Impact of Membrane Phosphoric Acid Doping Level on Transport Phenomena and Performance in High Temperature PEM Fuel Cells
Abstract
:1. Introduction
2. Model Description
2.1. Physical Model
2.2. Governing Equations
2.3. Numerical Implementation
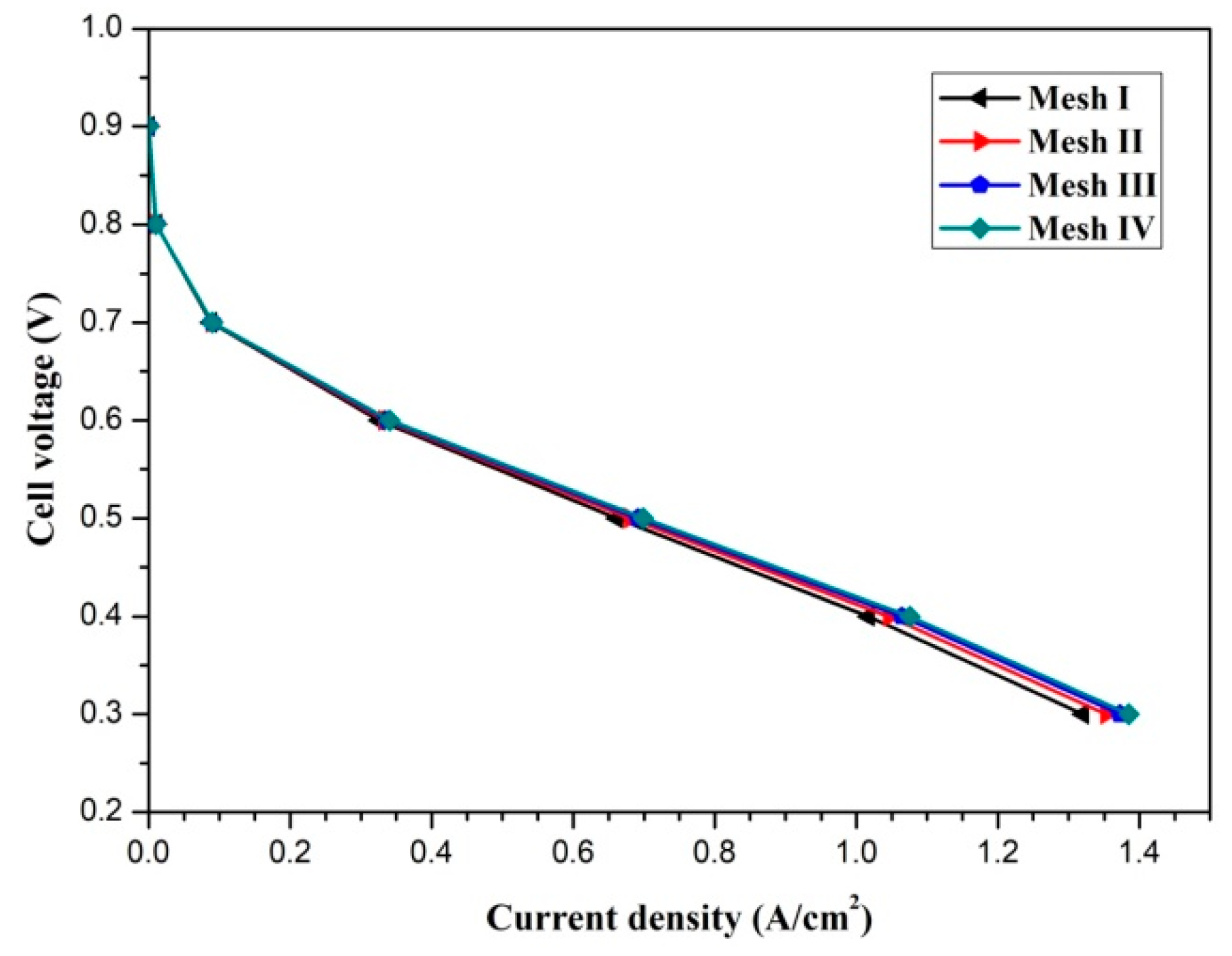
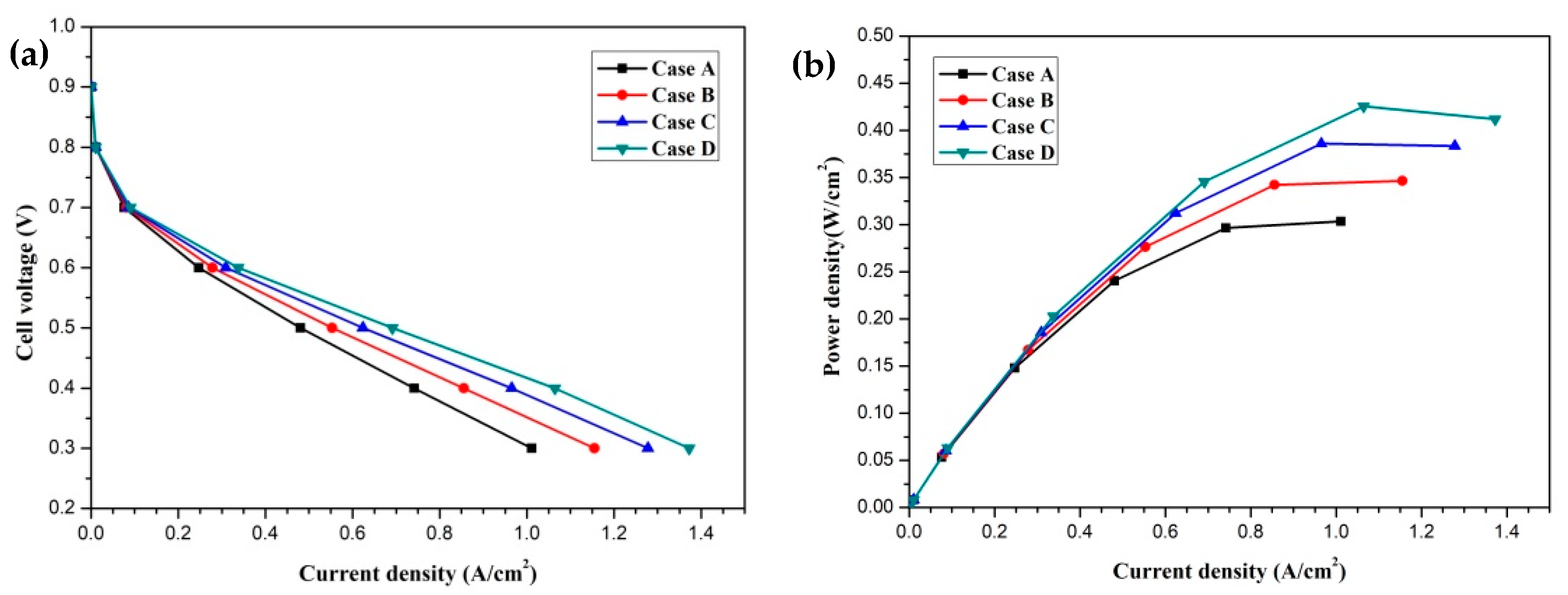
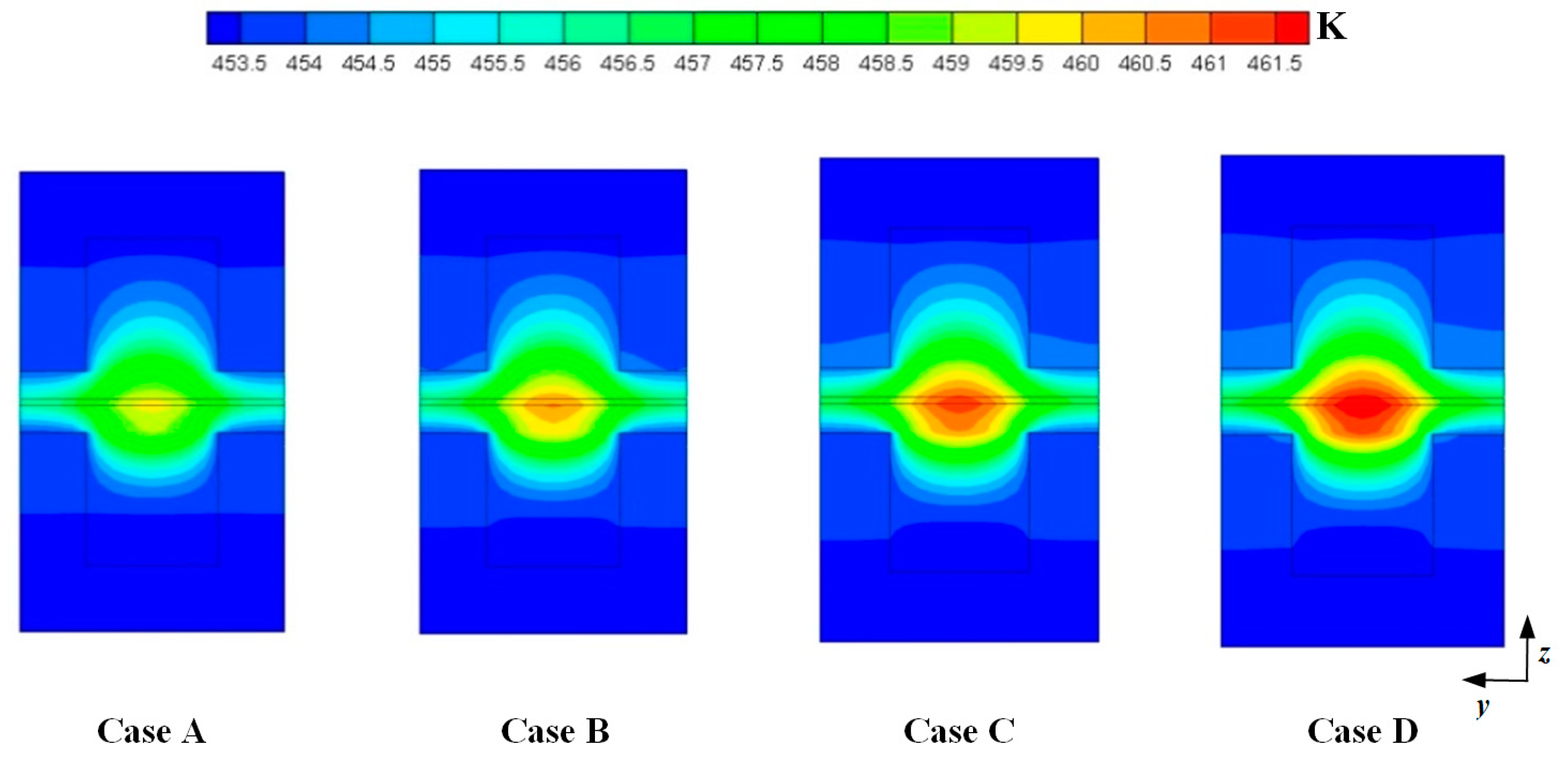
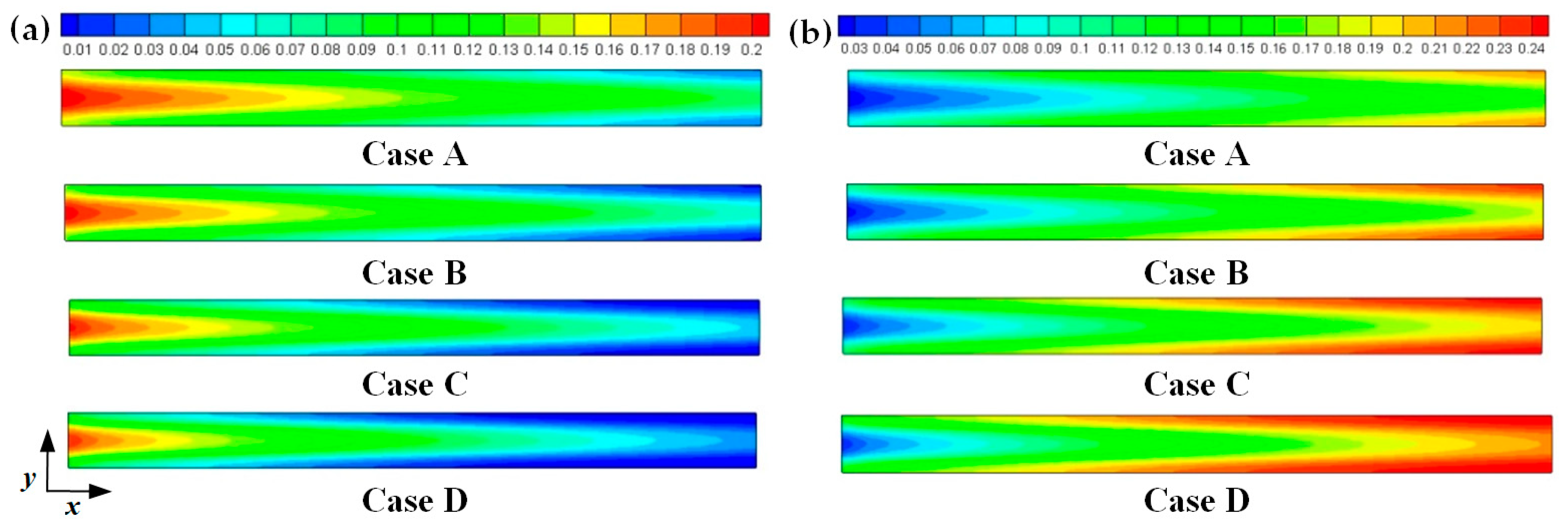
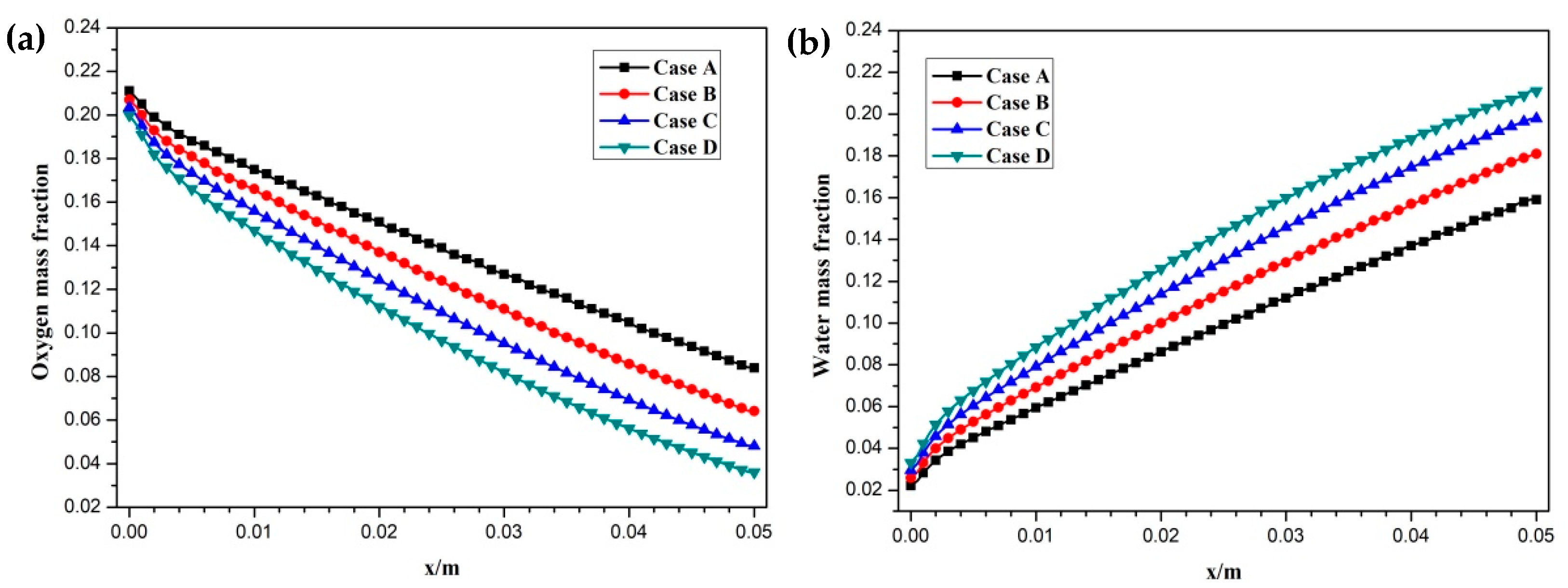
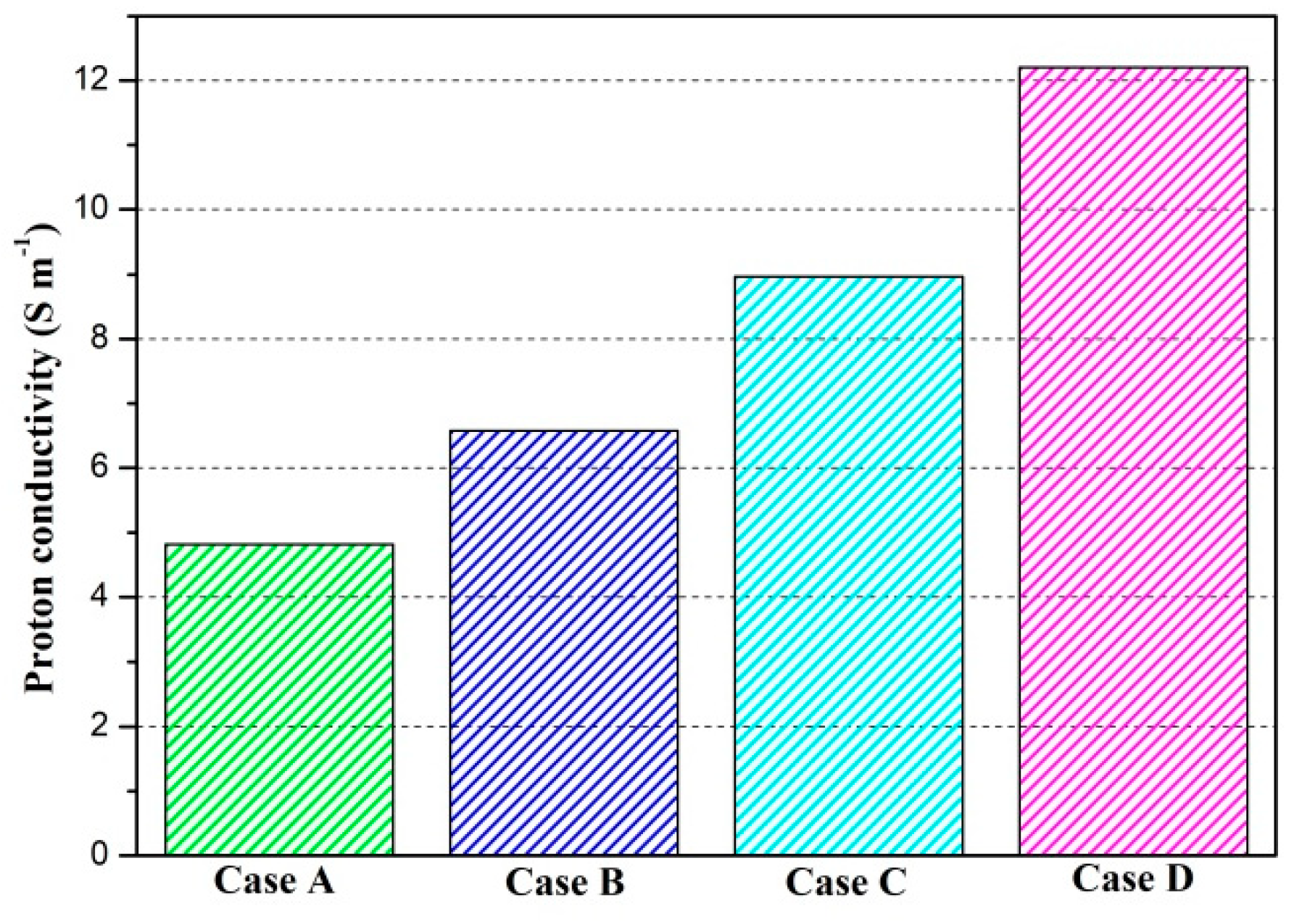
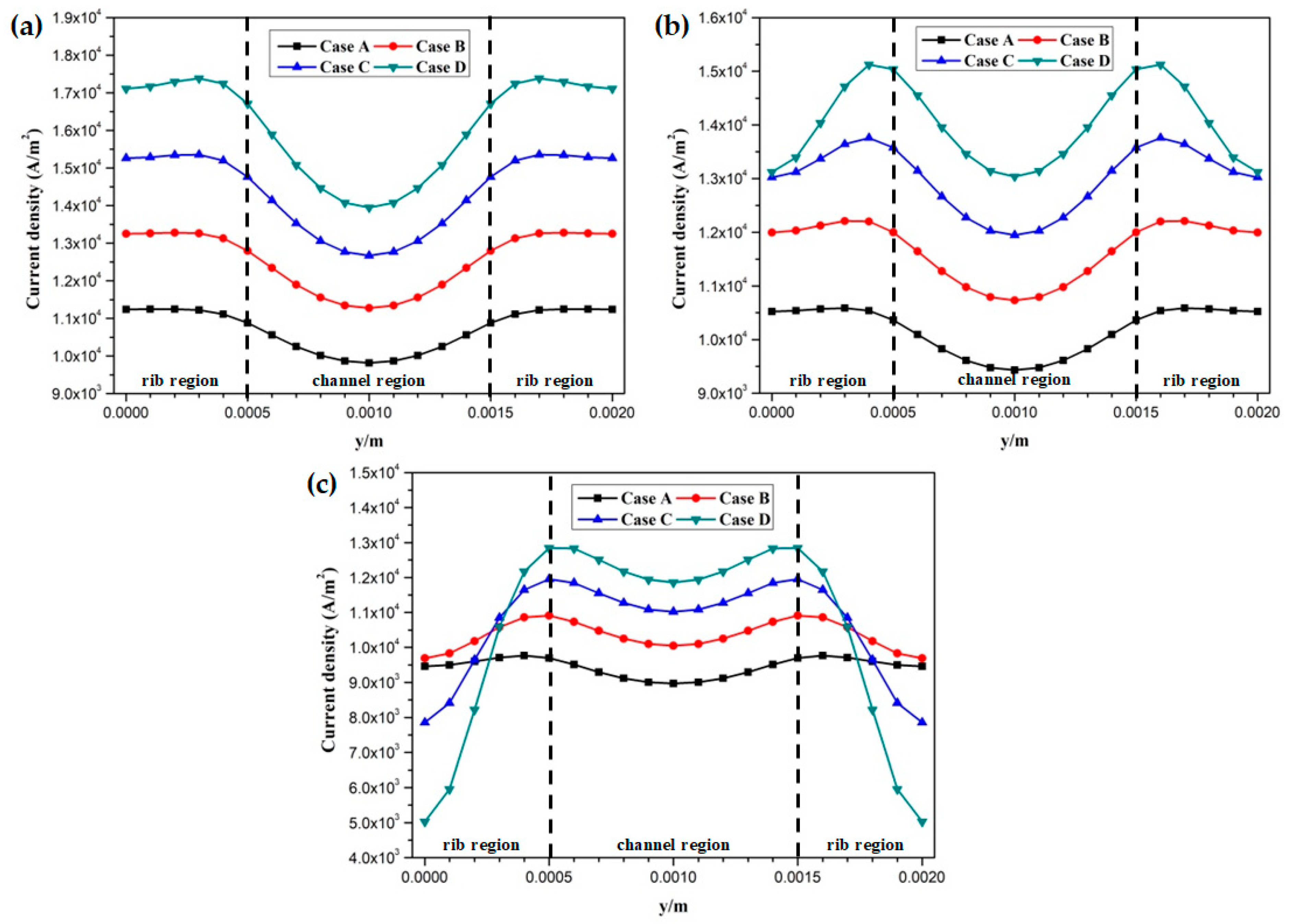
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Smith, G. Modelling the proton-conductive membrane in practical polymer electrolyte membrane fuel cell (PEMFC) simulation: A review. Membranes 2020, 10, 310. [Google Scholar] [CrossRef]
- Nguyen, T.V.; White, R.E. A water and heat management model for proton exchange membrane fuel cells. J. Electrochem. Soc. 1993, 140, 2178–2186. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.D.; Huang, S.; Vu, D.H.; Duy, V.N. Effects of gas channel design on water management and on the performance of polymer electrolyte membrane fuel cells: A review. Int. J. Electrochem. Sci. 2018, 13, 10480–10495. [Google Scholar] [CrossRef]
