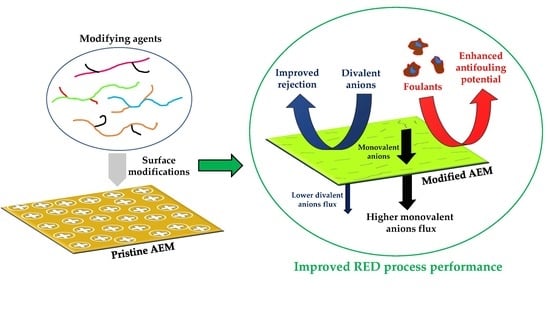
Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review
Abstract
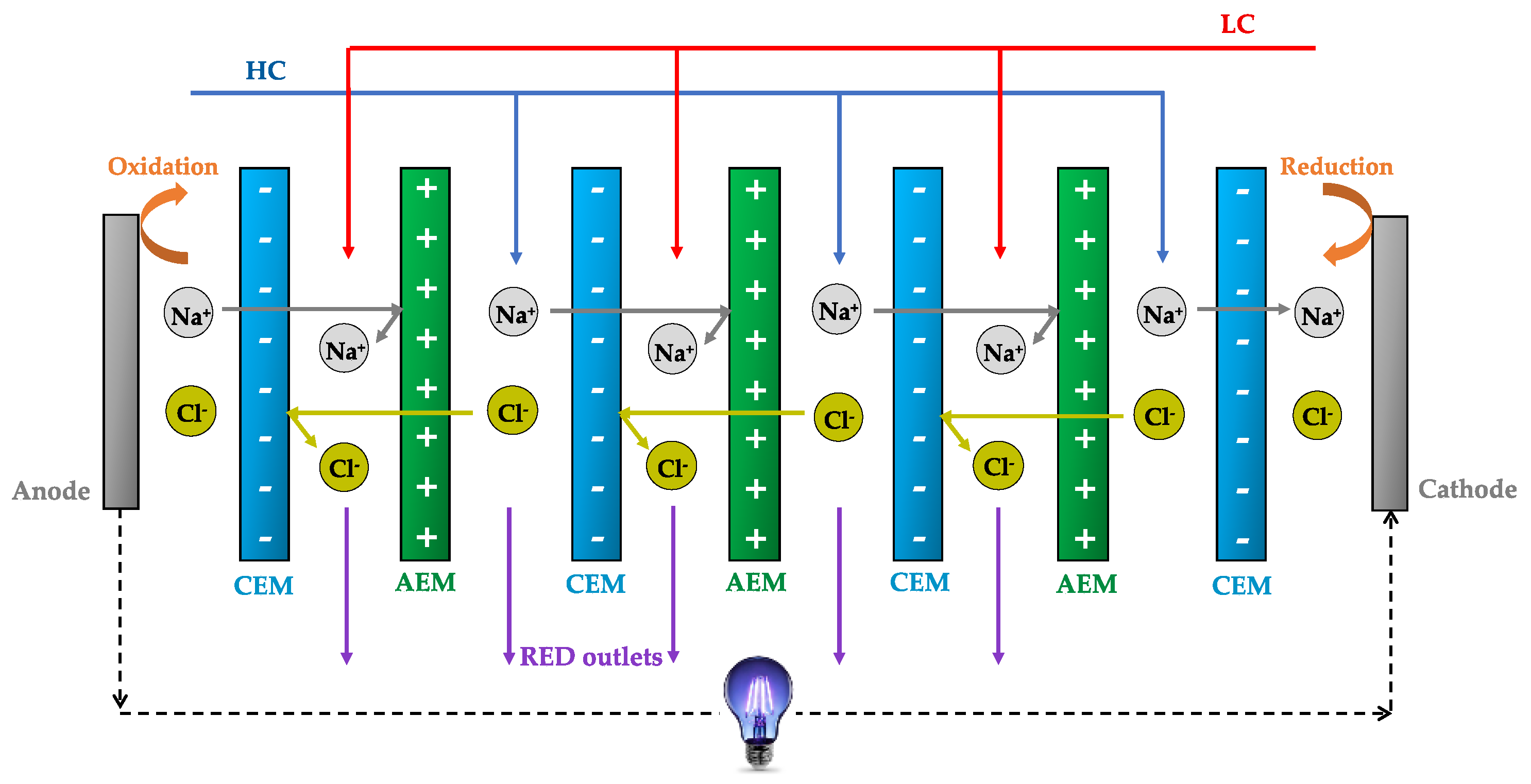
1. Introduction
2. Membrane Surface Modification Techniques
2.1. Surface Polymerization Methods
2.2. Dip Coating Strategies
2.3. Layer-by-Layer (LbL) Approaches
2.4. Electrodeposition Procedures
2.5. Alternative Modification Techniques
3. Selected Studies on Modified AEMs with Improved Performance
- Polymerization-based modification methods are capable of considerably improving the membrane behavior in terms of multivalent ions rejection (e.g., SO42−), i.e., the membrane permselectivity (Cl−/SO42−) is clearly enhanced after modification. However, an unfavorable impact in the membrane electro-resistance is often observed, which might be associated with an increased thickness of the modified AEMs compared to the pristine one. The effect of the modifying agent selected is clearly shown in Table 1. For example, the modification of a standard-grade homogeneous Fuji A membrane with AMPS and MBA via UV-curing with specific RED performance improvement purposes, resulted in an increased permselectivity (a comparable value with the one associated with a commercial Neosepta ACS membrane was reached), including enhanced hydrophilicity and antifouling characteristics, almost without compromising the membrane electro-resistance [21].
- Several AEMs were also proposed to be modified via immersion/dip coating-based strategies, with the purpose of enhancing their surface hydrophilicity, antifouling behavior, and rejection of divalent anions. Nevertheless, more comprehensive studies on membrane electro-resistances (preferably via electrochemical impedance spectroscopy) are required after modification to focus on developing AEMs with a lower electrical resistance for RED, which might lead to an increased obtainable net power density.
4. Future Outlook and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Tristán, C.; Pérez, G.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. A comprehensive study on the effects of operation variables on reverse electrodialysis performance. Desalination 2020, 482, 114389. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Pei, Y.; Crittenden, J.C. Unique applications and improvements of reverse electrodialysis: A review and outlook. Appl. Energy 2020, 262, 114482. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Crespo, J.G.; Nijmeijer, K.; Curcio, E. Progress and prospects in reverse electrodialysis for salinity gradient energy conversion and storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Veerman, J.; Vermaas, D.A. Reverse electrodialysis: Fundamentals. In Sustainable Energy from Salinity Gradients; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pattle, R. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled ion exchange membranes: A comprehensible review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Saakes, M.; Nijmeijer, K. Fouling in reverse electrodialysis under natural conditions. Water Res. 2013, 47, 1289–1298. [Google Scholar]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Chon, K.; Jeong, N.; Rho, H.; Nam, J.Y.; Jwa, E.; Cho, J. Fouling characteristics of dissolved organic matter in fresh water and seawater compartments of reverse electrodialysis under natural water conditions. Desalination 2020, 114478, in press. