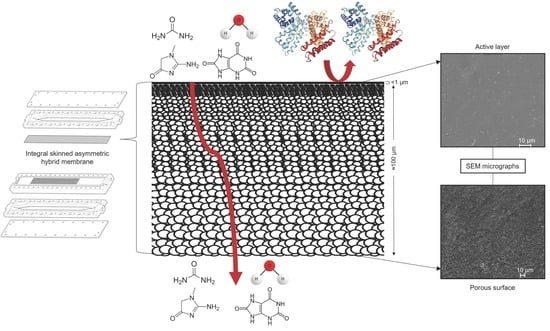
Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Membrane Characterization
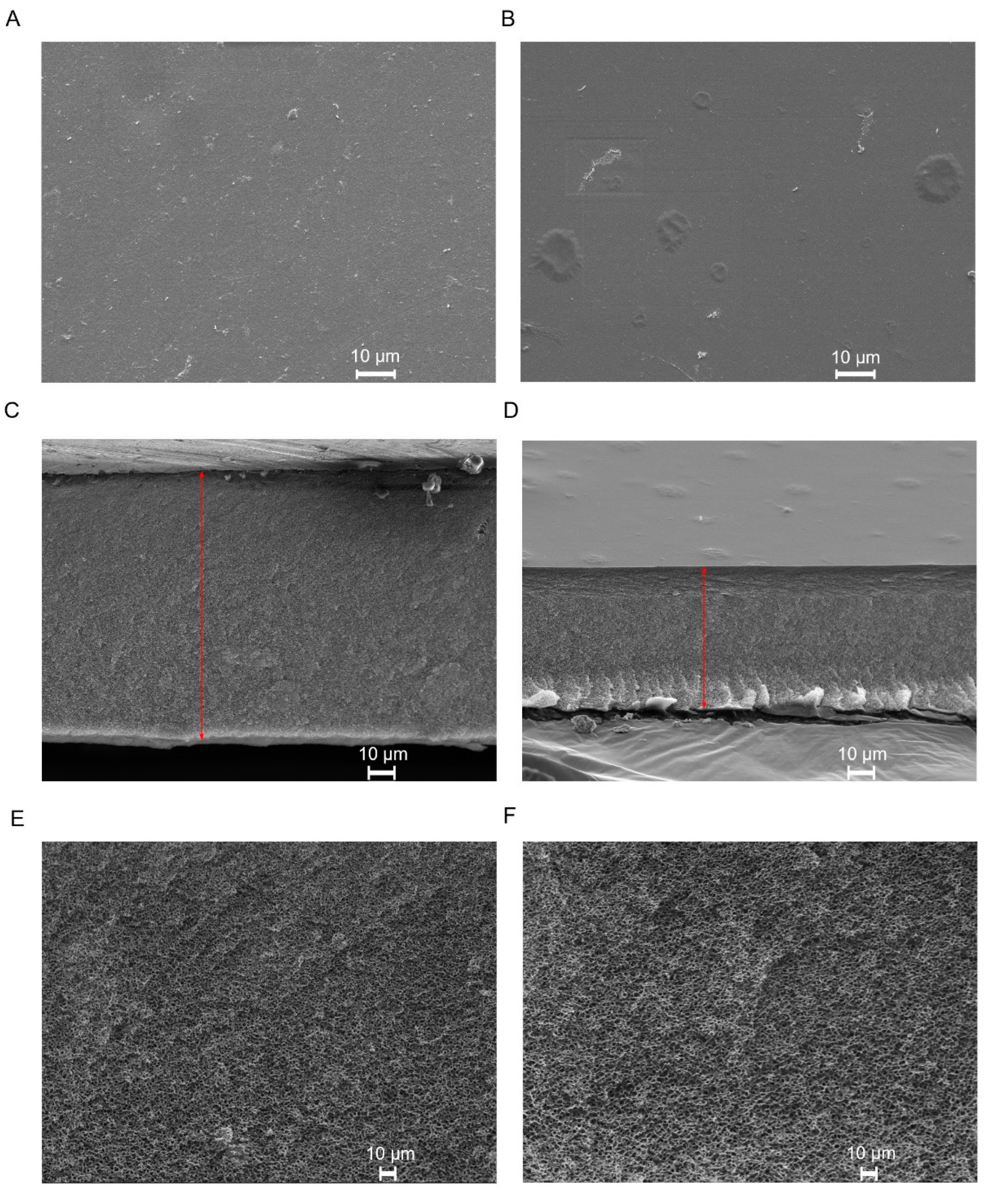
2.3.1. Scanning Electron Microscopy (SEM)
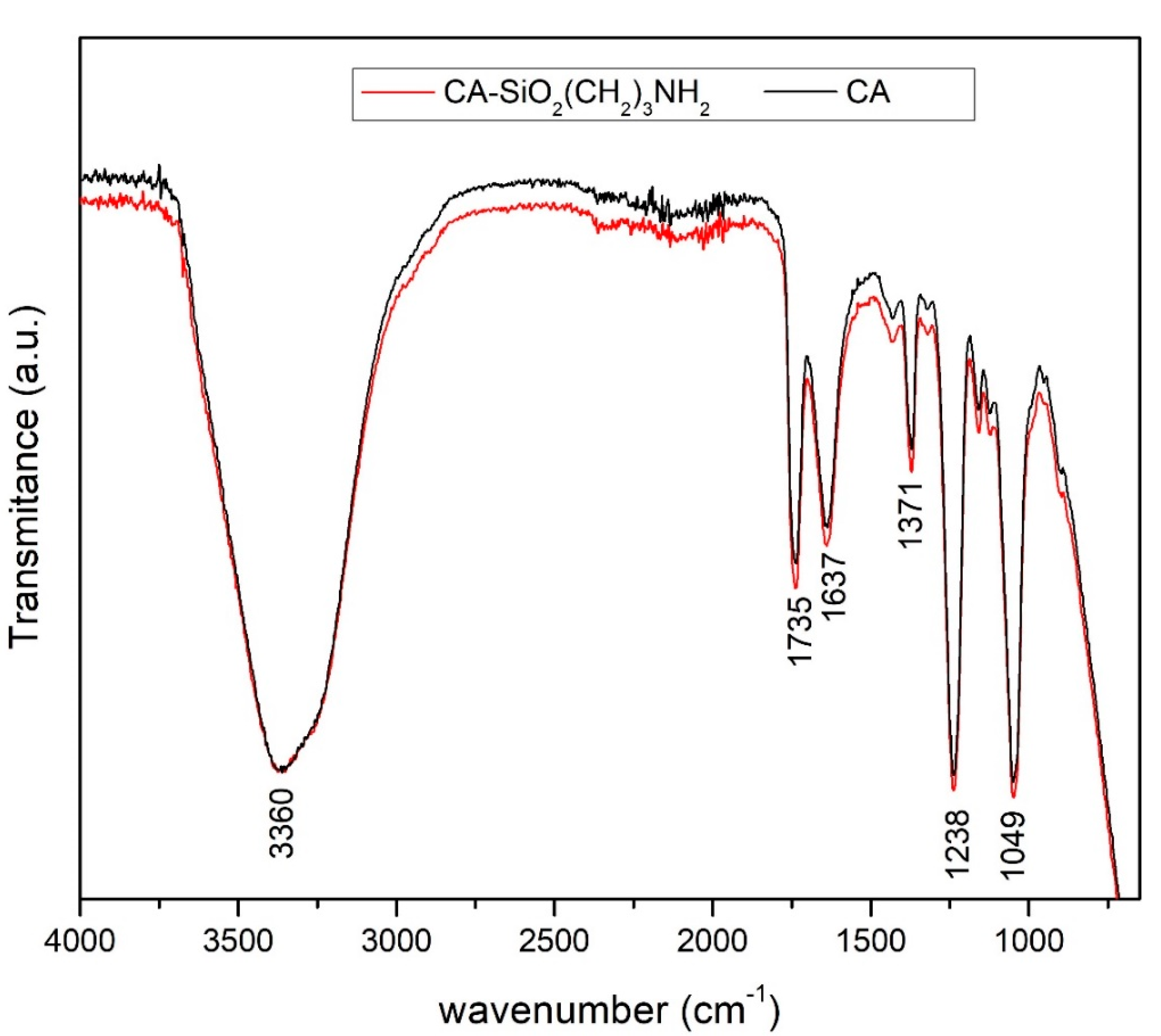
2.3.2. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
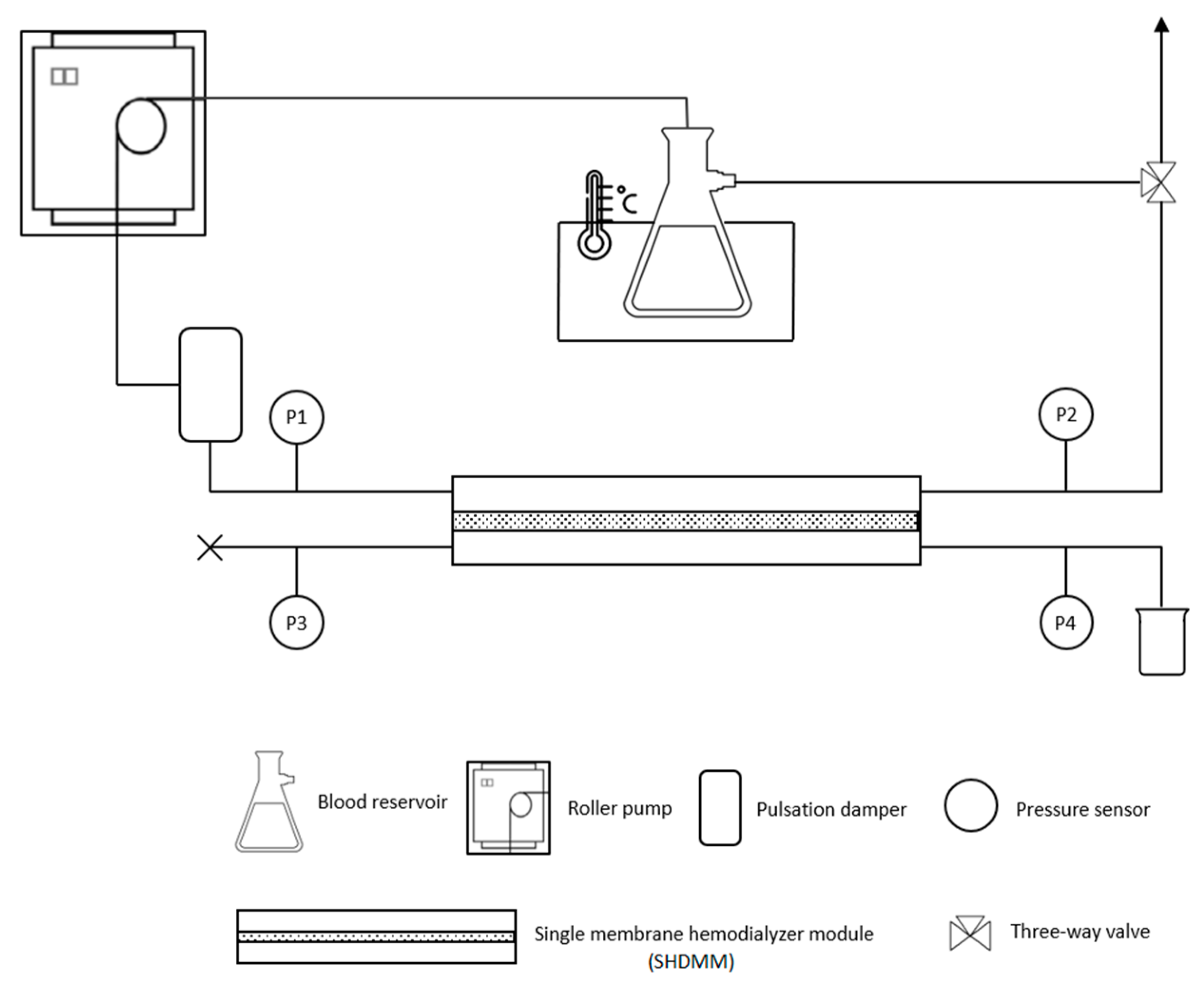
2.4. Experimental Setup
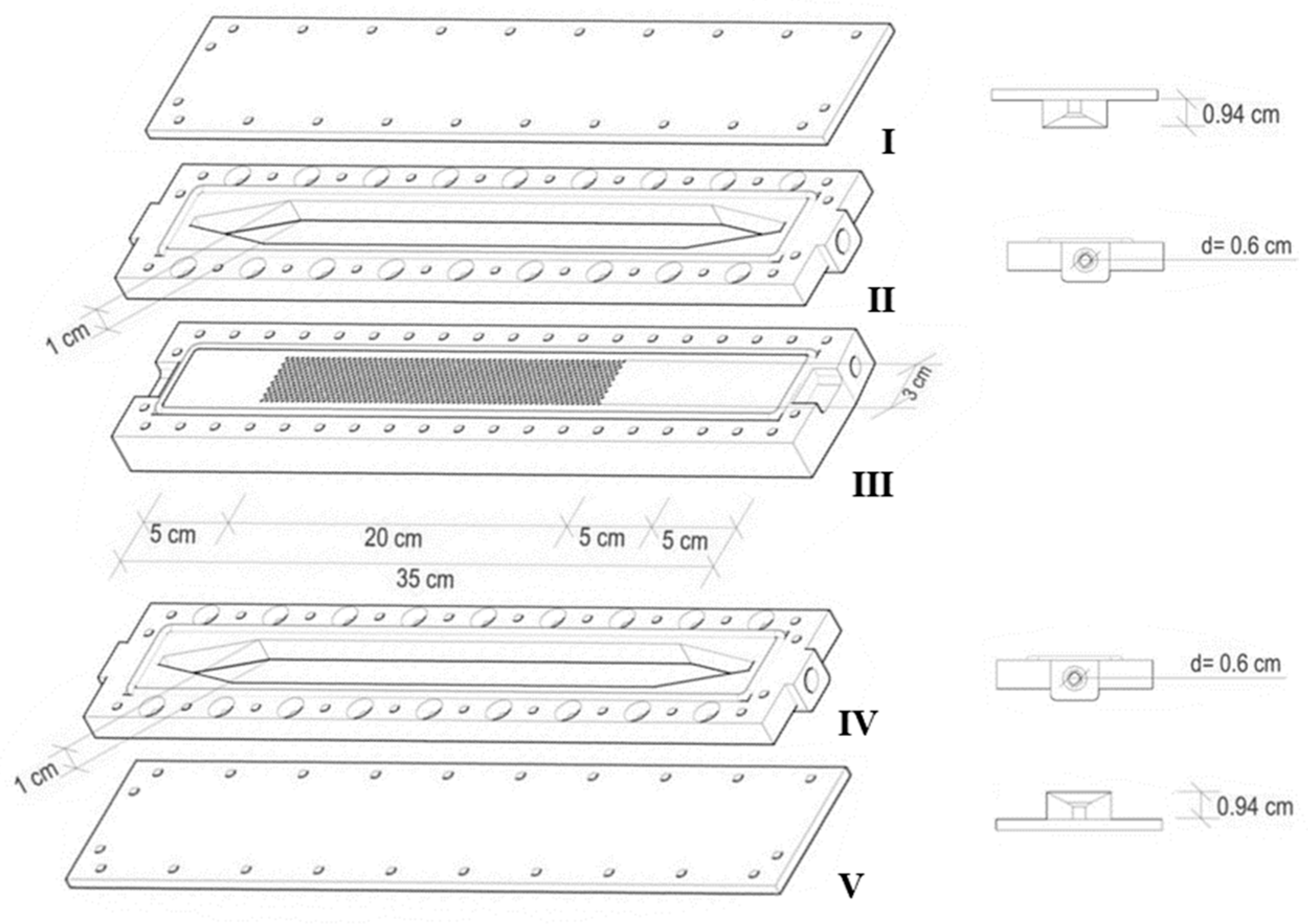
2.4.1. Single Hemodialysis Membrane Module (SHDMM)
