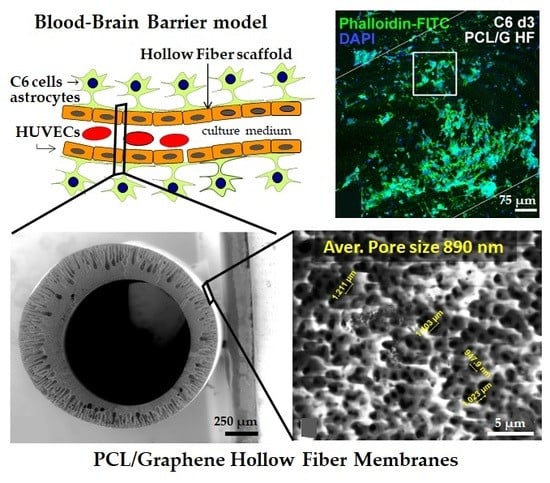
Hollow Fiber Membranes of PCL and PCL/Graphene as Scaffolds with Potential to Develop In Vitro Blood—Brain Barrier Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PCL/Graphene-Based Nanomaterial Hollow Fibers
2.2. Physicochemical Characterization of the Hollow Fibers
2.3. Mechanical, Electrical and Flux Properties Characterization of the Hollow Fibers
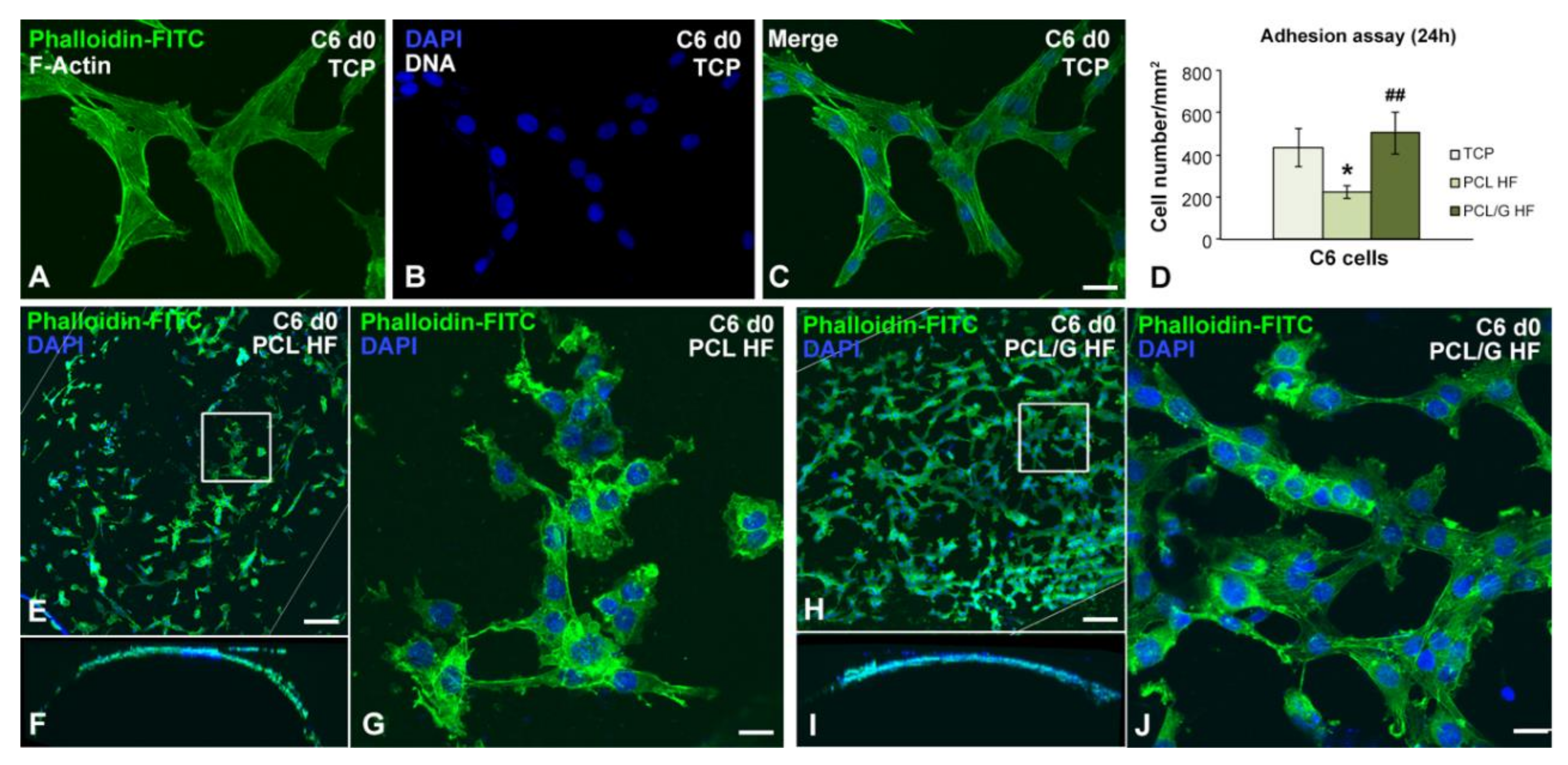
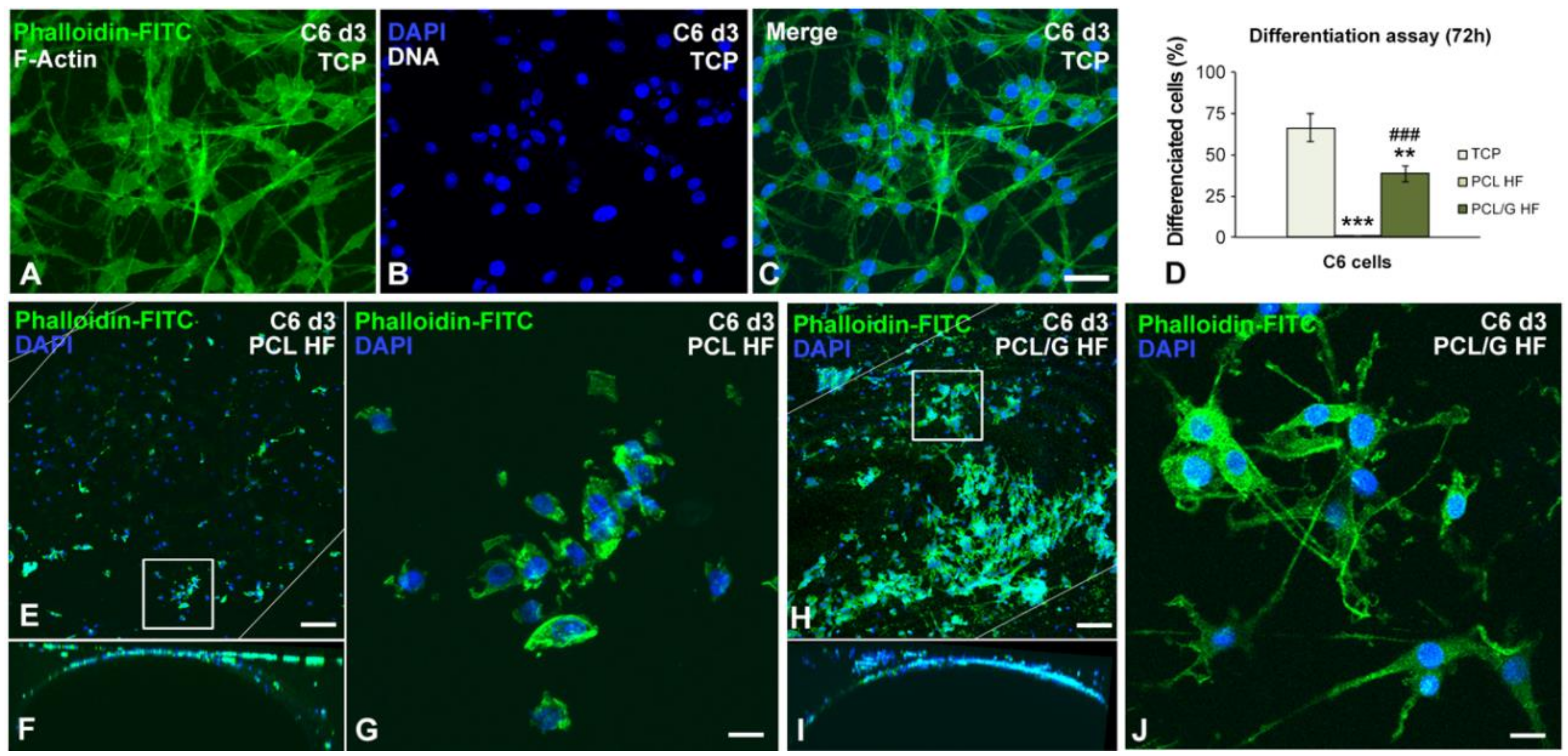
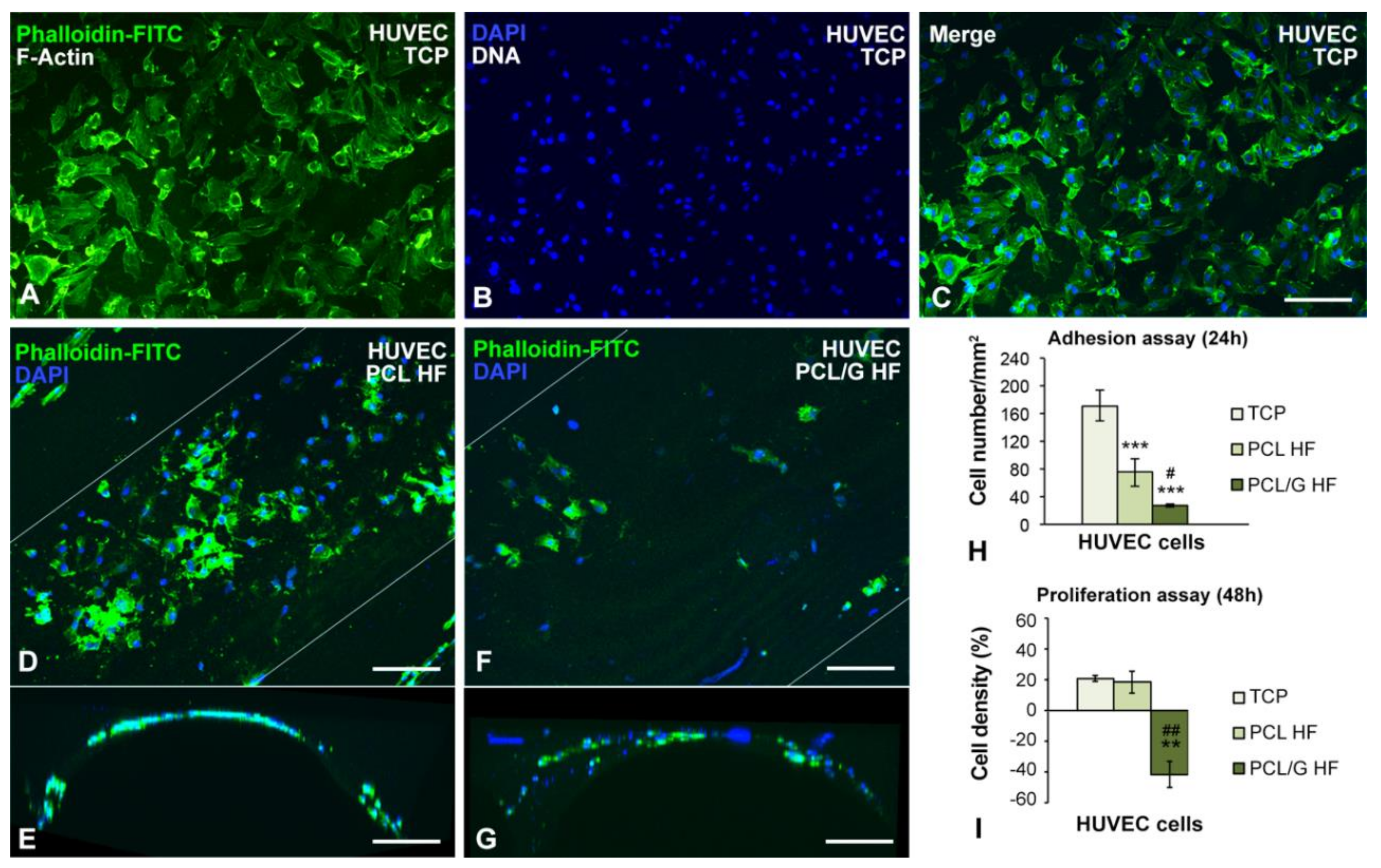
2.4. Cell Cultures
Immunofluorescence and Confocal Microscopy
2.5. Statistical Analysis
3. Results and Discussion
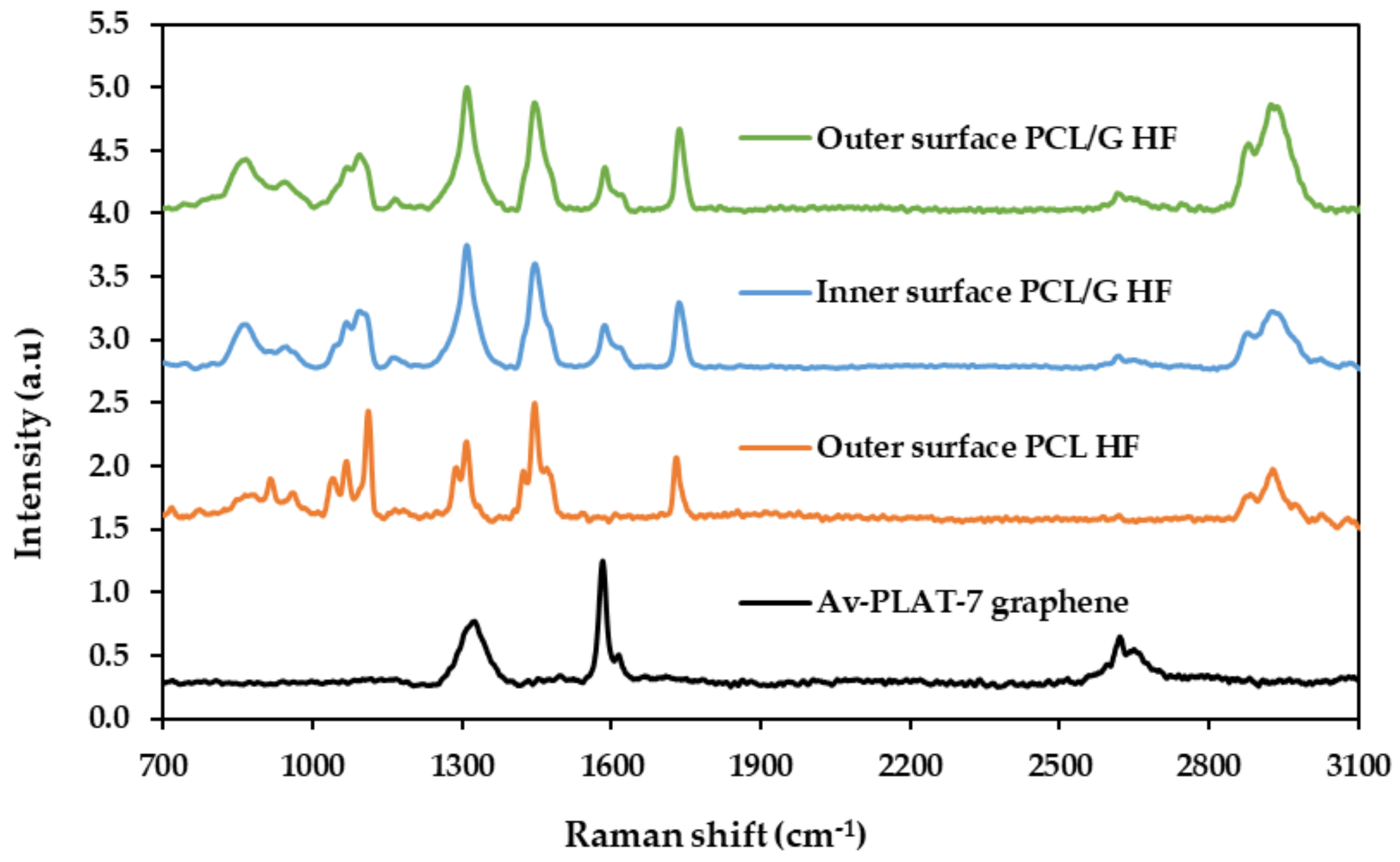
3.1. Physicochemical Characterization of the Hollow Fibers
3.2. Mechanical, Electrical and Flux Properties Characterization of the Hollow Fibers
3.3. Cell Cultures Viability on the Hollow Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Bartolo, L.; Leindlein, A.; Hofmann, D.; Bader, A.; de Grey, A.; Curcio, E.; Drioli, E. Bio-hybrid organs and tissues for patient therapy: A future vision for 2030. Chem. Eng. Process. Process. Intensif. 2012, 51, 79–87. [Google Scholar] [CrossRef]
- Diban, N.; Stamatialis, D. Polymeric hollow fiber membranes for bioartificial organs and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 633–643. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood–brain barrier models for drug screening and permeation studies: An overview. Drug Des. Devel. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L.; Maiti, P.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Parkinson, F.E.; Friesen, J.; Krizanac-Bengez, L.; Janigro, D. Use of a three-dimensional in vitro model of the rat blood–brain barrier to assay nucleoside efflux from brain. Brain Res. 2003, 980, 233–241. [Google Scholar] [CrossRef]