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, H.J.; Choi, S.J.; Geckeler, K.E.; Cho, J.; Moon, S.H. Fouling mitigation of anion exchange membrane by zeta potential control. J. Colloid Interface Sci. 2003, 259, 293–300. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Moreno, J.; Saakes, M.; de Vos, W.M.; Nijmeijer, K. Role of anion exchange membrane fouling in reverse electrodialysis using natural feed waters. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 198–204. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Liu, F.; Zhu, S.; Boggs, C.; Armstrong, M.D.; Call, D.F.; Coronell, O. Impact of natural organic matter and inorganic solutes on energy recovery from five real salinity gradients using reverse electrodialysis. J. Memb. Sci. 2017, 541, 621–632. [Google Scholar] [CrossRef]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Prediction of reverse electrodialysis performance by inclusion of 2D fluorescence spectroscopy data into multivariate statistical models. Sep. Purif. Technol. 2015, 150, 159–169. [Google Scholar] [CrossRef]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. 2D fluorescence spectroscopy for monitoring ion-exchange membrane based technologies—Reverse electrodialysis (RED). Water Res. 2016, 88, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.A. Uphill transport in improved reverse electrodialysis by removal of divalent cations in the dilute solution: A Nernst-Planck based study. J. Memb. Sci. 2020, 598, 117784. [Google Scholar] [CrossRef]
- Pintossi, D.; Chen, C.; Saakes, M.; Nijmeijer, K.; Borneman, Z. Influence of sulfate on anion exchange membranes in reverse electrodialysis. NPJ Clean Water 2020, 3, 29. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of monovalent ion selective membranes for reducing the impacts of multivalent ions in reverse electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Influence of multivalent ions on power production from mixing salt and fresh water with a reverse electrodialysis system. J. Memb. Sci. 2009, 330, 65–72. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Ortiz-Martínez, V.M.; Carmona, J.; Palacio, L.; Prádanos, P.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Modeling the influence of divalent ions on membrane resistance and electric power in reverse electrodialysis. J. Memb. Sci. 2019, 592, 117385. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Memb. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport characteristics of fujifilm ion-exchange membranes as compared to homogeneous membranes AMX and CMX and to heterogeneous membranes MK-40 and MA-41. Membranes 2019, 9, 84. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Pawlowski, S.; Huertas, R.M.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. On operation of reverse electrodialysis (RED) and membrane capacitive deionisation (MCDI) with natural saline streams: A critical review. Desalination 2020, 476, 114183. [Google Scholar] [CrossRef]
