Modifications on Promoting the Proton Conductivity of Polybenzimidazole-Based Polymer Electrolyte Membranes in Fuel Cells
Abstract
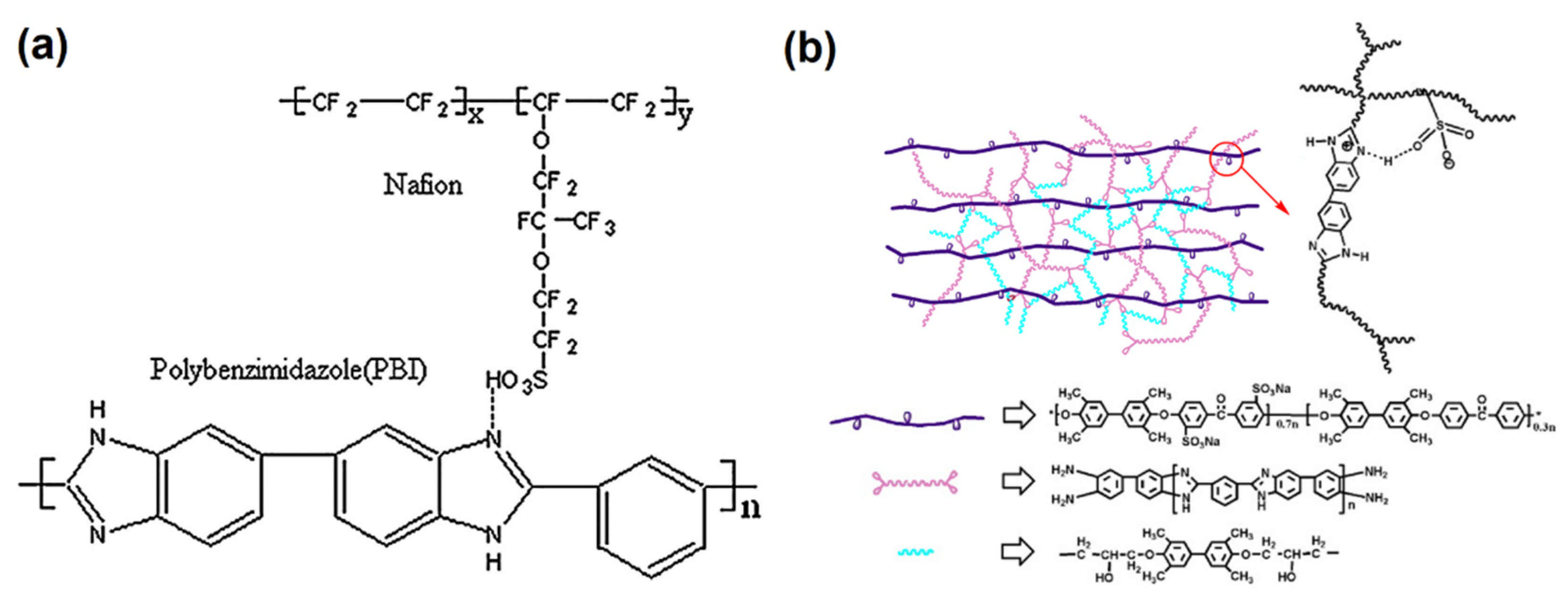
:1. Introduction
2. General Aspects of PBI-Based Polymer Electrolyte Membranes
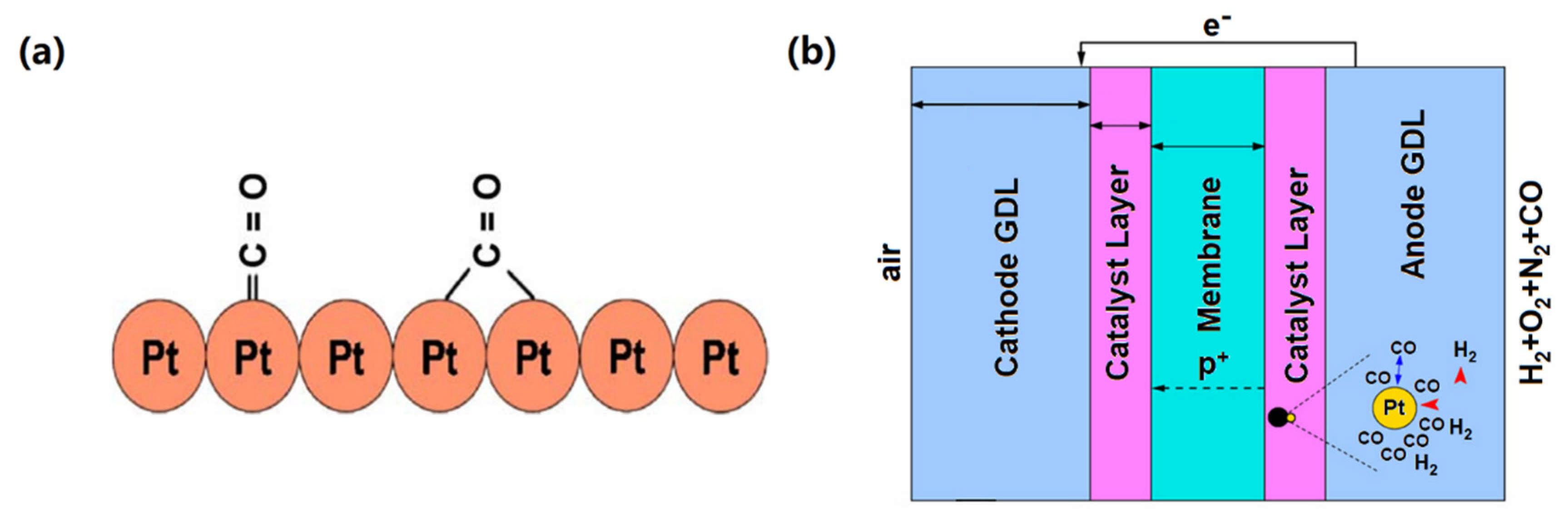
2.1. Mechanism of CO Tolerance in HT-PEMFCs
2.2. Mechanism of Methanol Crossover and Methanol Resistance in DMFCs
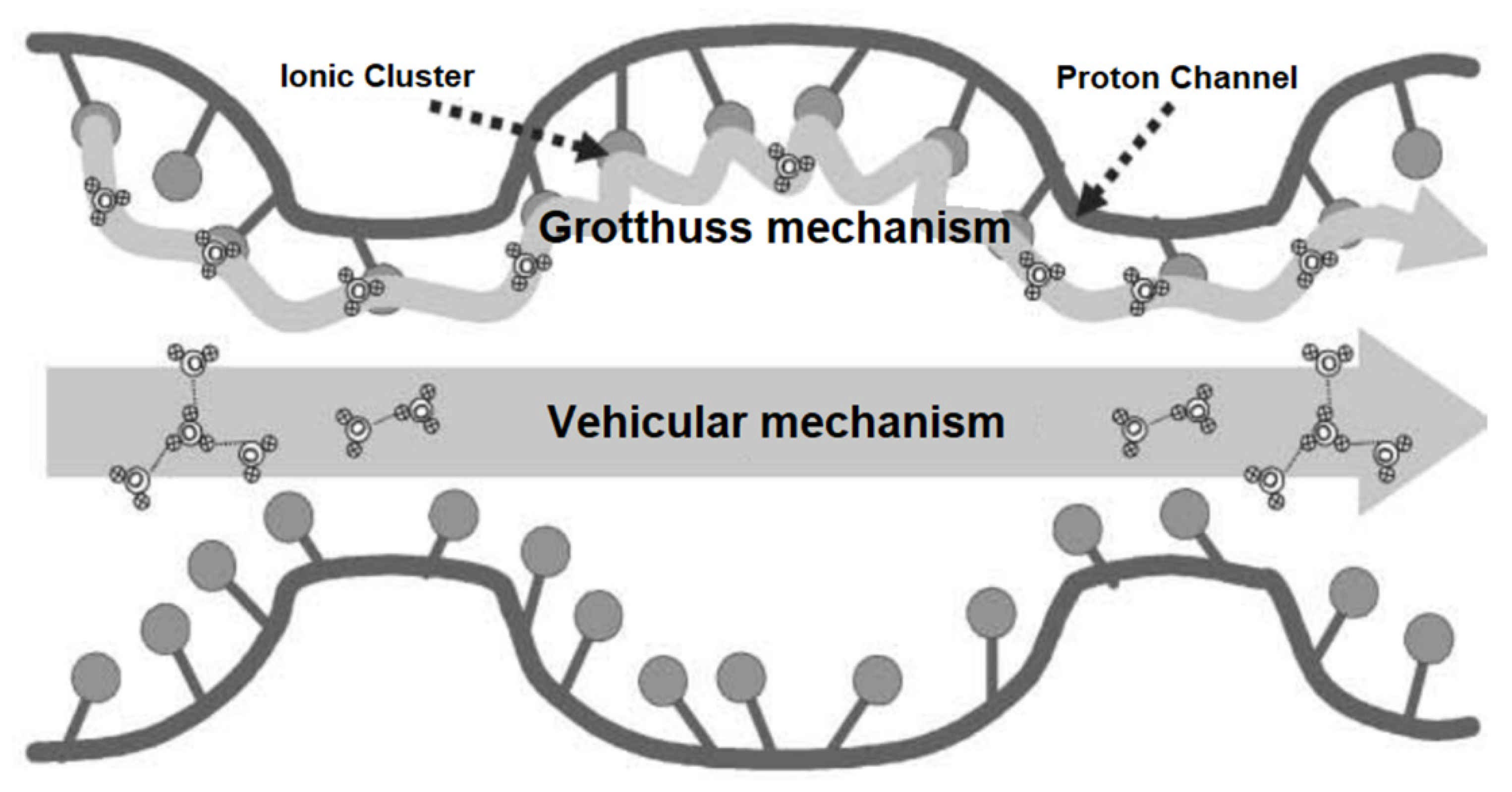
2.3. Methods to Optimize the Proton Conduction in PBI-Based PEMs
3. Progress of Modification of PBI-Based Membranes for Hydrogen-Air PEMFCs
3.1. Modification of PBI Membranes for Hydrogen-Air PEMFCs by Acid Doping
3.2. Modification of PBI Membranes for Hydrogen-Air PEMFCs by Synthesizing Modified Structures
3.3. Modification of PBI Membranes for Hydrogen-Air PEMFCs by Nanomaterials
3.4. Modification of PBI Membranes for Hydrogen-Air PEMFCs by Polymer Crosslinkers
3.5. Modification of PBI Membranes for Hydrogen-Air PEMFCs by Designing Porous Structures or Metal-Organic Frames
4. Progress of Modification of PBI-Based Membranes for DMFCs
4.1. Acid Doping in PBI Membranes for DMFCs
4.2. Modifying the Polymer Structure of PBI Membranes for DMFCs
4.3. Applying Inorganic Nanofillers to PBI Membranes for DMFCs
4.4. Applying Polymer Crosslinkers to PBI Membranes for DMFCs
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.; Calvin, K.; Thomson, A.; Clarke, L.; Bond-Lamberty, B.; Sands, R.; Smith, S.J.; Janetos, A.; Edmonds, J. Implications of limiting CO2 concentrations for land use and energy. Science 2009, 324, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.P.; Maynard, H.L. Design considerations for miniaturized PEM fuel cells. J. Power Sources 2002, 109, 76–88. [Google Scholar] [CrossRef]
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Viswanathan, B.; Uhbgcjbiz, B. Energy sources: Fundamentals of Chemical Conversion Processes and Applications; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, J.L.; Xie, Z.; Zhang, J.J.; Tang, Y.H.; Song, C.J.; Navessin, T.; Shi, Z.Q.; Song, D.T.; Wang, H.J.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Ahmed, M.; Dincer, I. A review on methanol crossover in direct methanol fuel cells: Challenges and achievements. Int. J. Energy Res. 2011, 35, 1213–1228. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. Overview of hybrid membranes for direct-methanol fuel-cell applications. Int. J. Hydrogen Energy 2010, 35, 2160–2175. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.F.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)-A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.F.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M.A. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, J.M.; Zhang, Y.F.; Liu, J.F.; Chen, J.Y.; Li, M.X.; Wang, W.Q.; Liu, X.W. Overcoming undesired fuel crossover: Goals of methanol-resistant modification of polymer electrolyte membranes. Renew. Sustain. Energy Rev. 2021, 138, 110660. [Google Scholar] [CrossRef]
- Li, Q.F.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.F.; He, R.H.; Jensen, J.O.; Bjerrum, N.J. The CO poisoning effect in PEMFCs operational at temperatures up to 200 degrees C. J. Electrochem. Soc. 2003, 150, A1599–A1605. [Google Scholar] [CrossRef] [Green Version]
- Carollo, A.; Quartarone, E.; Tomasi, C.; Mustarelli, P.; Belotti, F.; Magistris, A.; Maestroni, F.; Parachini, M.; Garlaschelli, L.; Righetti, P.P. Developments of new proton conducting membranes based on different polybenzimidazole structures for fuel cells applications. J. Power Sources 2006, 160, 175–180. [Google Scholar] [CrossRef]
- Wang, W.; Hsu, C. Enhanced high-temperature polymer electrolyte membrane for fuel cells based on polybenzimidazole and ionic liquids. Electrochim. Acta 2011, 56, 2842–2846. [Google Scholar] [CrossRef]
- Deborah, J.J.; Jacques, R. Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J. Membr. Sci. 2001, 185, 41–58. [Google Scholar]
- Ubong, E.U.; Shi, Z.; Wang, X. Three-Dimensional Modeling and Experimental Study of a High Temperature PBI-Based PEM Fuel Cell. J. Electrochem. Soc. 2009, 156, B1276–B1282. [Google Scholar] [CrossRef] [Green Version]
- Mamlouk, M.; Scott, K. The effect of electrode parameters on performance of a phosphoric acid-doped PBI membrane fuel cell. Int. J. Hydrogen Energy 2010, 35, 784–793. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.; Lee, S.; Ju, H. Studies of the methanol crossover and cell performance behaviors of high temperature-direct methanol fuel cells (HT-DMFCs). Int. J. Hydrogen Energy 2018, 43, 13999–14011. [Google Scholar] [CrossRef]