- Rasheed, R.K.A.; Liao, Q.; Zhang, C.Z.; Chan, S.H. A review on modeling of high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 3142–3165. [Google Scholar] [CrossRef]
- Seng, L.K.; Masdar, M.S.; Shyuan, L.K. Ionic liquid in phosphoric acid-doped polybenzimidazole (PA-PBI) as electrolyte membranes for PEM fuel cells: A review. Membranes 2021, 11, 728. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Emmanuel, O.; Thompson, J.; Olabi, A.G.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Maghrabie, H.M. Optimization of fuel cell performance using computational fluid dynamics. Membranes 2021, 11, 146. [Google Scholar] [CrossRef]
- Xia, L.; Xu, Q.; He, Q.; Ni, M.; Seng, M. Numerical study of high temperature proton exchange membrane fuel cell (HT-PEMFC) with a focus on rib design. Int. J. Hydrogen Energy 2021, 46, 21098–21111. [Google Scholar] [CrossRef]
- Perng, S.W.; Wu, H.W.; Chen, Y.B.; Zeng, Y.K. Performance enhancement of a high temperature proton exchange membrane fuel cell by bottomed-baffles in bipolar-plate channels. Appl. Energy 2019, 255, 113815. [Google Scholar] [CrossRef]
- Li, S.; Sunden, B. Three-dimensional modeling and investigation of high temperature proton exchange membrane fuel cells with metal foams as flow distributor. Int. J. Hydrogen Energy 2017, 42, 27323–27333. [Google Scholar] [CrossRef]
- Li, S.; Yuan, J.; Xie, G.; Sunden, B. Numerical investigation of transport phenomena in high temperature proton exchange membrane fuel cells with different flow field designs. Numer. Heat Transf. A 2017, 72, 807–820. [Google Scholar] [CrossRef]
- Taccani, R.; Zuliani, N. Effect of flow field design on performances of high temperature PEM fuel cells: Experimental analysis. Int. J. Hydrogen Energy 2011, 36, 10282–10287. [Google Scholar] [CrossRef]
- Singdeo, D.; Dey, T.; Gaikwad, S.; Andreasen, S.J.; Ghosh, P.C. A new modified serpentine flow field for application in high temperature polymer electrolyte fuel cell. Appl. Energy 2017, 195, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Desai, A.N.; Singh, S.; Ramadesigan, V.; Shaneeth, M. Effects of the membrane thickness and ionomer volume fraction on the performance of PEMFC with U-shaped serpentine channel. Int. J. Hydrogen Energy 2021, 46, 20650–20663. [Google Scholar] [CrossRef]