- Długołecki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current status of ion exchange membranes for power generation from salinity gradients. J. Memb. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Memb. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Memb. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis —Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Memb. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-conductive membranes for permeation enhancement and fouling mitigation: A short review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y.; Van der Bruggen, B. Layer-by-layer assembly of anion exchange membrane by electrodeposition of polyelectrolytes for improved antifouling performance. J. Memb. Sci. 2018, 558, 1–8. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; Van der Bruggen, B.; Shen, J. Preparation of a monovalent selective anion exchange membrane through constructing a covalently crosslinked interface by electro-deposition of polyethyleneimine. J. Memb. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiao, P.; Ruan, H.; Liao, J.; Gao, C.; Van der Bruggen, B.; Shen, J. Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property. Membranes 2019, 9, 36. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, B.; Tong, X.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Memb. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Nebavskaya, X.; Sarapulova, V.; Butylskii, D.; Larchet, C.; Pismenskaya, N. Electrochemical properties of homogeneous and heterogeneous anion exchange membranes coated with cation exchange polyelectrolyte. Membranes 2019, 9, 13. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yuan, S.; Zhu, J.; Houtmeyers, S.; Li, J.; Dewil, R.; Gao, C.; Van Der Bruggen, B. A chemically assembled anion exchange membrane surface for monovalent anion selectivity and fouling reduction. J. Mater. Chem. A 2019, 7, 6348–6356. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis—Experimental verification of theoretical predictions. J. Memb. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Van der Bruggen, B.; Sotto Díaz, A.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Memb. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Ahmad, M.; Tang, C.; Yang, L.; Yaroshchuk, A.; Bruening, M.L. Layer-by-layer modification of aliphatic polyamide anion-exchange membranes to increase Cl−/SO42− selectivity. J. Memb. Sci. 2019, 578, 209–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, C.; Van Der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Brugge, B.; Shen, J.; Gao, C. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity. J. Memb. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Lang, Q.; Tan, M.; Zhang, Y. Composite anion exchange membrane made by layer-by-layer method for selective ion separation and water migration control. Sep. Purif. Technol. 2018, 192, 278–286. [Google Scholar] [CrossRef]
- Sata, T.; Izuo, R.; Mizutani, Y.; Yamane, R. Transport properties of ion-exchange membranes in the presence of surface active agents. J. Colloid Interface Sci. 1972, 40, 317–328. [Google Scholar] [CrossRef]
- Guler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-made anion-exchange membranes for salinity gradient power generation using reverse electrodialysis. ChemSusChem 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Shahi, V.K.; Schubert, R.; Rangarajan, R.; Mehnert, R. Development of urethane acrylate composite ion-exchange membranes and their electrochemical characterization. J. Colloid Interface Sci. 2004, 270, 446–454. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with surface-enhanced antifouling properties for water purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Kedem, O.; Schechtmann, L.; Mirsky, Y.; Saveliev, G.; Daltrophe, N. Low-polarisation electrodialysis membranes. Desalination 1998, 118, 305–314. [Google Scholar] [CrossRef]