- Diaz, L.; Abuin, G.C.; Corti, H.R. Methanol sorption and permeability in Nafion and acid-doped PBI and ABPBI membranes. J. Membr. Sci. 2012, 411–412, 35–44. [Google Scholar] [CrossRef]
- Mack, F.; Aniol, K.; Ellwein, C.; Kerres, J.; Zeis, R. Novel phosphoric acid-doped PBI-blends as membranes for high-temperature PEM fuel cells. J. Mater. Chem. A 2015, 3, 10864. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, Y.N.; Fang, M.L.; Chen, J.L.; Liu, X.L.; Yin, B.B.; Wang, L. Synthesis and preparation of branched block polybenzimidazole membranes with high proton conductivity and single-cell performance for use in high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2020, 602, 117981. [Google Scholar] [CrossRef]
- Xu, C.X.; Cao, Y.C.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Chu, F.; Lin, B.; Qiu, B.; Si, Z.; Qiu, L.H.; Gu, Z.Z.; Ding, J.N.; Yan, F.; Lu, J.M. Polybenzimidazole/zwitterion-coated silica nanoparticle hybrid proton conducting membranes for anhydrous proton exchange membrane application. J. Mater. Chem. 2012, 22, 18411–18417. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Loh, K.S.; Wong, W.Y.; Daud, W.R.W.; Lee, T.K. Sulfonated graphene oxide as an inorganic filler in promoting the properties of a polybenzimidazole membrane as a high temperature proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 27510–27526. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Liu, Z.; Wang, S.; Li, M.Y.; Zhu, J.; Li, H.T.; Zhang, Y.; Lew, C.M.; Ma, H. Cross-linked polybenzimidazole with enhanced stability for high temperature proton exchange membrane fuel cells. J. Mater. Chem. 2011, 21, 2187. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Wang, S.; Yin, X.Y. Performance enhancement of polybenzimidazole based high temperature proton exchange membranes with multifunctional crosslinker and highly sulfonated polyaniline. J. Mater. Chem. 2018, 549, 660–669. [Google Scholar] [CrossRef]
- Gu, Z.Z.; Ding, J.N.; Yuan, N.Y.; Chu, F.Q.; Lin, B.C. Polybenzimidazole/zwitterion-coated polyamidoamine dendrimer composite membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 16410–16417. [Google Scholar] [CrossRef]
- Barati, S.; Mehdipourghazi, M.; Abdollahi, M.; Hooshyari, K.; Khoshandam, B. Preparation, characterization and proton transport of new porous nanocomposite membranes based on polybenzimidazole, lignin and TiO2 nanoparticles for high temperature PEM fuel cells. Int. J. Energy Res. 2021, 45, 20057–20072. [Google Scholar] [CrossRef]
- Jheng, L.C.; Hsu, S.L.C.; Tsai, T.Y.; Chang, W.J.Y. A novel asymmetric polybenzimidazole membrane for high temperature proton exchange membrane fuel cells. J. Mater. Chem. A 2014, 2, 4225–4233. [Google Scholar] [CrossRef]
- Geng, K.; Tang, H.; Ju, Q.; Qian, H.D.; Li, N.W. Symmetric sponge-like porous polybenzimidazole membrane for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2021, 620, 118981. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Bach, A.; Jensen, J.O.; Bjerrum, N.J. Physicochemical properties of phosphoric acid doped polybenzimidazole membranes for fuel cells. J. Membr. Sci. 2006, 277, 38–45. [Google Scholar] [CrossRef]
- Ainla, A.; Brandell, D. Nafion –polybenzimidazole (PBI) composite membranes for DMFC applications. Solid State Ion. 2007, 178, 581–585. [Google Scholar] [CrossRef]
- Chen, J.N.; Perez-Page, M.; Ji, Z.; Zhang, Z.; Guo, Z.M.; Holmes, S. One step electrochemical exfoliation of natural graphite flakes into graphene oxide for polybenzimidazole composite membranes giving enhanced performance in high temperature fuel cells. J. Power Sources 2021, 491, 229550. [Google Scholar] [CrossRef]
- Gogel, V.; Sakthivel, M.; Bender, J.; Salzmann, B.; Kerres, J.; Scholta, J.; Drillet, J.-F. New Materials and Flow Field Design for Middle-Temperature Direct Methanol Fuel Cell with Low Cathode Pressure. Fuel Cells 2019, 19, 256–267. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Zhao, X.S.; Fu, Y.Z.; Manthiram, A. Composite membranes based on sulfonated poly(ether ether ketone) and SDBS-adsorbed graphene oxide for direct methanol fuel cells. J. Mater. Chem. 2012, 22, 24862–24869. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhang, Y.F.; Chen, J.Y.; Yin, C.W.; Liu, X.W. Design, fabrication and performance evaluation of an integrated reformed methanol fuel cell for portable use. J. Power Sources 2018, 389, 37–49. [Google Scholar] [CrossRef]
- Wang, Q.H.; Yang, S.; Zhou, W.; Li, J.R.; Xu, Z.J.; Ke, Y.Z.; Yu, W.; Hu, G.H. Optimizing the porosity configuration of porous copper fiber sintered felt for methanol steam reforming micro-reactor based on flow distribution. Appl. Energy 2018, 216, 243–261. [Google Scholar] [CrossRef]