2.4.2. Feed Circulation Circuit
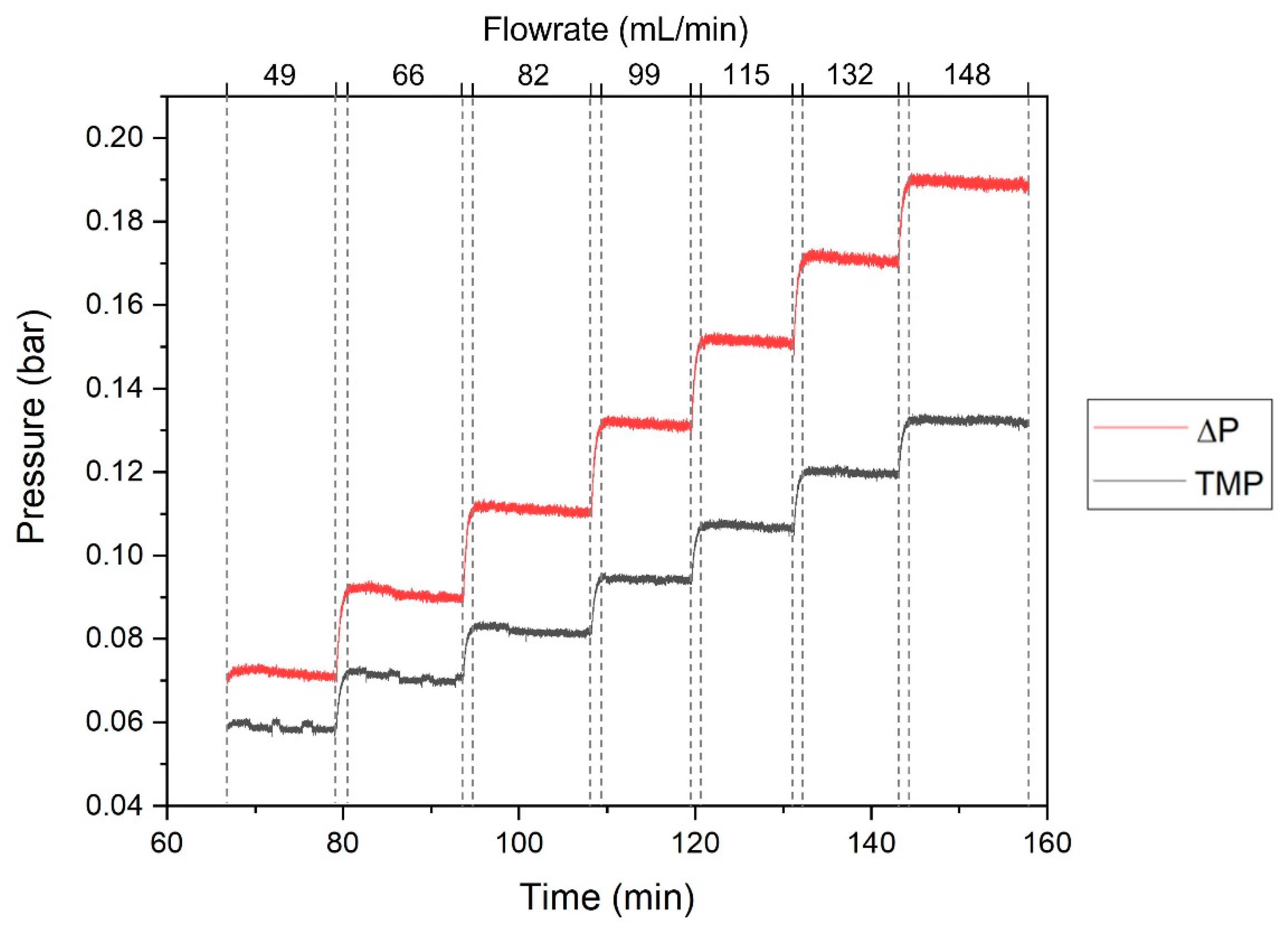
2.4.3. Characterization of the SHDMM: Pressure Profile, Pressure Drop, Transmembrane Pressure, Microchannel Height, Shear Rate and Shear Stress at the Wall
2.5. Permeation Experiments under Dynamic Conditions
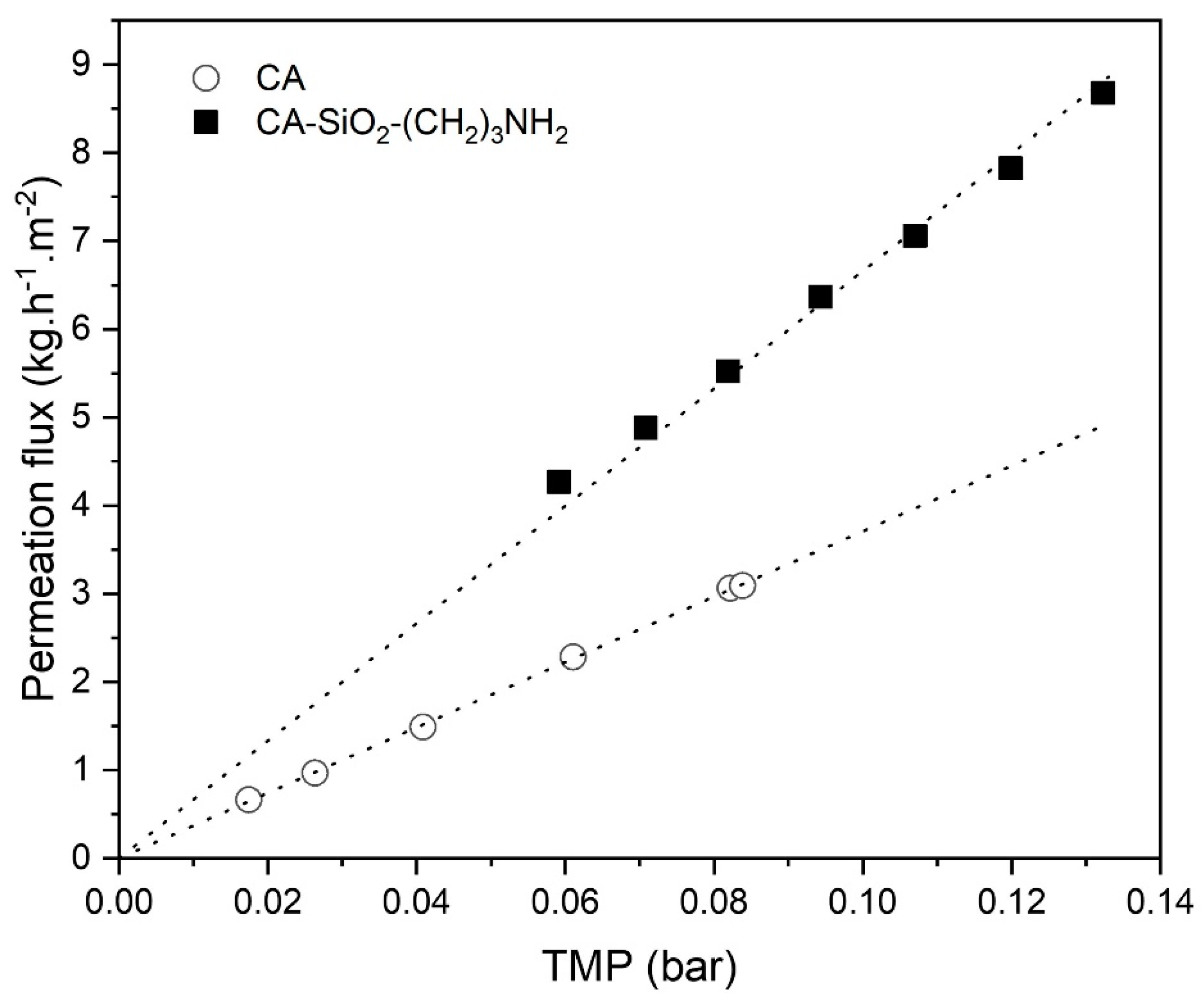
2.5.1. Water Permeability
2.5.2. Molecular Weight Cut-Off
2.5.3. Rejection Coefficients to Low-Molecular Weight Water-Soluble Uremic Toxins