- Liao, J.; Gao, X.; Yu, X.; Ruan, H.; Li, J.; Shen, J.; Gao, C. Developments on Monovalent Anion-Selective Membranes (MASMs): A Mini-review of Our Recent Contributions. J. Membr. Sci. Technol. 2019, 9, 192. [Google Scholar]
- Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G. Surface modification of ion-exchange membranes: Methods, characteristics, and performance. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar]
- Cao, R.; Shi, S.; Li, Y.; Xu, B.; Zhao, Z.; Duan, F.; Cao, H.; Wang, Y. The properties and antifouling performance of anion exchange membranes modified by polydopamine and poly (sodium 4-styrenesulfonate). Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124429. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Sata, T.; Yamaguchi, T.; Matsusaki, K. Interaction between anionic polyelectrolytes and anion exchange membranes and change in membrane properties. J. Memb. Sci. 1995, 100, 229–238. [Google Scholar] [CrossRef]
- Grebenyuk, V.D.; Chebotareva, R.D.; Peters, S.; Linkov, V. Surface modification of anion-exchange electrodialysis membranes to enhance anti-fouling characteristics. Desalination 1998, 115, 313–329. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling phenomena on anion exchange membranes under the reverse electrodialysis process. J. Memb. Sci. 2017, 530, 232–239. [Google Scholar] [CrossRef]
- De Giglio, E.; Cafagna, D.; Ricci, M.A.; Sabbatini, L.; Cometa, S.; Ferretti, C.; Mattioli-Belmonte, M. Biocompatibility of poly(acrylic acid) thin coatings electro-synthesized onto tiAlV-based implants. J. Bioact. Compat. Polym. 2010, 25, 374–391. [Google Scholar] [CrossRef]
- Diken, M.E.; Doğan, S.; Turhan, Y.; Doğan, M. Synthesis and Characterization of Poly(Acrylic Acid)/Organo-Modified Nanohydroxyapatite Nanocomposites: Thermal, Optical and Biocompatibility Properties. Adv. Mater. Sci. 2018, 18, 54–67. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Memb. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Zhao, Z.; Wen, H. Modification and properties characterization of heterogeneous anion-exchange membranes by electrodeposition of graphene oxide (GO). Appl. Surf. Sci. 2018, 442, 700–710. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Zhao, Z.; Su, C.; Wen, H. Improvement of the antifouling performance and stability of an anion exchange membrane by surface modification with graphene oxide (GO) and polydopamine (PDA). J. Memb. Sci. 2018, 566, 44–53. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, H.; Shi, S.; Li, Y.; Yao, L. Characterization of anion exchange membrane modified by electrodeposition of polyelectrolyte containing different functional groups. Desalination 2016, 386, 58–66. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, D.; Muhammad, A.S.; Hossain, M.M.; Ge, Z.; He, Y.; Feng, H.; Xu, T. Fouling deposition as an effective approach for preparing monovalent selective membranes. J. Memb. Sci. 2019, 580, 327–335. [Google Scholar] [CrossRef]
- Ariono, D. Khoiruddin Improving ion-exchange membrane properties by the role of nanoparticles. AIP Conf. Proc. 2017, 1788, 030003. [Google Scholar]
- Fernandez-Gonzalez, C.; Zhang, B.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A.; Chen, Y. Enhancing fouling resistance of polyethylene anion exchange membranes using carbon nanotubes and iron oxide nanoparticles. Desalination 2017, 411, 19–27. [Google Scholar] [CrossRef]