- Shah, A.A.; Sui, P.C.; Kim, G.S.; Ye, S. A transient PEMFC model with CO poisoning and mitigation by O2 bleeding and Ru-containing catalyst. J. Power Sources 2007, 166, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, H.M.; Zhai, Y.F.; Zhang, Y.; Xu, D.Y.; Shao, Z.G. Pt4ZrO2/C cathode catalyst for improved durability in high temperature PEMFC based on H3PO4 doped PBI. Electrochem. Commun. 2007, 9, 135–141. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Singh, A.; Chu, D. Kinetic characterization of a Pt-Ni/C catalyst with a phosphoric acid doped PBI membrane in a proton exchange membrane fuel cell. J. Power Sources 2008, 176, 112–117. [Google Scholar] [CrossRef]
- Peron, J.; Ruiz, E.; Jones, D.J.; Roziere, J. Solution sulfonation of a novel polybenzimidazole, a proton electrolyte for fuel cell application. J. Membr. Sci. 2008, 314, 247–256. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Havas, D.; Zhang, H.; Xu, P.; Han, J.; Cho, J.; Wu, G. High-performance direct methanol fuel cells with precious-metal-free cathode. Adv. Sci. 2016, 3, 1600140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Xu, Y.; Xia, Y.; Xi, J.; Wang, S. Ultra-small Fe2N nanocrystals embedded into mesoporous nitrogen-doped graphitic carbon spheres as a highly active, stable, and methanol-tolerant electrocatalyst for the oxygen reduction reaction. Nanomater. Energy 2016, 24, 121–129. [Google Scholar] [CrossRef]
- Tung, S.P.; Hwang, B.J. Synthesis and characterization of hydrated phosphor–silicate glass membrane prepared by an accelerated sol-gel process with water/vapor management. J. Mater. Chem. 2005, 15, 3532–3538. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Bouchet, R.; Miller, S.; Deulot, M.; Sonquet, J.L. A thermodynamic approach to proton conductivity in acid-doped polybenzimidazole. Solid State Ion. 2001, 145, 69–78. [Google Scholar] [CrossRef]
- Ryu, S.W.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Effect of type and stoichiometry of fuels on performance of polybenzimidazole-based proton exchange membrane fuel cells operating at the temperature range of 120–160 °C. Energy 2022, 128, 121791. [Google Scholar] [CrossRef]
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ion. 1999, 118, 287–299. [Google Scholar] [CrossRef]
- Chtioui, A.; Jouini, A. Structure, thermal behavior and IR investigations of (C6H9N2)H2PO4. J. Chem. Crystallogr. 2004, 34, 43–49. [Google Scholar] [CrossRef]
- Hughes, C.E.; Haufe, S.; Angerstein, B.; Kalim, R.; Mähr, U.; Reiche, A.; Baldus, M. Probing Structure and Dynamics in Poly[2,2‘-(m-phenylene)-5,5‘-bibenzimidazole] Fuel Cells with Magic-Angle Spinning NMR. J. Phys. Chem. B 2004, 108, 13626–13631. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.L.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Conductivity of PBI Membranes for High-Temperature Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2004, 151, A8–A16. [Google Scholar] [CrossRef]
- Ye, H.; Huang, J.; Xu, J.J.; Kodiweera, N.K.A.C.; Jayakody, J.R.P.; Greenbaum, S.G. New membranes based on ionic liquids for PEM fuel cells at elevated temperatures. J. Power Sources 2008, 178, 651–660. [Google Scholar] [CrossRef]
- Escorihuela, J.; Sahuquillo, Ó.; García-Bernabé, A.; Gimenez, E.; Compan, V. Phosphoric acid doped polybenzimidazole (PBI)/Zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [Green Version]
- Uregen, N.; Pehlivanoglu, K.; Ozdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Kerres, J.; Hein, M.; Zhang, W. Development of new blend membranes for polymer electrolyte fuel cell applications. J. New Mater. Electrochem. Syst. 2003, 6, 223–230. [Google Scholar]
- Kerres, J.; Ullrich, A.; Hein, M.; Gogel, V.; Friedrich, K.A.; Jorissen, L. Crosslinked polyaryl blend membranes for polymer electrolyte fuel cells. Fuel Cells 2004, 4, 105–112. [Google Scholar] [CrossRef]
- Weber, J.; Kreuer, K.D.; Maier, J.; Thomas, A. Proton Conductivity Enhancement by Nanostructural Control of Poly(benzimidazole)-Phosphoric Acid Adducts. Adv. Mater. 2018, 20, 2595–2598. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, A.; Jana, T.; Das, S.K. Fabricating a MOF material with polybenzimidazole into an efficient proton exchange membrane. ACS Appl. Energy Mater. 2020, 3, 7964–7977. [Google Scholar] [CrossRef]