- Nanadegani, F.S.; Lay, E.N.; Sunden, B. Computational analysis of the impact of a micro porous layer (MPL) on the characteristics of a high temperature PEMFC. Electrochim. Acta 2020, 333, 135552. [Google Scholar] [CrossRef]
- Das, S.K.; Gibson, H.A. Three dimensional multi-physics modeling and simulation for assessment of mass transport impact on the performance of a high temperature polymer electrolyte membrane fuel cell. J. Power Sources 2021, 499, 229844. [Google Scholar] [CrossRef]
- Elden, G.; Celik, M.; Genc, G.; Yapici, H. The effects of temperature on transport phenomena in phosphoricacid doped polybenzimidazole polymer membrane fuel cell. Energy 2016, 103, 772–783. [Google Scholar] [CrossRef]
- Xing, B.; Savadogo, O. The effect of acid doping on the conductivity of polybenzimidazole. New Mater. Electrochem. Syst. 1999, 2, 95–102. [Google Scholar]
- Li, Q.F.; Hjuler, H.A.; Bjerrum, N. Phosphoric acid doped polybenzimidazole membranes: Physiochemical characterization and fuel cell applications. J. Appl. Electrochem. 2001, 31, 773–779. [Google Scholar] [CrossRef]
- Wu, H.W.; Ho, T.Y.; Han, Y.J. Parametric optimization of wall-mounted cuboid rows installed in interdigitated flow channel of HT-PEM fuel cells. Energy 2021, 216, 119261. [Google Scholar] [CrossRef]
- Huang, T.; Wang, W.; Yuan, Y.; Huang, J.; Chen, X.; Zhang, J.; Kong, X.; Zhang, Y.; Wan, Z. Optimization of high-temperature proton exchange membrane fuel cell flow channel based on genetic algorithm. Energy Rep. 2021, 7, 1374–1384. [Google Scholar] [CrossRef]
- Sezgin, B.; Caglayan, D.G.; Devrim, Y.; Steenberg, T.; Eroglu, I. Modeling and sensitivity analysis of high temperature PEM fuel cells by using Comsol multiphysics. Int. J. Hydrogen Energy 2016, 41, 10001–10009. [Google Scholar] [CrossRef]
- Chippar, P.; Ju, H. Three-dimensional non-isothermal modeling of a phosphoric acid-doped polybenzimidazole (PBI) membrane fuel cell. Solid State Ion. 2012, 225, 30–39. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Hao, D.; Ni, M.; Huang, S.; Liu, D.; Zheng, Y. 3D non-isothermal dynamic simulation of high temperature proton exchange membrane fuel cell in the start-up process. Int. J. Hydrogen Energy 2021, 46, 2577–2593. [Google Scholar] [CrossRef]
- Diaz, L.A.; Abuin, G.C.; Corti, H.R. Acid-doped ABPBI membranes prepared by low-temperature casting: Proton conductivity and water uptake properties compared with other polybenzimidazole-based membranes. J. Electrochem. Soc. 2016, 163, F485–F491. [Google Scholar] [CrossRef]
- Cheddie, D.F.; Munroe, N.D.H. A two-phase model of an intermediate temperature PEM fuel cell. Int. J. Hydrogen Energy 2007, 32, 832–841. [Google Scholar] [CrossRef]



| Parameter | Value | Unit |
|---|---|---|
| Cell length/width | 50/2 | mm |
| GFC height/width | 1.0/1.0 | mm |
| Anode/Cathode GDL thickness | 0.2 | mm |
| Anode/Cathode CL thickness | 0.01 | mm |
| Membrane thickness | 0.05 | mm |
| Description | Units |
|---|---|
| (Anode CL) (Cathode CL) (Cathode CL) | kg m−3 s−1 |
| (GDLs and CLs) | kg m−2 s−2 |
| (Anode CL) | kg m−3 s−1 |
| (Cathode CL) | kg m−3 s−1 |
| (Cathode CL) | kg m−3 s−1 |
| (Anode CL) (Cathode CL) (Membrane) (GDLs and CCs) | W m−3 |