2.5.4. Bovine Serum Albumin Filtration
3. Results and Discussion
3.1. Membrane Chacracterization
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
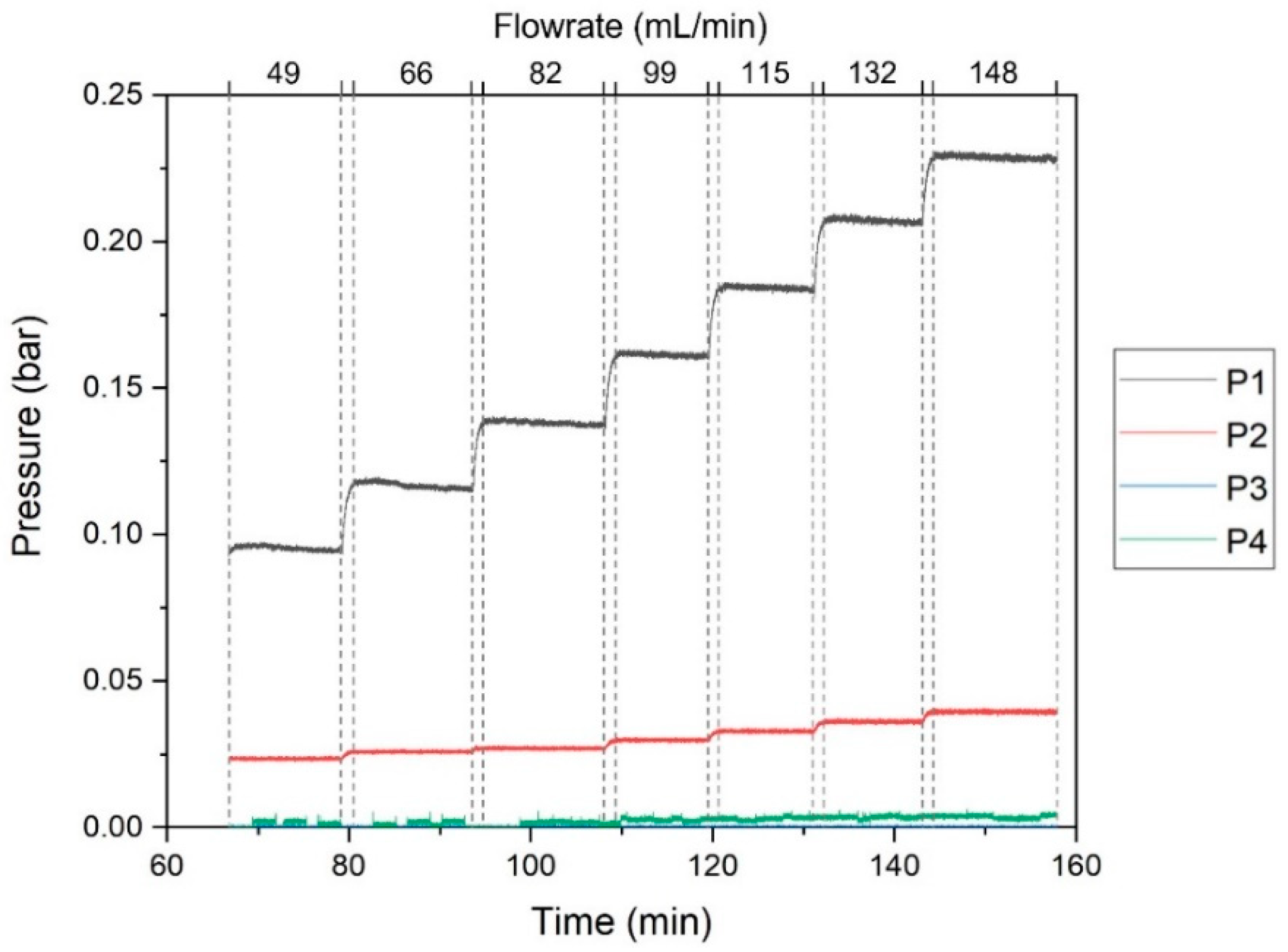
3.2. Characterization of the SHDMM: Pressure Profile, Pressure Drop, Transmembrane Pressure, Microchannel Height, Shear Rate and Shear Stress at the Wall
3.3. Permeation Experiments under Dynamic Conditions
3.3.1. Water Permeability
3.3.2. Molecular Weight Cut-Off
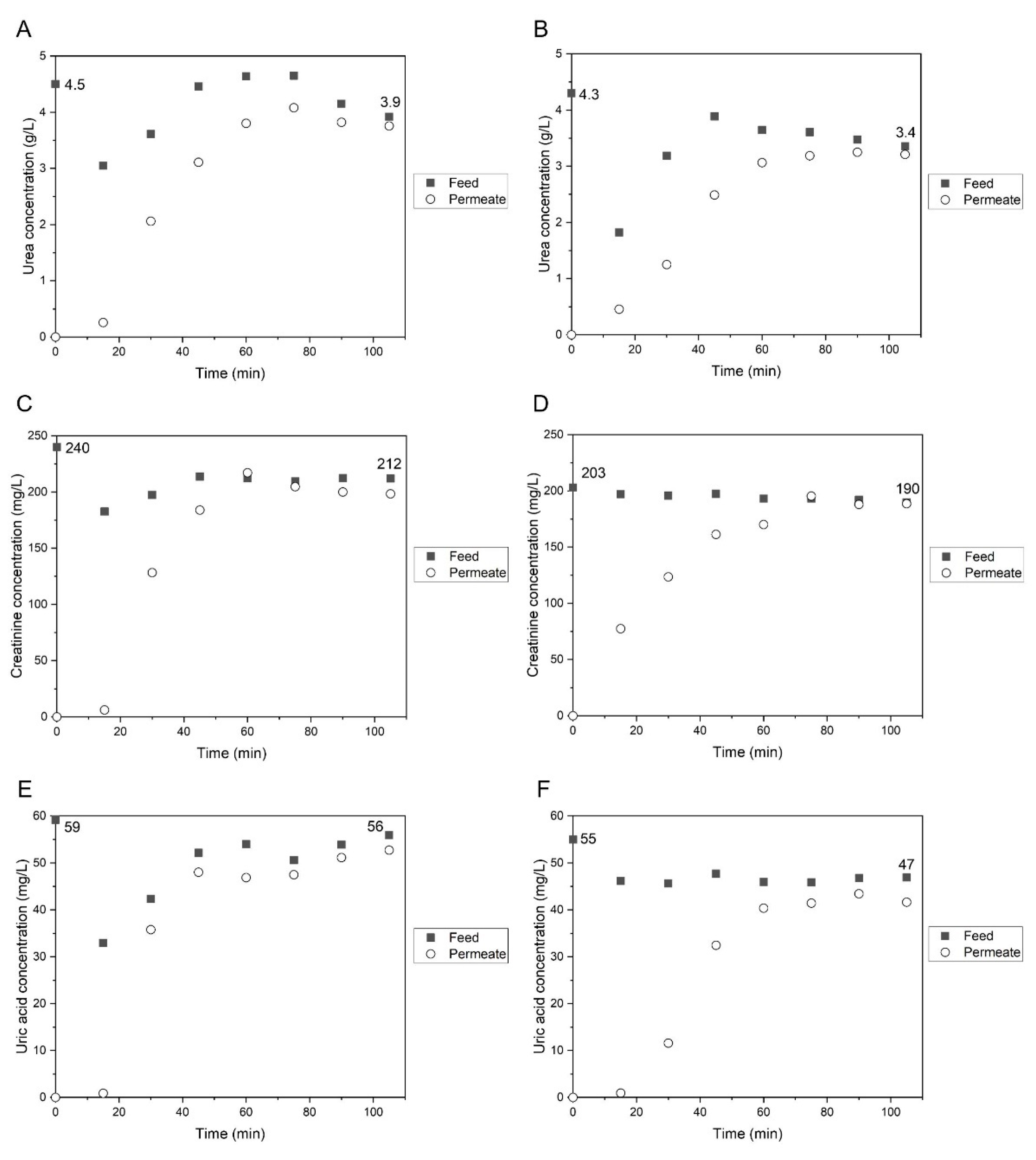