- Hooshyari, K.; Rezania, H.; Vatanpour, V.; Salarizadeh, P.; Askari, B.M.; Beydaghi, H.; Enhesari, M. High temperature membranes based on PBI/sulfonated polyimide and doped-perovskite nanoparticles for PEM fuel cells. J. Membr. Sci. 2020, 612, 118436. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-based Polymer Membranes for High Temperature Fuel Cells–Preparation, Characterization and Fuel Cell Demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Lobato, J.; Caizares, P.; Rodrigo, M.A.; Linares, J.J.; Manjavacas, G. Synthesis and characterisation of poly[2,2-(m-phenylene)-5,5-bibenzimidazole] as polymer electrolyte membrane for high temperature PEMFCs. J. Membr. Sci. 2006, 280, 351–362. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Gubler, L.; Kramer, D.; Belack, J.; Ünsal, Ö.; Schmidt, T.J.; Scherer, G.G. Celtec-V: A polybenzimidazole-based membrane for the direct methanol fuel cell. J. Electrochem. Soc. 2007, 154, B981–B987. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Liu, Y.L. Preparation and properties of nanocomposite membranes of polybenzimidazole/sulfonated silica nanoparticles for proton exchange membranes. J. Membr. Sci. 2009, 332, 121–128. [Google Scholar]
- Li, Q.F.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating Above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef] [Green Version]
- Seland, F.; Berning, T.; Borresen, B. Improving the performance of high-temperature PEM fuel cells based on PBI electrolyte. J. Power Sources 2006, 160, 27–36. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhao, C.; Fu, T.Z.; Shi, Y.H.; Na, H. Composite membranes based on highly sulfonated PEEK and PBI: Morphology characteristics and performance. J. Membr. Sci. 2008, 308, 66–74. [Google Scholar] [CrossRef]
- Hao, C.; Wang, S.; Li, J.S.; Liu, F.X.; Tian, X.; Wang, X.; Mao, T.J.; Xu, J.M.; Wang, Z. Novel cross-linked membranes based on polybenzimidazole and polymeric ionic liquid with improved proton conductivity for ht-pemfc applications. J. Taiwan Inst. Chem. Eng. 2019, 95, 185–194. [Google Scholar]
- Venugopalan, G.; Chang, K.; Nijoka, J.; Livingston, S.; Geise, G.M.; Arges, C.G. Stable and highly conductive polycation–polybenzimidazole membrane blends for intermediate temperature polymer electrolyte membrane fuel cells. ACS Appl. Energy Mater. 2020, 3, 573–585. [Google Scholar] [CrossRef]
- Rajabi, Z.; Javanbakht, M.; Hooshyari, K.; Adibi, M.; Badiei, A. Phosphoric acid doped polybenzimidazole based polymer electrolyte membrane and functionalized SBA-15 mesoporous for elevated temperature fuel cell. Int. J. Hydrogen Energy 2021, 46, 33241–33259. [Google Scholar] [CrossRef]
- Hedberg, F.L.; Marvel, C.S. New single-step process for polybenzimidazole synthesis. J. Polym. Sci. 1974, 12, 1823–1828. [Google Scholar] [CrossRef]
- Kovar, R.F.; Arnold, F.E. Para-ordered polybenzimidazole. J. Polym. Sci. Polym. Chem. 1976, 14, 2807–2817. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, S.; Ghorpade, R.V.; Konovalova, A.; Jang, J.H.; Kim, H.J.; Han, J.H.; Henkensmeier, D.; Han, H. Polybenzimidazole (PBI-OO) based composite membranes using sulfophenylated TiO2 as both filler and crosslinker, and their use in the HT-PEM fuel cell. J. Membr. Sci. 2018, 560, 11–20. [Google Scholar] [CrossRef]
- Berber, M.R.; Nakashima, N. Bipyridine-based polybenzimidazole membranes with outstanding hydrogen fuel cell performance at high temperature and non-humidifying conditions. J. Membr. Sci. 2019, 591, 117354. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, D.Y.; Kim, H.J.; Nam, S.Y.; Choi, J.J.; Kim, J.Y.; Jang, J.H.; Cho, E.A.; Kim, S.K.; Hong, S.A.; et al. Synthesis and characterization of acid-doped polybenzimidazole membranes by sol–gel and post-membrane casting method. J. Membr. Sci. 2010, 357, 130–133. [Google Scholar] [CrossRef]
- Mack, F.; Heissler, S.; Laukenmann, R.; Zeis, R. Phosphoric acid distribution and its impact on the performance of polybenzimidazole membranes. J. Power Sources 2014, 270, 627–633. [Google Scholar] [CrossRef]
- Yu, S.H.; Benicewicz, B.C. Synthesis and Properties of Functionalized Polybenzimidazoles for High-Temperature PEMFCs. Marcomolecules 2009, 42, 8640–8648. [Google Scholar] [CrossRef]
- Liu, C.Z.; Khan, S.B.; Lee, M.J.; Kim, K.I.; Akhtar, K.; Han, H.; Asiri, A.M. Fuel Cell Based on Novel Hyper-Branched Polybenzimidazole Membrane. Macromol. Res. 2013, 21, 35–41. [Google Scholar] [CrossRef]
- Lin, H.L.; Hu, C.R.; Lai, S.W.; Yu, L. Polybenzimidazole and butylsulfonate grafted polybenzimidazole blends for proton exchange membrane fuel cells. J. Membr. Sci. 2012, 389, 399–406. [Google Scholar] [CrossRef]
- Klaehn, J.R.; Luther, T.A.; Orme, C.J.; Jones, M.G.; Wertsching, A.K.; Peterson, E.S. Soluble N-substituted organosilane polybenzimidazoles. Macromolecules 2007, 40, 7487–7492. [Google Scholar] [CrossRef]