| (Anode CL) (Cathode CL) | A m−3 |
| (Anode CL) (Cathode CL) | A m−3 |
| Parameter | Value | Units |
|---|---|---|
| Porosity of GDL/CL | 0.6/0.4 | - |
| Volume fraction of membrane in the CL | 0.3 | - |
| Anode/cathode reference exchange current density | 1 × 109/1 × 104 | A m−3 |
| Anode transfer coefficient | 0.5/1 | - |
| Reference hydrogen concentration | 40.88 | mol m−3 |
| Reference oxygen concentration | 40.88 | mol m−3 |
| Thermal conductivity of CC/GDL/CL/membrane | 20/1.2/1.5/0.95 | W m−1 K−1 |
| Electrical conductivity of CC/GDL/CL | 10,000/1250/300 | S m−1 |
| Permeability of GDL/CL | 1.18 × 10−11/2.36 × 10−12 | m2 |
| Hydrogen diffusivity | 1.055 × 10−4 (T/333.15)1.5 (101,325/P) | m2 s−1 |
| Oxygen diffusivity | 2.652 × 10−5 (T/333.15)1.5 (101,325/P) | m2 s−1 |
| Water diffusivity | 2.982 × 10−5 (T/333.15)1.5 (101,325/P) | m2 s−1 |
| Description | Conditions | Value | Units |
|---|---|---|---|
| Anode terminal | ϕs | 0 | V |
| Cathode terminal | ϕs | Vcell | V |
| Anode GFC inlet | Y | H2 = 1 | - |
| T | 453.15 | K | |
| Cathode GFC inlet | Y | O2:N2 = 0.233:0.767 | - |