- Cucullo, L.; Couraud, P.O.; Weksler, B.; Romero, I.A.; Hossain, M.; Rapp, E.; Janigro, D. Immortalized human brain endothelial cells and flow-based vascular modeling: A marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2008, 28, 312–328. [Google Scholar] [CrossRef]
- Stanness, K.A.; Westrum, L.E.; Fornaciari, E.; Mascagni, P.; Nelson, J.A.; Stenglein, S.G.; Myers, T.; Janigro, D. Morphological and functional characterization of an in vitro blood–brain barrier model. Brain Res. 1997, 771, 329–342. [Google Scholar] [CrossRef]
- Pinho, A.C.; Fonseca, A.C.; Serra, A.C.; Santos, J.D.; Coelho, J.F.J. Peripheral Nerve Regeneration: Current Status and New Strategies Using Polymeric Materials. Adv. Healthc. Mater. 2016, 5, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, L. Polymers for fabricating nerve conduits. Int. J. Polym. Sci. 2010, 2010, 20. [Google Scholar] [CrossRef]
- Morelli, S.; Piscioneri, A.; Salerno, S.; Al-Fageeh, M.B.; Drioli, E.; De Bartolo, L. Neuroprotective effect of didymin on hydrogen peroxide-induced injury in the neuronal membrane system. Cells Tissues Organs 2014, 199, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.; Leal, C.V.; Derami, M.S.; de Rezende Duek, E.A.; Ceragioli, H.J.; de Oliveira, A.L.R. Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Diban, N.; Haimi, S.; Bolhuis-Versteeg, L.; Teixeira, S.; Miettinen, S.; Poot, A.; Grijpma, D.; Stamatialis, D. Development and characterization of poly(ε-caprolactone) hollow fiber membranes for vascular tissue engineering. J. Memb. Sci. 2013, 438, 29–37. [Google Scholar] [CrossRef]
