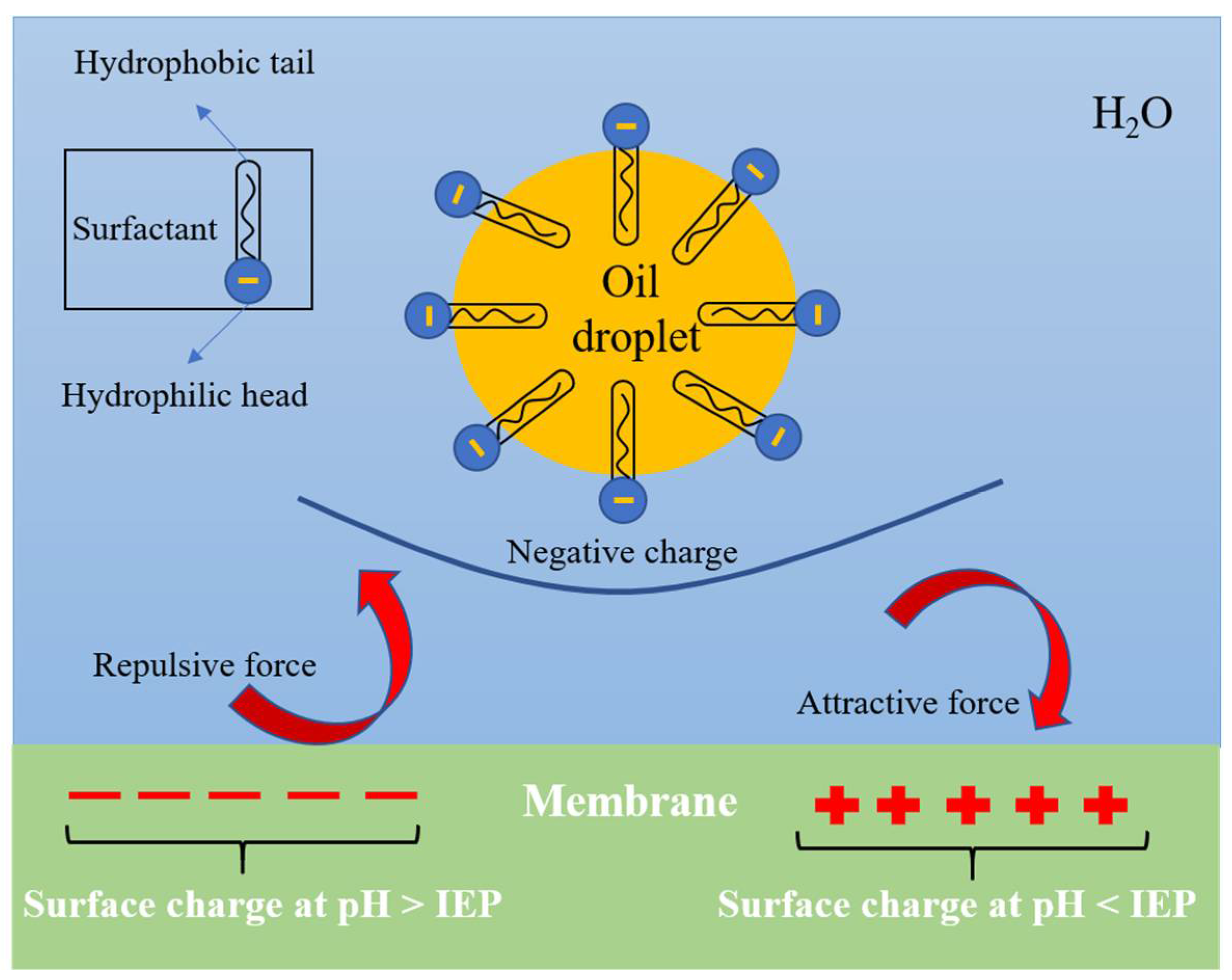


| Ceramic Materials | α-Al2O3 | TiO2 | ZrO2 | SiO2 | SiC | Si3N4 |
|---|---|---|---|---|---|---|
| IEP | 8–9 | 3.9–8.2 | 4–6 | 1.8–2.7 | 2.6 | 3.3 |
| Membrane | Configuration | Method | Function | Pore Size (µm) | Cf (mg/L) | NFi (LMH/bar) | NFs (LMH/bar) | Rd (%) | FD (%) | FT (min) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe2O3/ ZrO2 | Disc | Pulsed-laser deposition | Hydrophilic | 50 kDa | Crude oil, 100 | ~ | ~ | 95COD | 12 | 30 | [32] |
| γ-Al2O3/α-Al2O3 | Tubular | Dip-coating | Hydrophilic/charge | 0.14 | Engine oil, 1000 | 450 | 315 | 98.5 | 30 | 60 | [46] |
| ZrO2/α-Al2O3 | Tubular | Dip-coating | Hydrophilic/charge | 0.2 | Engine oil, 1000 | 316 | 276 | 97.8 | 12.6 | 100 | [37] |
| TiO2/ α-Al2O3 | Tubular | Dip-coating | Hydrophilic/charge | 0.2 | Hydraulic oil, 4000 | 244 | 213 | 99.75 | 12.5 | 120 | [38] |
| GO/Al2O3 | Tubular | Vacuum filtration | Hydrophilic | 0.2 | Machine oil, 1000 | 950 | 728 | 98.7 | 23.3 | 140 | [120] |
| NaA/Al2O3 | Tubular | Hydrothermal synthesis | Hydrophilic | 1.2 | Lubricant oil, 1000 | 109 | 60 | 99.5 | 45 | 50 | [108] |
| TiO2/Al2O3 | Disc | PVD + Hydrothermal reaction | Super-hydrophilic | 0.98 | Toluene, 1/30 mL | 287 | ~ | 93 | ~ | ~ | [131] |
| SiO2/ceramic mixture | Disc | Sol-gel | Super-hydrophilic | ~2 | Oilfield water | ~ | ~ | 99.95 | ~ | ~ | [122] |
| TiO2-doped α-Al2O3 | Tubular | Doping + sintering | Negative charge | 0.2 | Soybean oil, 5000 | 705 | 605 | ~ | 14 | 120 | [50] |
| CNT/YSZ | Disc | CVD | Increase rejection | 0.7 | Blue oil based ink, 210 | 36 | 26 | 100 | 28.6 | 550 | [92] |