| T | 453.15 | K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Peng, C.; Shen, Q.; Wang, C.; Cheng, Y.; Yang, G. Impact of Membrane Phosphoric Acid Doping Level on Transport Phenomena and Performance in High Temperature PEM Fuel Cells. Membranes 2021, 11, 817. https://doi.org/10.3390/membranes11110817
Li S, Peng C, Shen Q, Wang C, Cheng Y, Yang G. Impact of Membrane Phosphoric Acid Doping Level on Transport Phenomena and Performance in High Temperature PEM Fuel Cells. Membranes. 2021; 11(11):817. https://doi.org/10.3390/membranes11110817
Chicago/Turabian StyleLi, Shian, Chengdong Peng, Qiuwan Shen, Chongyang Wang, Yuanzhe Cheng, and Guogang Yang. 2021. "Impact of Membrane Phosphoric Acid Doping Level on Transport Phenomena and Performance in High Temperature PEM Fuel Cells" Membranes 11, no. 11: 817. https://doi.org/10.3390/membranes11110817
APA StyleLi, S., Peng, C., Shen, Q., Wang, C., Cheng, Y., & Yang, G. (2021). Impact of Membrane Phosphoric Acid Doping Level on Transport Phenomena and Performance in High Temperature PEM Fuel Cells. Membranes, 11(11), 817. https://doi.org/10.3390/membranes11110817