- Salerno, S.; Tasselli, F.; Drioli, E.; De Bartolo, L. Poly(ε-Caprolactone) Hollow Fiber Membranes for the Biofabrication of a Vascularized Human Liver Tissue. Membranes (Basel) 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Xie, K.; Ji, X.; Xu, B.; Wang, C.; Shi, P. Vascularized neural constructs for ex-vivo reconstitution of blood–brain barrier function. Biomaterials 2020, 245, 119980. [Google Scholar] [CrossRef]
- Akhavan, O. Graphene scaffolds in progressive nanotechnology/stem cell-based tissue engineering of the nervous system. J. Mater. Chem. B 2016, 4, 3169–3190. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, 263–267. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable one-step wet-spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: Towards multifunctional textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Apollo, N.V.; Maturana, M.I.; Tong, W.; Nayagam, D.A.X.; Shivdasani, M.N.; Foroughi, J.; Wallace, G.G.; Prawer, S.; Ibbotson, M.R.; Garrett, D.J. Soft, Flexible Freestanding Neural Stimulation and Recording Electrodes Fabricated from Reduced Graphene Oxide. Adv. Funct. Mater. 2015, 25, 3551–3559. [Google Scholar] [CrossRef]
- Diban, N.; Sánchez-González, S.; Lázaro-Díez, M.; Ramos-Vivas, J.; Urtiaga, A. Facile fabrication of poly(ε-caprolactone)/graphene oxide membranes for bioreactors in tissue engineering. J. Memb. Sci. 2017, 540, 219–228. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic Degradation and Mechanical Stability of Poly(ε-Caprolactone)/Reduced Graphene Oxide Membranes as Scaffolds for in Vitro Neural Tissue Regeneration. Membranes (Basel) 2018, 8, 12. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Bianchi, F.; Ye, H.; Urtiaga, A. Evidences of the Effect of GO and rGO in PCL Membranes on the Differentiation and Maturation of Human Neural Progenitor Cells. Macromol. Biosci. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer–Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 16. [Google Scholar] [CrossRef]
- Diban, N.; Haimi, S.; Bolhuis-Versteeg, L.; Teixeira, S.; Miettinen, S.; Poot, A.; Grijpma, D.; Stamatialis, D. Hollow fibers of poly(lactide-co-glycolide) and poly(ε-caprolactone) blends for vascular tissue engineering applications. Acta Biomater. 2013, 9, 6450–6458. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Nijmeijer, K.; Ortiz, I.; Urtiaga, A. Hydrogen separation from multicomponent gas mixtures containing CO, N2 and CO2 using Matrimid® asymmetric hollow fiber membranes. J. Memb. Sci. 2012, 419–420, 49–56. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Poly(ε-caprolactone)/graphene oxide biocomposites: Mechanical properties and bioactivity. Biomed. Mater. 2011, 6, 8. [Google Scholar] [CrossRef]
- Bullock, T.H. Signals and signs in the nervous system: The dynamic anatomy of electrical activity is probably information-rich. Proc. Natl. Acad. Sci. USA 1997, 94, 1–6. [Google Scholar] [CrossRef]
- Suhaimi, H. Glucose Diffusivity in Tissue Engineering Membranes and Scaffolds: Implications for Hollow Fibre Membrane Bioreactor. Ph.D. Thesis, Loughborough University, Leicester, UK, 2015. [Google Scholar]