- Shabanikia, A.; Javanbakht, M.; Amoli, H.S.; Hooshyari, K.; Enhessari, M. Effect of La2Ce2O7 on the physicochemical properties of phosphoric acid doped polybenzimidazole nanocomposite membranes for high temperature proton exchange membrane fuel cells applications. J. Electrochem. Soc. 2014, 161, F1403–F1408. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Karthikeyan, C.S.; Nunes, S.P.; Prado, L.A.S.A.; Ponce, M.L.; Silva, H.; Ruffmann, B.; Schulte, K. Polymer nanocomposite membranes for DMFC application. J. Membr. Sci 2005, 254, 139–146. [Google Scholar] [CrossRef]
- Wang, S.W.; Sun, P.; Li, Z.F.; Liu, G.H.; Yin, X.Y. Comprehensive performance enhancement of polybenzimidazole based high temperature proton exchange membranes by doping with a novel intercalated proton conductor. Int. J. Hydrogen Energy 2018, 43, 9994–10003. [Google Scholar] [CrossRef]
- Wang, L.; Deng, N.; Wang, G.; Ju, J.; Cheng, B.; Kang, W. Constructing Amino-Functionalized Flower-like Metal Organic Framework Nanofibers in Sulfonated Poly(ether sulfone) Proton Exchange Membrane for Simultaneously Enhancing Interface Compatibility and Proton Conduction. ACS Appl. Mater. Interfaces 2019, 11, 39979. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Wang, S.; Wang, D.; Liu, G.; Cui, Y.H.; Liang, D.; Wang, X.D.; Yong, Z.P.; Wang, Z. Multifunctional poly (ionic liquid) s cross-linked polybenzimidazole membrane with excellent long-term stability for high temperature-proton exchange membranes fuel cells. J. Power Sources 2021, 494, 229732. [Google Scholar] [CrossRef]
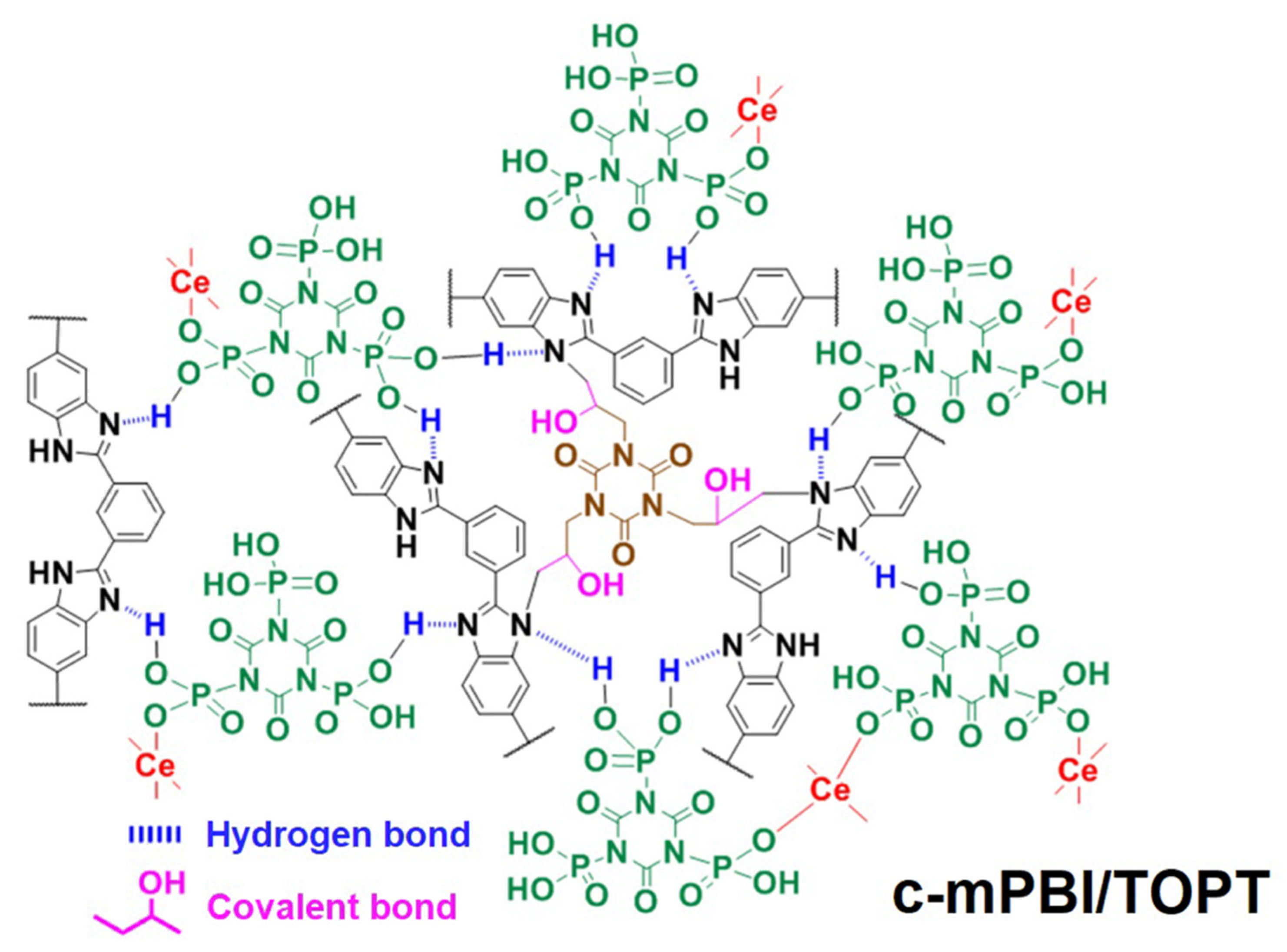
- Guo, H.; Li, Z.F.; Sun, P.; Pei, H.C.; Zhang, L.; Cui, W.H.; Yin, X.Y.; Hui, H.S. Enhancing proton conductivity and durability of crosslinked PBI-Based high-temperature PEM: Effectively doping a novel cerium triphosphonic-isocyanurate. J. Electrochem. Soc. 2021, 168, 024510. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.F.; Jin, L.; Yang, Y.G.; Wang, S.W.; Yin, X.Y.; Wang, Y.X. Pre-Oxidized Acrylic Fiber Reinforced Ferric Sulfophenyl Phosphate-Doped Polybenzimidazole-Based High-Temperature Proton Exchange Membrane. Macromol. Mater. Eng. 2017, 302, 1600468. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.F.; Jin, L.; Wang, S.W.; Yin, X.Y. Construction of proton channels and reinforcement of physicochemical properties of oPBI/FeSPP/GF high temperature PEM via building hydrogen bonding network. Int. J. Hydrogen Energy 2017, 42, 14572–14582. [Google Scholar] [CrossRef]
- Song, M.; Lu, X.; Li, Z.; Liu, G.; Yin, X.; Wang, Y. Compatible ionic crosslinking composite membranes based on SPEEK and PBI for high temperature proton exchange membranes. Int. J. Hydrogen Energy 2016, 41, 12069–12081. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Y.L. Polyelectrolyte composite membranes of polybenzimidazole and crosslinked polybenzimidazole-polybenzoxazine electrospun nanofibers for proton exchange membrane fuel cells. J. Mater. Chem. A 2013, 1, 1171–1178. [Google Scholar] [CrossRef]
- Ven, E.; Chairuna, A.; Merle, G.; Benito, S.P.; Borneman, Z.; Nijmeijer, K. Ionic liquid doped polybenzimidazole membranes for high temperature Proton Exchange Membrane fuel cell applications. J. Power Sources 2013, 222, 202–209. [Google Scholar]
- Kim, K.; Choi, S.W.; Park, J.O.; Kim, S.K.; Lim, M.Y.; Kim, K.H.; Ko, T.; Lee, J.C. Proton conductive cross-linked benzoxazine-benzimidazole copolymers as novel porous substrates for reinforced pore-filling membranes in fuel cells operating at high temperatures. J. Membr. Sci. 2017, 536, 76–85. [Google Scholar]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Shigematsu, A.; Yamada, T.; Kitagawa, H. Wide control of proton conductivity in porous coordination polymers. J. Am. Chem. Soc. 2011, 133, 2034–2036. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Ko, T.; Han, J.; Lee, J.C. Polybenzimidazole composite membranes containing imidazole functionalized graphene oxide showing high proton conductivity and improved physicochemical properties. Int. J. Hydrogen Energy 2021, 46, 12254–12262. [Google Scholar] [CrossRef]
- Pan, H.Y.; Chen, S.X.; Jin, M.; Chang, Z.H.; Pu, H.T. Preparation and properties of sulfonated polybenzimidazole-polyimide block copolymers as electrolyte membranes. Ionics 2018, 24, 1629–1638. [Google Scholar] [CrossRef]
- Hooshyari, K.; Rezania, H.; Vatanpour, V.; Rastgoo-Deylami, M.; Rajabi, H.R. New blend nanocomposite membranes based on PBI/sulfonated poly (ether keto imide sulfone) and functionalized quantum dot with improved fuel cell performance at high temperatures. Int. J. Energy Res. 2021, 1–19. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.T.; Wang, D.; Savinell, R.F.; Litt, M. Acid-doped Polybenzimidazoles-a New Polymer Electrolyte. J. Electrochem. Soc. 1995, 142, L121–L123. [Google Scholar] [CrossRef]