3.3.3. Rejection Coefficients to Low-Molecular Weight Water-Soluble Uremic Toxins
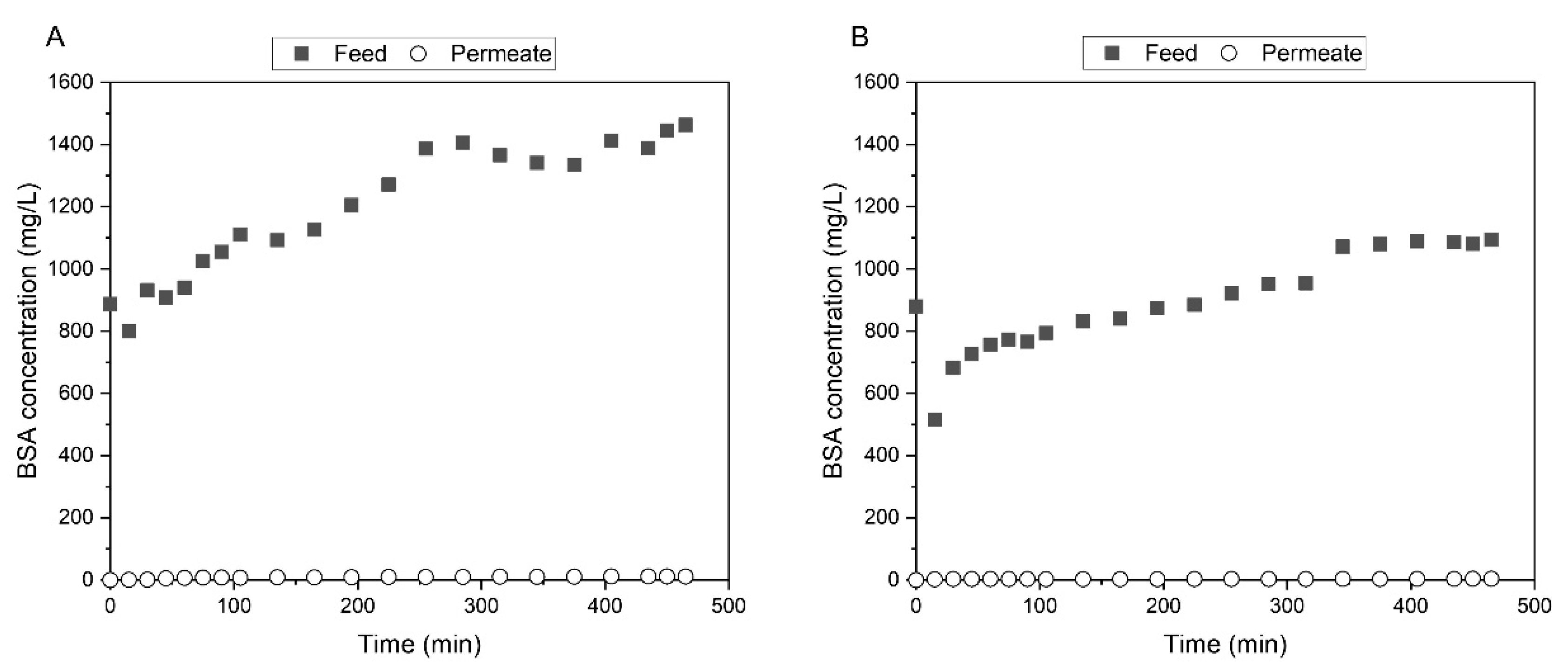
3.3.4. Bovine Serum Albumin Filtration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.; O’Callaghan, C.A.; Lasserson, D.; Hobbs, R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Faria, M.; de Pinho, M.N. Challenges of reducing protein-bound uremic toxin levels in chronic kidney disease and end stage renal disease. Transl. Res. 2021, 229, 115–134. [Google Scholar] [CrossRef]
- Peinemann, K.V.; Nunes, S.P. (Eds.) Membranes for Life Sciences; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Bonomini, V.; Berland, Y. (Eds.) Dialysis Membranes: Structure and Predictions. International Workshop, Marseille, June 1994; Contrib Nephrol: Basel, Karger, 1995; Volume 113, pp. 25–31. [Google Scholar] [CrossRef]
- Drukker, W.; Parsons, F.M.; Maher, J.F. Replacement of Renal Function by Dialysis; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- D’Amico, G.; Colasanti, G. (Eds.) Nephrology and Dialysis Updated. 18th Course on Advances in Nephrology and Dialysis, Milano, December 1986; Contrib Nephrol: Basel, Karger, 1988; Volume 61, pp. 1–17. [Google Scholar] [CrossRef]
- Guidance for the Content of Premarket Notifications for Hemodialysis Delivery Systems. Available online: https://www.fda.gov/media/116852/download (accessed on 27 October 2021).