- Hu, W.; Onuma, T.; Birukawa, N.; Abe, M.; Ito, E.; Chen, Z.; Urano, A. Change of morphology and cytoskeletal protein gene expression during dibutyryl cAMP-induced differentiation in C6 glioma cells. Cell. Mol. Neurobiol. 2008, 28, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M. Preparation of Synthetic Membranes. In Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; pp. 54–109. [Google Scholar]
- Lee, J.; Chae, H.R.; Won, Y.J.; Lee, K.; Lee, C.H.; Lee, H.H.; Kim, I.C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Lan, C.Q.; Matsuura, T. Effects of Inorganic Nano-Additives on Properties and Performance of Polymeric Membranes in Water Treatment. Sep. Purif. Rev. 2016, 45, 141–167. [Google Scholar] [CrossRef]
- Karande, T.S.; Ong, J.L.; Agrawal, C.M. Diffusion in musculoskeletal tissue engineering scaffolds: Design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng. 2004, 32, 1728–1743. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single-and Few-Layer Graphene. Nano Lett. 2007, 17, 238–242. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Kesarwani, A.K.; Panwar, O.S.; Dhakate, S.R.; Singh, V.N.; Rakshit, R.K.; Bisht, A.; Kumar, A. Determining the number of layers in graphene films synthesized by filtered cathodic vacuum arc technique. Fullerenes Nanotub. Carbon Nanostruct. 2016, 24, 725–731. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon N. Y. 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Chaudhuri, B. Biopolymers-graphene Qxide Nanoplatelets Composites with Enhanced Conductivity and Biocompatibility Suitable for Tissue Engineering Applications. In Fullerenes, Graphenes and Nanotubes: A Pharmaceutical Approach; Elsevier: Norwich, UK; New York, NY, USA, 2018; pp. 457–544. ISBN 9780128136911. [Google Scholar]