- Wang, J.T.; Wasmus, S.; Savinell, R.F. Real-time mass spectrometric study of the methanol crossover in a direct methanol fuel cell. J. Electrochem. Soc. 1996, 143, 1233–1239. [Google Scholar] [CrossRef]
- Maity, S.; Singha, S.; Jana, T. Low acid leaching PEM for fuel cell based on polybenzimidazole nanocomposites with protic ionic liquid modified silica. Polymer 2015, 66, 76–85. [Google Scholar] [CrossRef]
- Kawahara, M.; Morita, J.; Rikukawa, M.; Sanui, K.; Ogata, N. Synthesis and proton conductivity of thermally stable polymer electrolyte: Poly(benzimidazole) complexes with strong acid molecules. Electrochim. Acta 2000, 45, 1395–1398. [Google Scholar] [CrossRef]
- Wycisk, R.; Chisholm, J.; Lee, J.; Lin, J.; Pintauro, P.N. Direct methanol fuel cell membranes from Nafion–polybenzimidazole blends. J. Power Sources 2006, 163, 9–17. [Google Scholar] [CrossRef]
- Qian, G.Q.; Smith, D.W., Jr.; Benicewicz, B.C. Synthesis and characterization of high molecular weight perfluorocyclobutyl-containing polybenzimidazoles (PFCB–PBI) for high temperature polymer electrolyte membrane fuel cells. Polymer 2009, 50, 3911–3916. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Lu, S.; Jiang, S.P. Significantly enhanced performance of direct methanol fuel cells at elevated temperatures. J. Power Sources 2020, 450, 227620. [Google Scholar] [CrossRef]
- Aili, D.; Vassiliev, A.; Jensen, J.O.; Schmidt, T.J.; Li, Q.F. Methyl phosphate formation as a major degradation mode of direct methanol fuel cells with phosphoric acid based electrolytes. J. Power Sources 2015, 279, 517–521. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, W.; Wu, Q.; Sun, H.Y.; Luo, Z.K.; Fu, H. High-temperature passive direct methanol fuel cells operating with concentrated fuels. J. Power Sources 2015, 273, 517–521. [Google Scholar] [CrossRef]
- Chuang, S.W.; Hsu, L.C.; Hsu, C.L. Synthesis and properties of fluorine-containing polybenzimidazole/montmorillonite nanocomposite membranes for direct methanol fuel cell applications. J. Power Sources 2007, 168, 172–177. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, H.J.; Kim, H.J.; Hwang, I.; Jang, J.H.; Cho, E.A.; Kim, S.K.; Henkensmeier, D.; Hong, S.A.; Lim, T.H. Sulfonated poly(ether sulfone)/sulfonated polybenzimidazole blend membrane for fuel cell applications. Eur. Polym. J. 2010, 46, 1633–1641. [Google Scholar] [CrossRef]
- Gao, C.M.; Hu, M.S.; Fang, M.L.; Chen, J.L.; Wang, L. Alkali-doped hyperbranched cross-linked polybenzimidazoles containing benzyltrimethyl ammoniums with improved ionic conductivity as alkaline direct methanol fuel cell membranes. Energy Res. 2020, 44, 4677–4686. [Google Scholar] [CrossRef]
- Hu, F.Q.; Lin, B.C.; Feng, T.Y.; Wang, C.Y.; Zhang, S.; Yuan, N.Y.; Liu, Z.F.; Ding, J.N. Zwitterion-coated graphene-oxide-doped composite membranes for proton exchange membrane applications. J. Membr. Sci. 2015, 496, 31–38. [Google Scholar]
- Silva, V.S.; Weisshaar, S.; Reissner, R.; Ruffmann, B.; Vetter, S.; Mendes, A.; Madeira, L.M.; Nunes, S. Performance and efficiency of a DMFC using non-fluorinated composite membranes operating at low/medium temperatures. J. Power Sources 2005, 145, 485–494. [Google Scholar] [CrossRef]
- Yu, X.X.; Lou, F.; Yang, Z.H. Bottom-up design of a stable CO-tolerant platinum electrocatalyst with enhanced fuel cell performance in direct methanol fuel cells. RSC Adv. 2016, 101, 98554–99614. [Google Scholar] [CrossRef]
- Okamoto, M.; Fujigaya, T.; Nakashima, N. Design of an Assembly of Poly(benzimidazole), Carbon Nanotubes, and Pt Nanoparticles for a Fuel-Cell Electrocatalyst with an Ideal Interfacial Nanostructure. Small 2009, 5, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.S.; Ruffmann, B.; Vetter, S.; Mendes, A.; Madeire, L.M.; Nunes, S.P. Characterization and application of composite membranes in DMFC. Catal. Today 2005, 104, 205–212. [Google Scholar] [CrossRef]
- Kumar, S.R.; Wang, J.J.; Wu, Y.S.; Yang, C.C.; Lue, S.J.J. Synergistic role of graphene oxide-magnetite nanofillers contribution on ionic conductivity and permeability for polybenzimidazole membrane electrolytes. J. Power Sources 2020, 445, 227293. [Google Scholar] [CrossRef]
- Feng, S.G.; Shang, Y.M.; Wang, S.B.; Xie, X.F.; Wang, Y.Z.; Wang, Y.; Xu, J.M. Novel method for the preparation of ionically crosslinked sulfonated poly (arylene ether sulfone)/polybenzimidazole composite membranes via in situ polymerization. J. Membr. Sci. 2010, 346, 105–112. [Google Scholar] [CrossRef]