- Salehi, E.; Hosseini, S.M.; Ansari, S.; Hamidi, A. Surface modification of sulfonated polyvinylchloride cation-exchange membranes by using chitosan polymer containing Fe3O4 nanoparticles. J. Solid State Electrochem. 2016, 20, 371–377. [Google Scholar] [CrossRef]
- Hong, J.G.; Park, T.W. Electrochemical characterizations and reverse electrodialysis performance of hybrid anion exchange membranes for salinity gradient energy. J. Electroanal. Chem. 2018, 817, 134–140. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Ding, J.; Shi, W.; Pan, J.; Gao, C.; Shen, J.; van der Bruggen, B. Surface layer modification of AEMs by infiltration and photo-cross-linking to induce monovalent selectivity. AIChE J. 2018, 64, 993–1000. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, R.; Zhang, Y.; Shi, B.; Wang, J.; Liu, J. Stably coating loose and electronegative thin layer on anion exchange membrane for efficient and selective monovalent anion transfer. Desalination 2017, 410, 55–65. [Google Scholar] [CrossRef]
- Ruan, H.; Zheng, Z.; Pan, J.; Gao, C.; Van der Bruggen, B.; Shen, J. Mussel-inspired sulfonated polydopamine coating on anion exchange membrane for improving permselectivity and anti-fouling property. J. Memb. Sci. 2018, 550, 427–435. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Mulyati, S.; Takagi, R.; Matsuyama, H. Improved antifouling of anion-exchange membrane by polydopamine coating in electrodialysis process. Desalination 2014, 332, 126–133. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Zhao, Y.; Molina, S.; García-Calvo, E.; Van der Bruggen, B. Alternating current enhanced deposition of a monovalent selective coating for anion exchange membranes with antifouling properties. Sep. Purif. Technol. 2019, 229, 115807. [Google Scholar] [CrossRef]
- Sata, T.; Yamaguchi, T.; Kawamura, K.; Matsusaki, K. Transport numbers of various anions relative to chloride ions in modified anion-exchange membranes during electrodialysis. J. Chem. Soc. -Faraday Trans. 1997, 93, 457–462. [Google Scholar] [CrossRef]
- Melnikov, S.; Shkirskaya, S. Transport properties of bilayer and multilayer surface-modified ion-exchange membranes. J. Memb. Sci. 2019, 590, 117272. [Google Scholar] [CrossRef]
- Guesmi, F.; Hannachi, C.; Hamrouni, B. Selectivity of anion exchange membrane modified with polyethyleneimine. Ionics 2012, 18, 711–717. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.L.; Jia, Y.X.; Liu, X. An attempt for improving electrodialytic transport properties of a heterogeneous anion exchange membrane. Desalination 2014, 351, 163–170. [Google Scholar] [CrossRef]
- Hao, L.; Liao, J.; Jiang, Y.; Zhu, J.; Li, J.; Zhao, Y.; Van der Bruggen, B.; Sotto, A.; Shen, J. “Sandwich”-like structure modified anion exchange membrane with enhanced monovalent selectivity and fouling resistant. J. Memb. Sci. 2018, 556, 98–106. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Van der Bruggen, B.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Memb. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Tang, K.; Jin, Y.; Pan, J.; Van Der Bruggen, B.; Shen, J.; Gao, C. Mimicking the cell membrane: Bio-inspired simultaneous functions with monovalent anion selectivity and antifouling properties of anion exchange membrane. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]