- Wang, G.; Wei, Z.; Sang, L.; Chen, G.; Zhang, W.; Dong, X.; Qi, M. Morphology, crystallization and mechanical properties of poly(ɛ-caprolactone)/graphene oxide nanocomposites. Chin. J. Polym. Sci. 2013, 31, 1148–1160. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon N. Y. 2013, 52, 296–304. [Google Scholar] [CrossRef]
- Zirak Hassan Kiadeh, S.; Ghaee, A.; Mashak, A.; Mohammadnejad, J. Preparation of chitosan–silica/PCL composite membrane as wound dressing with enhanced cell attachment. Polym. Adv. Technol. 2017, 28, 1396–1408. [Google Scholar] [CrossRef]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in vitro modeling of the central nervous system. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.Y.; Wang, Y.J.; Mao, H.; Zhao, Z.P. Fabrication of PP hollow fiber membrane: Via TIPS using environmentally friendly diluents and its CO2 degassing performance. RSC Adv. 2019, 9, 19164–19170. [Google Scholar] [CrossRef]
- Patel, A.; Xue, Y.; Mukundan, S.; Rohan, L.C.; Sant, V.; Stolz, D.B.; Sant, S. Cell-Instructive Graphene-Containing Nanocomposites Induce Multinucleated Myotube Formation. Ann. Biomed. Eng. 2016, 44, 2036–2048. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; She, X.; Yang, D.; Wu, X.; Su, F.; Chen, Y. Synthesis of network reduced graphene oxide in polystyrene matrix by a two-step reduction method for superior conductivity of the composite. J. Mater. Chem. 2012, 22, 17254–17261. [Google Scholar] [CrossRef]
- Morelli, S.; Salerno, S.; Piscioneri, A.; Tasselli, F.; Drioli, E.; De Bartolo, L. Neuronal membrane bioreactor as a tool for testing crocin neuroprotective effect in Alzheimer’s disease. Chem. Eng. J. 2016, 305, 69–78. [Google Scholar] [CrossRef]