- Mondal, S.; Soam, S.; Kundu, P.P. Reduction of methanol crossover and improved electrical efficiency in direct methanol fuel cell by the formation of a thin layer on Nafion 117 membrane: Effect of dip-coating of a blend of sulphonated PVdF-co-HFP and PBI. J. Membr. Sci. 2015, 474, 140–147. [Google Scholar] [CrossRef]
- Pasupathi, S.; Ji, P.S.; Bladergroen, B.J.; Linkov, V. High DMFC performance output using modified acid–base polymer blend. Int. J. Hydrogen Energy 2008, 33, 3132–3136. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Li, M.Y.; Wang, S.; Liu, Z.G.; Li, H.T.; Zhang, Y.; Xu, D.; Wang, J.; Ni, J.; et al. Sulfonated poly (ether ether ketone)/polybenzimidazole oligomer/epoxy resin composite membranes in situ polymerization for direct methanol fuel cell usages. J. Power Sources 2011, 196, 9916–9923. [Google Scholar] [CrossRef]
- Haghighi, A.H.; Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Bahlakeh, G.; Shakeri, E.; Majedi, F.S.; Emami, S.H.; Moaddel, H. Direct methanol fuel cell performance of sulfonated poly (2,6-dimethyl-1,4-phenylene oxide)-polybenzimidazole blend proton exchange membranes. Int. J. Hydrogen Energy 2011, 36, 3688–3696. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. A novel hybrid Nafion-PBI-ZP membrane for direct methanol fuel cells. Int. J. Hydrogen Energy 2011, 36, 14668–14677. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.F.; Sun, P.; Pei, H.C.; Yin, X.Y. Preparation and Properties of Covalently Crosslinked Polybenzimidazole High Temperature Proton Exchange Membranes Doped with High Sulfonated Polyphosphazene. J. Electrochem. Soc. 2020, 167, 104617. [Google Scholar] [CrossRef]
- Wycisk, R.; Lee, J.K.; Pintauro, P.N. Sulfonated polyphosphazene-polybenzimidazole membranes for DMFCs. J. Electrochem. Soc. 2005, 152, A892–A898. [Google Scholar] [CrossRef]
- Wang, S.H.; Lin, H.L. Poly (vinylidene fluoride-co-hexafluoropropylene)/polybenzimidazole blend nanofiber supported Nafion membranes for direct methanol fuel cells. J. Power Sources 2014, 257, 254–263. [Google Scholar] [CrossRef]


| Type of Membrane | Synthesis Method | σ (S·cm−1) | Operating Temperature (°C) | Publishing Year | Ref. |
|---|---|---|---|---|---|
| PBI/ZrP + H3PO4 PBI/SiWA + SiO2 + H3PO4 PBI/PWA + SiO2 + H3PO4 | solvent casting | 9 × 10−2 (RH = 5%) 3–4 × 10−2 (RH = 5%) 1.5 × 10−3 (RH = 5%) | 200 200 150 | 2003 | [70] |
| PBI/H3PO4 | solvent casting | 5.90 × 10−2 (RH = 30%) | 150 | 2004 | [55] |
| PBI/H3PO4 (solvent:DMAc) | solvent casting | 6.1~6.3 × 10−2 | 140 | 2006 | [34] |
| PBI/H3PO4 | solvent casting | 6.8 × 10−2 | 200 | 2006 | [71] |
| PBI/H3PO4 | solvent casting | 0.039 | 190 | 2006 | [65] |
| H3PO4/PMIH2PO4/PBI | solvent casting | 2.0 × 10−3 | 150 | 2008 | [55] |
| SPEEK/PBI/H3PO4 | solvent casting | 0.080 | 80 | 2008 | [72] |
| PBI/HMI-Tf/H3PO4 | solvent casting | 1.6 × 10−2 | 250 | 2011 | [17] |
| cPBI–BF4 + H3PO4 | solvent casting | 0.117 | 170 | 2019 | [73] |
| QPPSf-PBI + H3PO4 | solvent casting | 0.09 | 200 | 2020 | [74] |
| PBI/Mim7+/PAM2 + H3PO4 | solvent casting | 0.226 | 180 | 2021 | [75] |
| Type of Membrane | Synthesis Method | σ (S·cm−1) | Energy Density | Operating Temperature (°C) | Publishing Year | Ref. |
|---|---|---|---|---|---|---|
| 2OH-PBI/PPA | solvent casting | 0.350 | 0.294 W·cm−2 | 180 | 2009 | [82] |
| OPBI/AMS | solvent casting | 0.125 | N/A | 160 | 2011 | [69] |
| HB-PBI | solvent casting | 0.168 | 0.346 W·cm−2 | 150 | 2013 | [83] |
| P-PBI | solvent casting | 0.160 | N/A | 150 | 2010 | [80] |
| AB-PBI | N/A | N/A | 0.305 W·cm−2 | 120 | 2014 | [81] |
| F6PBI | solvent casting | N/A | 0.322 W·cm−2 | 160 | 2015 | [23] |
| c-sTiO2-PBI-OO | solvent casting | 0.098 | 0.356 W·cm−2 | 160 | 2018 | [78] |
| Bipy-PBI | solvent casting | 0.037 | 0.779 W·cm−2 | 120 | 2019 | [79] |
| PBI/ZC-SiO2 | solvent casting | 0.190 | N/A | 160 | 2012 | [26] |
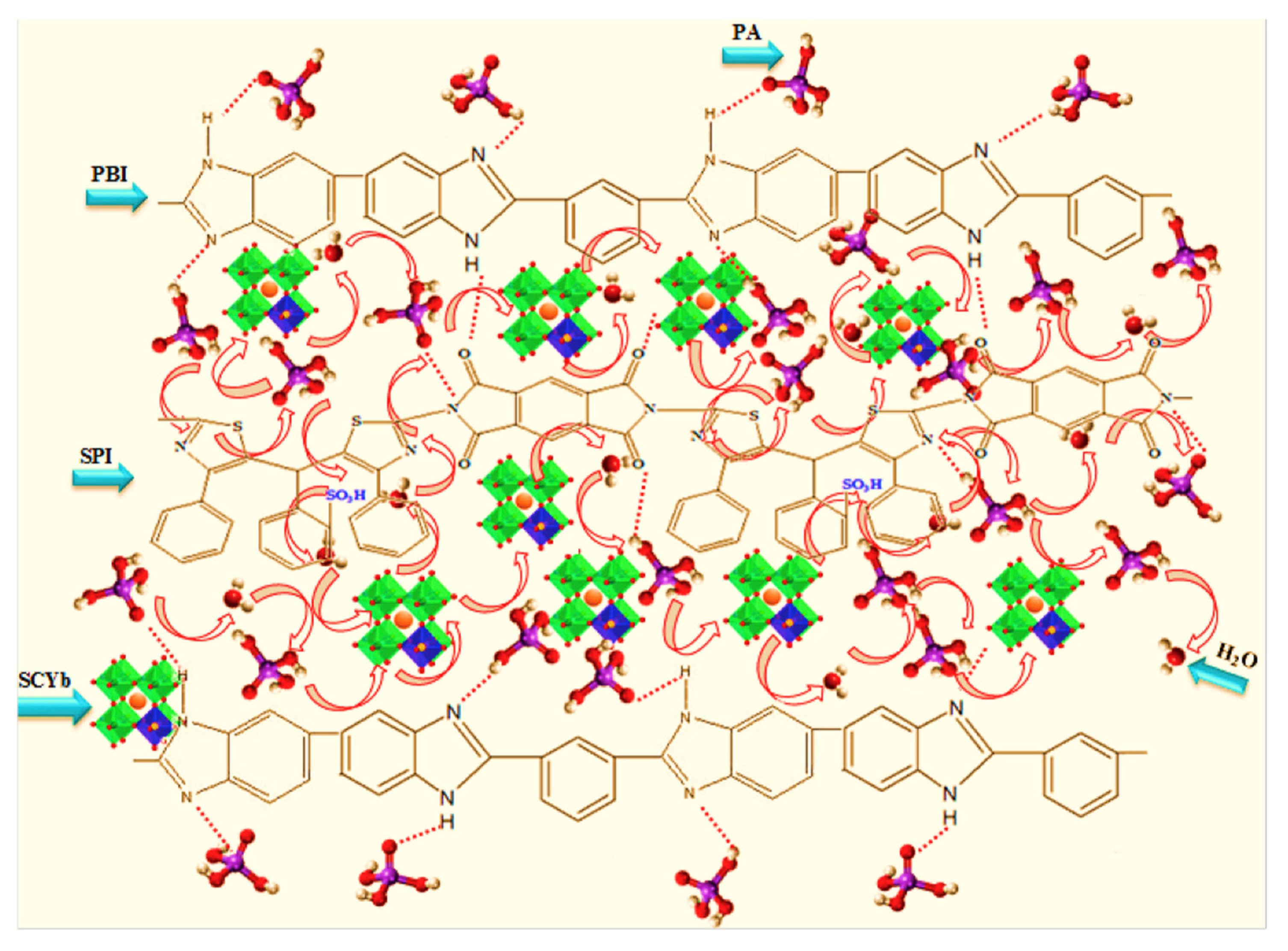
| PPBI-SPI25-SCYbx | solvent casting | 0.131 | 0.59 W·cm−2 | 180 | 2020 | [63] |
| ILGO/PBI | hot-pressed | 0.035 | 0.32 W·cm−2 | 175 | 2015 | [25] |
| PBI-SGO | solvent casting | 0.029 | 40% higher than pure PBI membrane | 150 | 2020 | [27] |
| PBI/ImGO | solvent casting | 0.078 | N/A | 150 | 2021 | [101] |
| PBI-TMBP | 160 ℃ heating | 0.051 | N/A | 200 | 2011 | [28] |
| PBI/FeSPP-PWA | hot-pressed | 0.110 | N/A | 170 | 2018 | [89] |
| PBI-TGIC/SPAN | solvent casting | 0.130 | N/A | 180 | 2018 | [29] |
| NbPBI-TPAm | solvent casting | 0.072 | 0.385 W·cm−2 | 160 | 2021 | [90] |
| PSM-OPBI | solution casting | 0.308 | N/A | 160 | 2020 | [62] |