| Membrane | Modification Method/ Modifying Agent | Permselectivity or Other Figures of Merit | Special Improvement/s | Membrane Electro-Resistance Change (Ω·cm2) | Reference |
|---|---|---|---|---|---|
| JMA-II-07 (Tingrun Co. Ltd. Beijing, China) | Infiltration and cross-linking under UV irradiation/ 4,4-diazostilbene-2,2-disulfonic acid disodium salt (DAS) | From 0.55 to 11.21 (better behavior than Selemion® ASV) | The modified layer was stable after 80 h of operation. | From 3.53 to 4.50 | [70] |
| Polyvinyl alcohol and quaternized-chitosan based AEM | Electronegative coating through interfacial polymerization/ 3,5-diaminobenzoic acid (DMA) | From 1.80 to ~9.30 | Improved antifouling potential transition time (from 55 min to 92 min), enhanced hydrophilicity (contact angle decreased from 56 to 40 ) and high thermal/mechanical membrane stability. | From 1.88 to 4.29 | [71] |
| Polyvinyl alcohol and quaternized-chitosan based AEM | Electronegative coating through interfacial polymerization/ 2,5 diaminobenzenesulfonic acid (DSA) | From 1.80 to 10.30 | Improved antifouling potential transition time (from 55 min to 95 min), enhanced hydrophilicity (contact angle decreased from 56 to 38 ) and high thermal/mechanical membrane stability. | From 1.88 to 3.21 | [71] |
| AEM Type I (Fujifilm Corp.) | Rapid deposition and polymerization/ L-polydopamine (L-PDA), and 4-amino-benzenesulfonic acid monosodium salt (ABS) | From 1.00 to 4.66 | Improved organic antifouling potential (electrical resistance due to fouling decreased from 4.78 Ω·cm2 to 0.53 Ω·cm2), enhanced hydrophilicity (contact angle decreased from 105.2 to 68.6 ). Separation efficiency enhanced from 2% to 63%. | N.A. | [40] |
| Fuji A (Fujifilm Corp.) | Coating by UV-curing/ 2-acryloylamido-2-methylpropane sulfonic acid (AMPS) and N,N-methylenebis(acrylamide) (MBA) | decreased by 10% and was comparable to that of Neosepta ACS | Improved organic antifouling potential transition time from 50 min to 90 min, increased hydrophilicity (contact angle reduced from 63 to 24 ). | From 0.93 to 1.10 | [21] * |
| Heterogeneous Ralex AM-PP (Mega a.s.) | Physical coating/ sPPO, sulfonated -Fe2O3 and oxidized carbon nanotubes (CNTs) | N.A. | Antifouling resistance improved by 53%. Enhanced hydrophilicity properties (contact angle decreased from 100.1 to 57.9 ) with 40–60% energy savings were achieved. | N.A. | [67] |
| AEM Type I (Fujifilm Corp.) | Self-adhesion deposition/ Sulfonated polydopamine (SPDA) | From 1.00 to 34.02 (improving both Neosepta ACS and Selemion ASV performances) | Higher anti-organic fouling potential (transition time improved from 76 min to 112 min). | From 1.02 to 6.83 | [72] |
| AEM Type I (Fujifilm Corp.) | Self-adhesion deposition/ Polydopamine (PDA) | From 1.00 to 11.59 (better results than Neosepta ACS and Selemion ASV) | Higher anti-organic fouling potential (transition time improved from 76 min to 106 min). | From 1.02 to 4.84 | [72] |
| Neosepta AMX (Astom Corp.) | Immersion/ Polydopamine (PDA) | N.A. | Improved anti-organic fouling (transition time increased from less than 25 min to ~300 min) and anti-biofouling properties. Enhanced hydrophilicity (contact angle decreased from 70 to 45 ). | From 2.5 to 5.0 | [58,73] * |
| Neosepta AM-1, AM-2 and AM-3 (Astom Corp.) | Immersion/Sodium naphthalene sulfate and formaldehyde or polystyrene sulphonic acid | From 1.25 to 3.33, approximately | Higher ion exchange capacity. | N.A. | [56] |