- Clark, W.R.; Hamburger, R.J.; Lysaght, M.J. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 1999, 56, 2005–2015. [Google Scholar] [CrossRef] [Green Version]
- Lipps, B.J.; Stewart, R.D.; Perkins, H.A.; Holmes, G.W.; McLain, E.A.; Rolfs, M.R.; Oja, P.D. The Hollow Fiber Artificial Kidney. Trans. Am. Soc. Artif. Organs 1967, 13, 200–207. [Google Scholar]
- Ward, R.A.; Klein, E.; Harding, G.B.; Murchison, K.E. Response of complement and neutrophils to hydrophilized synthetic membranes. ASAIO Trans. 1988, 34, 334–337. [Google Scholar]
- Pedrini, L.A.; Krisp, C.; Gmerek, A.; Wolters, D.A. Patterns of Proteins Removed with High-Flux Membranes on High-Volume Hemodiafiltration Detected with a MultiDimensional LC-MS/MS Strategy. Blood Purif. 2014, 38, 115–126. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane, in: Saline Water Conversion—II. Am. Chem. Soc. 1963, 38, 117–132. [Google Scholar] [CrossRef]
- Kunst, B.; Sourirajan, S. An approach to the development of cellulose acetate ultrafiltration membranes. J. Appl. Polym. Sci. 1974, 18, 3423–3434. [Google Scholar] [CrossRef]
- McCray, S.B.; Vilker, V.; Nobe, K. Reverse osmosis cellulose acetate membranes II. Dependence of transport properties on acetyl content. J. Membr. Sci. 1991, 59, 317–330. [Google Scholar] [CrossRef]
- Ingram, A.J.; Parbtani, A.; Churchill, D.N. Effects of two low-flux cellulose acetate dialysers on plasma lipids and lipoproteins-a cross-over trial. Nephrol. Dial. Transplant. 1998, 13, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Hoenich, N.A.; Woffindin, C.; Matthews, J.N.S.; Goldfinch, M.E.; Turnbull, J. Clinical comparison of high-flux cellulose acetate and synthetic membranes. Nephrol. Dial. Transplant. 1994, 9, 60–66. [Google Scholar]
- Hoenich, N.A.; Woffindin, C.; Cox, P.J.; Goldfinch, M.; Roberts, S.J. Clinical characterization of Dicea a new cellulose membrane for haemodialysis. Clin. Nephrol. 1997, 48, 253–259. [Google Scholar]
- Weissinger, E.M.; Kaiser, T.; Meert, N.; De Smet, R.; Walden, M.; Mischak, H.; Vanholder, R.C.; for the European Uremic Toxin Work Group. Proteomics: A novel tool to unravel the patho-physiology of uraemia. Nephrol. Dial. Transplant. 2004, 19, 3068–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speeckaert, M.M.; Glorieux, G.L.; Vanholder, R.; Van Biesen, W.; Taes, Y.E.; Clément, F.; Wehlou, C.; Delanghe, J.R. Vitamin D Binding Protein and the Need for Vitamin D in Hemodialysis Patients. J. Ren. Nutr. 2008, 18, 400–407. [Google Scholar] [CrossRef]
- Elsen, R.; Silva, L.K. Advantages of meltspun membrane technology compared to solution spinning. Contrib. Nephrol. 1993, 103, 125–138. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Gastaldello, K.; Melot, C.; Kahn, R.-J.; Vanherweghem, J.-L.; Vincent, J.-L.; Tielemans, C. Comparison of cellulose diacetate and polysulfone membranes in the outcome of acute renal failure. A prospective randomized study. Nephrol. Dial. Transplant. 2000, 15, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Vos, K.D.; Hatcher, A.P.; Merten, U. Lifetime of Cellulose Acetate Reverse Osmosis Membranes. Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 211–218. [Google Scholar] [CrossRef]
- Mendes, G.; Faria, M.; Carvalho, A.; Gonçalves, M.C.; de Pinho, M.N. Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties. Carbohydr. Polym. 2018, 189, 342–351. [Google Scholar] [CrossRef]
- Faria, M.; Moreira, C.; Eusébio, T.; Brogueira, P.; De Pinho, M.N. Hybrid flat sheet cellulose acetate/silicon dioxide ultrafiltration membranes for uremic blood purification. Cellulose 2020, 27, 3847–3869. [Google Scholar] [CrossRef]
- Peixoto, I.; Faria, M.; Gonçalves, M. Synthesis and Characterization of Novel Integral Asymmetric Monophasic Cellulose–Acetate/Silica/Titania and Cellulose–Acetate/Titania Membranes. Membranes 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.; Pereira, J.C.; de Almeida, N.; Marques, P.; Faria, M.; Gonçalves, M.C. Improving hydraulic permeability, mechanical properties, and chemical functionality of cellulose acetate-based membranes by co-polymerization with tetraethyl orthosilicate and 3-(aminopropyl)triethoxysilane. Carbohydr. Polym. 2021, 261, 117813. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, M.; Faria, M.; Boi, C.; Semiao, V.; de Pinho, M.N. The effect of ultrafiltration transmembrane permeation on the flow field in a surrogate system of an artificial kidney. Exp. Results 2021, 2, 16. [Google Scholar] [CrossRef]
- Brinker, J.; Scherer, G. The Physics and Chemistry of Sol-Gel Processing; Gulf Professional Publishing: Oxford, UK, 1990. [Google Scholar]
- Gonçalves, M.C. Sol-gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [Green Version]
- Lui, A.; Talbot, F.D.F.; Fouda, A.; Matsuura, T.; Sourirajan, S. Studies on the solvent exchange technique for making dry cellulose acetate membranes for the separation of gaseous mixtures. J. Appl. Polym. Sci. 1988, 36, 1809–1820. [Google Scholar] [CrossRef]