| Porous PBI | VIPS+ solvent casting | 0.071 | 0.485 W·cm−2 | 180 | 2021 | [33] |
| asymmetric PBI | soft-template | 0.066 | 0.295 W·cm−2 | 160 | 2014 | [32] |
| PBI@ZIF | solvent casting | 0.091 ± 0.002 | N/A | 200 | 2018 | [59] |
| mp-PBI | hard templating | 0.011 | N/A | 180 | 2008 | [61] |
| PBI-PBz-NF- X | electrospinning | 0.17 | 0.67W·cm−2 | 160 | 2013 | [96] |
| Spbi-b-PIs | Solvent evaporation | 0.2 | N/A | 160 | 2018 | [102] |
| PBI-BS | solvent casting | 0.031 | 0.551W·cm−2 | 160 | 2012 | [84] |
| PBI/IL | solvent casting | 1.86 | 0.039 W·cm−2 | 190 | 2013 | [97] |
| SPF-70 | solvent casting | N/A | 0.186 W·cm−2 | 120 | 2017 | [98] |
| PBI/La2Ce2O7 | solvent casting | 0.093 | 0.43 W cm−2 | 180 | 2014 | [86] |
| PPBI-SPI-SCYb | solvent casting | 0.131 | N/A | 180 | 2020 | [63] |
| PPBI-SPE-ZQD | solvent casting | 0.162 | 0.67 W/cm2 | 180 | 2021 | [103] |
| Type of Membrane | Synthesis Method | ρ (cm2·s−1) | σ (S·cm−1) | Operating Temperature (℃) | Publishing Year | Ref. |
|---|---|---|---|---|---|---|
| 90 μm H3PO4/PBI | solvent casting | 5~11 mA·cm−2 (CO2) | N/A | 180 | 1996 | [105] |
| PBI/H3PO4 | N/A | N/A | 10−5 | 160 | 2000 | [107] |
| mPBI/H3PO4 | solvent casting | N/A | 10−4~10−1 | 150 | 2015 | [111] |
| PBI/H3PO4 | N/A | 5~35 mA cm−2 | N/A | 140~160 | 2015 | [112] |
| PBI/H3PO4 | N/A | 6~26 mA cm−2 | N/A | 175 | 2018 | [21] |
| Type of Membrane | Synthesis Method | ρ (cm2·s−1) | σ (S·cm−1) | Energy Density | Operating Temperature (°C) | Publishing Year | Ref. |
|---|---|---|---|---|---|---|---|
| PBI_4N | solvent casting | 2 × 10−8 | >0.1 | N/A | 80 | 2006 | [16] |
| fluorine-containing PBI/m-MMT | solvent casting | 6.2 × 10−9 | 7.94 × 10−5 | N/A | 160 | 2007 | [113] |
| PES/MS-p-PBI | solvent casting | N/A | 0.072 | 506.9 mW cm−2 | 70 | 2010 | [114] |
| SPEEK/o-PBI | solvent casting | 2.38 × 10−8 | 0.14 | N/A | 80 | 2011 | [13] |
| AB-PBI | High-temperature casting | 1.63~2.07 × 10−7 | 0.041 | N/A | 60 | 2012 | [22] |
| QOPBI-x | solvent casting | 1.86 × 10−8 | 0.122 | 75.6 mW cm−2 | 60 | 2020 | [115] |
| Type of Membrane | Synthesis Method | ρ (cm2·s−1) | σ (S·cm−1) | Energy Density | Operating Temperature (°C) | Publishing Year | Ref. |
|---|---|---|---|---|---|---|---|
| PBI/ZC-GO | solvent casting | 1.38 × 10−7 | 1.83 × 10−2 | N/A | 25–90 | 2015 | [116] |
| PBI/SiO2 | casting & pre-heat treatment | N/A | 2.9–4.1 × 10−2 | 237 mW cm−2 (260 °C) | 200–250 | 2020 | [110] |
| PBI/SA-SNP | polycondensation reaction | 3.3 × 10−7 | N/A | N/A | N/A | 2009 | [69] |
| PBI/GO-Fe3O4 | solvothermal | 9.6 × 10−7 | 4.6 × 10−2 | 233 mW cm−2 | 80 | 2020 | [121] |
| Nafion-PBI-ZP | solvent casting | 2.34 × 10−7 | 0.020 | N/A | room temperature | 2011 | [127] |
| (PBI)/ZC-PAMAM | solvent casting | 5.23 × 10−8 | 1.83 × 10−2 | N/A | 80 | 2013 | [30] |
| M-SPVdF-co-HFP/PBI | solution polymerization | 1.22 × 10−6~9.71 × 10−7 | 0.0042- 0.0301 | 36–39 mW cm−2 | 60–90 | 2015 | [123] |
| SPAES-PBI | in situ polymerization | 2.15 × 10−7 | 0.077 | N/A | 30 | 2010 | [124] |
| SPPO-PBI | catalyst painting technique | 5.5 × 10−7 | 0.012 | 57.6 mW cm−2 | 25–70 | 2011 | [126] |
| SPOP/PBI | solvent casting | 1 × 10−7~2 × 10−6 | 0.005–0.08 | N/A | 60 | 2005 | [129] |
| SPEEK/PBI | solvent casting | N/A | 0.0046 | N/A | 60 | 2008 | [124] |
| SPEEK/o-PBI/TMBP | solvent casting | 2.38 × 10−8 | 0.14 | N/A | 80 | 2011 | [125] |
| N/PVFP-BI | Electrospinning process | 1.88 ± 0.02 × 10−8 | 1.3 ± 0.1 × 10−2 | 84.6–106.2 mW cm−2 | 70–90 | 2014 | [130] |
| sPEEK/ZrPh/ PBI | solvent casting | N/A | 0.0294 | N/A | 25 | 2005 | [117] |
| sPEEK/ZrPh/ PBI | solvent casting | 4.7 × 10−7 | 18.2 × 10−2 | N/A | 110 | 2005 | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cao, J.; Zhang, R.; Zhou, J.; Wang, S.; Liu, X.; Zhang, T.; Tao, X.; Zhang, Y. Modifications on Promoting the Proton Conductivity of Polybenzimidazole-Based Polymer Electrolyte Membranes in Fuel Cells. Membranes 2021, 11, 826. https://doi.org/10.3390/membranes11110826
Chen J, Cao J, Zhang R, Zhou J, Wang S, Liu X, Zhang T, Tao X, Zhang Y. Modifications on Promoting the Proton Conductivity of Polybenzimidazole-Based Polymer Electrolyte Membranes in Fuel Cells. Membranes. 2021; 11(11):826. https://doi.org/10.3390/membranes11110826
Chicago/Turabian StyleChen, Junyu, Jiamu Cao, Rongji Zhang, Jing Zhou, Shimin Wang, Xu Liu, Tinghe Zhang, Xinyuan Tao, and Yufeng Zhang. 2021. "Modifications on Promoting the Proton Conductivity of Polybenzimidazole-Based Polymer Electrolyte Membranes in Fuel Cells" Membranes 11, no. 11: 826. https://doi.org/10.3390/membranes11110826