| Neosepta ASE (Astom Corp.) | Co-deposition by immersion/ Mixed solution of polydopamine (PDA) and poly(sodium 4-styrene sulfonate) (PSS) | N.A. | Excellent organic antifouling properties (transition time increased from 240 min to 1200 min). Improved hydrophilicity (contact angle decreased from 78 to 58 ) and stability. | From ~3.6 to ~4.5 | [54] |
| Neosepta AMX (Astom Corp.) | Dip coating/ Polydopamine (PDA) | From 0.8 to 4.5 | Validation of a theoretical model to obtain the charge density of the negatively charged layer | From 1.15 to 2.85 | [41] |
| Neosepta AMX (Astom Corp.) | Dip coating/ L-PDA and 4,4′-diamino-2,2′-biphenyldisulfonic acid (DBSA) | From 1.25 to 2.13 | Enhancement of the organic fouling resistance. Electrical resistance due to fouling reduced from 1.14 Ω·cm2 to 0.01 Ω·cm2 | From 1.49 to 3.62 | [74] |
| Home-made AEM from copolymer membranes composed of chloromethylstyrene and divinylbenzene | Immersion and refluxing/ Polyethylene polyamines (PEPDA) | From 1.20 to 3.03 | Membrane hydrophilicity improved. | From 1.80 to 5.6 | [75] |
| Heterogeneous Ralex AM-PES (Mega a.s.) | Coating (direct contact)/ Poly(acrylic) acid (PAA) | Sulfate rejection increased by 35% | Improved hydrophilicity (water contact angle decreased from 96 to 66 ) | From 5.0 to 5.4 | [55] * |
| Heterogeneous Ralex AMH (Mega a.s.) | Coating (sequential diffusion)/ Polyaniline (PANi) and perfluorocarbon cation-exchanger MF4-SK/PANi | N.A. | Increased ion exchange capacity, electrical conductivity and limiting current density. High mechanical and chemical stability. | N.A. | [76] |
| Neosepta AMX (Astom Corp.) | Adsorption/ Poly(ethyleneimine) (PEI) | Selectivity coefficients for SO42−/Cl−, NO3−/Cl−, and SO42−/NO3− are reduced from 0.11 to 0.04, 0.71 to 0.24, and 0.21 to 0.08, respectively | The modified membrane became more selective towards monovalent anions | N.A. | [77] |
| AEM** (Ionics) | Coating by adsorption/ Olygourethane surfactants and Disodium salt α,ω-oligooxipropylene-bis(o-urethane-2.4,2.6-tolueneurylbenzene sulphonic acid) | N.A. | Power consumption reduced 1.7 times. Excellent anti-organic fouling properties | From 2.5 to 5.7 | [57] |
| CJMA-2 (Hefei Chemjoy Polymer Material Co., Ltd., Hefei, China) | Layer-by-layer (LbL) deposition (7.5 bilayers)/ Poly(styrene sulfonate) and poly(ethyleneimine) (PEI) | From 1.10 to 2.44 | Anti-organic fouling potential transition time improved by 38.4%. Enhanced hydrophilicity (contact angle decreased from 82.47 to 68.63 ), and gross power density increased by 10% compared to Neosepta ACS. | From 2.8 to 3.3 | [37] * |
| AEM Type I (Fujifilm Corp.) | Coating by LbL/ Poly(4-styrene sulfonate) and protonated poly(allylamine) | From 1.3 to 7.4 | Increased Cl−/SO42− permselectivity in Diffusion dialysis | N.A. | [43] |
| TWEDA1 (Tianwei Membrane Technology Co.) | Coating via LbL (10.5 layers)/ Poly (sodium-p-styrene sulfonate), Poly (diallyldimethyl ammonium chloride) (PDDA), and graphene | From 1 to 11.5 (better performance than Neosepta ACS) | Improved separation efficiency of monovalent ions. Controlled water migration | From 1.81 to 2.31 | [46] |
| Heterogeneous AEM ** (Zhe-jiang Qianqiu Environmental Protection & Water Treatment Co. Ltd.) | LbL deposition (10 layers max.)/ Glutaraldehyde (GA) and poly(ethyleneimine) (PEI) | From 0.42 to 0.55 | Increased hydrophilicity (water contact angle decreased from 102.3 to 73.2) and improved surface homogeneity | From 4.47 to 4.81 | [78] |