| IrO2/Al2O3 | ~ | Dip-coating | Electrochemical degradation | <1 | Peanut oil, 200 | 414 | 331 | 96.3COD | 20 | 160 | [159] |
| Ti4O7/Al2O3 | Tubular | Dip-coating | Electrochemical degradation | 0.2 | Peanut oil, 200 | 1018 | 914 | 97.9COD | 9.2 | 60 | [43] |
| CuO/CeO2/ Al2O3 | Hollow fiber | Sol-gel | Photocatalytic degradation | 0.05 | 1000 | 1815 | 1422 | 92 | 22 | 240 | [151] |
| g-C3N4/Al2O3 | Hollow fiber | Electro-spinning | Photocatalytic degradation | 0.25 | Crude oil, 1000 | 816 | 640 | 99TOC | 21 | 180 | [152] |
| PZT | Disc | ~ | Generated ultrasound | 0.3 | Soybean oil, 500 | 86 | 73.1 | 95.3TOC | 15 | 180 | [155] |
| Al2O3/ PZT | Disc | Dip-coating | Generated ultrasound | 0.1 | Soybean oil, 200 | 230 | 185 | 99.5TOC | 20 | 120 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Heijman, S.G.J.; Rietveld, L.C. State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application. Membranes 2021, 11, 888. https://doi.org/10.3390/membranes11110888
Chen M, Heijman SGJ, Rietveld LC. State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application. Membranes. 2021; 11(11):888. https://doi.org/10.3390/membranes11110888
Chicago/Turabian StyleChen, Mingliang, Sebastiaan G. J. Heijman, and Luuk C. Rietveld. 2021. "State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application" Membranes 11, no. 11: 888. https://doi.org/10.3390/membranes11110888
APA StyleChen, M., Heijman, S. G. J., & Rietveld, L. C. (2021). State-of-the-Art Ceramic Membranes for Oily Wastewater Treatment: Modification and Application. Membranes, 11(11), 888. https://doi.org/10.3390/membranes11110888