- Faria, M.; Liu, Y.; Leonard, E.F. Particle Spallation in a Microfluidic Blood Processing Device: The Problem of Using Peristaltic Pumps and Silicon-based Microfilters. Int. J. Artif. Organs 2017, 40, 589–593. [Google Scholar] [CrossRef]
- Liu, Y.; Faria, M.; Leonard, E. Spallation of Small Particles From Peristaltic Pump Tube Segments. Artif. Organs 2016, 41, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley&Sons Inc.: Chichester, UK, 2002. [Google Scholar]
- Rosa, M.J. Separação Seletiva de Compostos Orgânicos de Correntes Aquosas por Ultrafiltração e Nanofiltração. Ph.D. Thesis, Instituto Superior Técnico, University of Lisbon, Lisbon, Portugal, 1995. [Google Scholar]
- Gallant, R.W.; Railey, J.M. Physical Properties of Hydrocarbons, 2nd ed.; Gulf Publishing Company: Oxford, UK, 1970. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; on behalf of the European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Filutowicz, Z.; Łukaszewski, K.; Pieszyński, K. Remarks on spectra-photometric monitoring of urea in dialysate. J. Med. Inform. Technol. 2004, 8, MM105–MM110. [Google Scholar]
- ter Beek, O.; van Gelder, M.; Lokhorst, C.; Hazenbrink, D.; Lentferink, B.; Gerritsen, K.; Stamatialis, D. In vitro study of dual layer mixed matrix hollow fiber membranes for outside-in filtration of human blood plasma. Acta Biomater. 2021, 123, 244–253. [Google Scholar] [CrossRef]
- Montaseri, H.; Khajehsharifi, H.; Yousefinejad, S. UV Determination of epinephrine, uric acid, and acetaminophen in pharmaceutical formulations and some human body fluids using multivariate calibration. Química Nova 2014, 37, 1404–1409. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.; de Pinho, M.N. An ATR-FTIR study of water in cellulose acetate membranes prepared by phase inversion. J. Membr. Sci. 1995, 106, 245–257. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Garcia, A.R.; Minhalma, M.; Ilharco, L.; de Pinho, M. The Ultrafiltration Performance of Cellulose Acetate Asymmetric Membranes: A New Perspective on the Correlation with the Infrared Spectra. J. Membr. Sci. Res. 2020, 6, 70–80. [Google Scholar] [CrossRef]
- Nyquist, R. (Ed.) Chapter 8—Aliphatic Amines. In Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001; pp. 143–148. [Google Scholar] [CrossRef]
- Albalate, M.; Pérez-García, R.; De Sequera, P.; Corchete, E.; Alcázar, R.; Ortega, M.; Puerta, M. Is it useful to increase dialysate flow rate to improve the delivered Kt? BMC Nephrol. 2015, 16, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.Y.; Kim, S.-H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.K.; Kim, Y.-S.; Kang, S.-W.; Kim, N.-H.; et al. The impact of blood flow rate during hemodialysis on all-cause mortality. Korean J. Intern. Med. 2016, 31, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, J.; Martin-Malo, A.; Pedrini, L.; Basci, A.; Canaud, B.; Fouque, D.; Haage, P.; Konner, K.; Kooman, J.; Pizzarelli, F.; et al. EBPG guideline on dialysis strategies. Nephrol. Dial. Transplant. 2007, 22, ii5–ii21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kroll, M.; Hellums, J.; McIntire, L.; Schafer, A.; Moake, J. Platelets and shear stress. Blood 1996, 88, 1525–1541. [Google Scholar] [CrossRef] [Green Version]
- Holme, P.A.; Ørvim, U.; Hamers, M.J.A.G.; Solum, N.O.; Brosstad, F.R.; Barstad, R.M.; Sakariassen, K.S. Shear-Induced Platelet Activation and Platelet Microparticle Formation at Blood Flow Conditions as in Arteries With a Severe Stenosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 646–653. [Google Scholar] [CrossRef]
- Flythe, J.; Kimmel, S.E.; Brunelli, S.M. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011, 79, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Magee, C.C.; Tucker, J.K.; Singh, A.K. (Eds.) Core Concepts in Dialysis and Continuous Therapies; Springer: Boston, MA, USA, 2016. [Google Scholar] [CrossRef]
- Belinskaia, D.; Voronina, P.; Batalova, A.; Goncharov, N. Serum Albumin. Encyclopedia 2020, 1, 65–75. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janeca, A.; Rodrigues, F.S.C.; Gonçalves, M.C.; Faria, M. Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes 2021, 11, 825. https://doi.org/10.3390/membranes11110825
Janeca A, Rodrigues FSC, Gonçalves MC, Faria M. Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes. 2021; 11(11):825. https://doi.org/10.3390/membranes11110825
Chicago/Turabian StyleJaneca, Adriana, Flávia S. C. Rodrigues, Maria Clara Gonçalves, and Mónica Faria. 2021. "Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions" Membranes 11, no. 11: 825. https://doi.org/10.3390/membranes11110825
APA StyleJaneca, A., Rodrigues, F. S. C., Gonçalves, M. C., & Faria, M. (2021). Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions. Membranes, 11(11), 825. https://doi.org/10.3390/membranes11110825