| Neosepta AMX (Astom Corp.) | LbL deposition/Poly(sodium 4-styrene sulfonate) (PSS) and poly(allylamine hydrochloride) (PAH) | From 0.8 to 2.6 | Improved antifouling properties (transition time increased from almost zero to ~150 min). | N.A. | [61] |
| AEM Type I (Fujifilm Corp.) | Electric-pulse LbL deposition (7.5 bilayers)/ Hydroxypropyltrimethyl ammonium chloride chitosan (HACC) and N-O-sulfonic acid benzyl chitosan (NSBC) | From 0.81 to 47.04 (higher value than those associated with Neosepta ACS and Selemion ASV) | Separation efficiency rising from –8.93% to 94.43% | From 1.31 to ~3.53 | [45] |
| AEM Type I (Fujifilm Corp.) | Alternating current LbL assembly/ Poly(4-styrenesulphonic acid-co-maleic acid) sodium salt, 2-hydroxypropyltrimethyl ammonium chloride chitosan, and 1,4-bis(2′,3′-epoxypropyl) perfluoro-1-butane | From 0.81 to 4.87 | Improved separation efficiency (from −8% to 62%). Improved antifouling characteristics against three foulants. The modified layer was stable after 96 h of operation. | N.A. | [44] |
| AEM Type I (Fujifilm Corp.) | Deposition/ Polydopamine (PDA) and sandwich alternating bilayers of poly(sodium 4-styrene sulfonate) (PSS)/hydroxypropyl trimethyl ammonium chloride chitosan-nano silver particles (HACC-Ag Np) | From 0.98 to 5.1 | Higher anti-organic fouling potential (transition time enhanced from 60 min to 125 min). Improved hydrophilicity (contact angle decreased from 101.8 to 95.5 ) | From 1.70 to 3.93 | [79] |
| JAM-II-07 (Yanrun Co.) | Coating by Deposition/ Sulfonated reduced graphene oxide (S-rGO) nanosheets | From 0.72 to 2.30 | Separation efficiency increased from −0.07 to 0.28 | From 3.06 to 3.72 | [80] |
| AEM ** (Fujifilm Corp.) | Electrodeposition/ Polydopamine (PDA) and N-O-sulfonic acid benzyl chitosan (NSBC) | From 0.78 to 2.20 | Enhanced anti-organic fouling properties | From 1.3 to 1.94 | [81] |
| Neosepta AEM *** | Electrodeposition/ Poly(ethyleneimine) (PEI) | From 0.79 to 4.2 | The modified layer was stable up to 70 h of operation. | From 4.63 to 6.05 | [35] |
| AEM ** (Fujifilm Corp.) | Alternate electrodeposition (9 bilayers)/ poly(sodium 4-styrene sulfonate) (PSS) and hydroxypropyltrimethyl ammonium chloride chitosan (HACC) | From 0.66 to 2.90 | Separation efficiency improved from −0.19 to 0.28 | From 1.31 to 4.52 | [42] |
| Neosepta AMX (Astom Corp.) | Fouling deposition/ Sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) (SPPO) | From 1.95 to 52.44, higher value than the one associated with Neosepta ACS | N.A. | From 2.4 to 2.83 | [65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotoka, F.; Merino-Garcia, I.; Velizarov, S. Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes 2020, 10, 160. https://doi.org/10.3390/membranes10080160
Kotoka F, Merino-Garcia I, Velizarov S. Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes. 2020; 10(8):160. https://doi.org/10.3390/membranes10080160
Chicago/Turabian StyleKotoka, Francis, Ivan Merino-Garcia, and Svetlozar Velizarov. 2020. "Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review" Membranes 10, no. 8: 160. https://doi.org/10.3390/membranes10080160
APA StyleKotoka, F., Merino-Garcia, I., & Velizarov, S. (2020). Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes, 10(8), 160. https://doi.org/10.3390/membranes10080160