- Morelli, S.; Piscioneri, A.; Salerno, S.; Rende, M.; Campana, C.; Tasselli, F.; di Vito, A.; Giusi, G.; Canonaco, M.; Drioli, E.; et al. Flat and tubular membrane systems for the reconstruction of hippocampal neuronal network. J. Tissue Eng. Regen. Med. 2012, 6, 299–313. [Google Scholar] [CrossRef]
- Shearer, H.; Ellis, M.J.; Perera, S.P.; Chaudhuri, J.B. Effects of common sterilization methods on the structure and properties of poly(D,L lactic-co-glycolic acid) scaffolds. Tissue Eng. 2006, 12, 2717–2727. [Google Scholar] [CrossRef]
- Moriya, A.; Maruyama, T.; Ohmukai, Y.; Sotani, T.; Matsuyama, H. Preparation of poly(lactic acid) hollow fiber membranes via phase separation methods. J. Membr. Sci. 2009, 342, 307–312. [Google Scholar] [CrossRef]
- Lasocka, I.; Szulc-Dąbrowska, L.; Skibniewski, M.; Skibniewska, E.; Strupinski, W.; Pasternak, I.; Kmieć, H.; Kowalczyk, P. Biocompatibility of pristine graphene monolayer: Scaffold for fibroblasts. Toxicol. Vitr. 2018, 48, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Raghavan, G.; Yang, G.; Cohen-Karni, T. Effect of Graphene on Nonneuronal and Neuronal Cell Viability and Stress. Nano Lett. 2017, 17, 3297–3301. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Swaroop, S.; Chandran, P.; Nair, S.; Koyakutty, M. Cellular and molecular mechanistic insight into the DNA-damaging potential of few-layer graphene in human primary endothelial cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated Functional Group Density on Graphene Oxide: Its Effect on Cell Toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- Murray, E.; Thompson, B.C.; Sayyar, S.; Wallace, G.G. Enzymatic degradation of graphene/polycaprolactone materials for tissue engineering. Polym. Degrad. Stab. 2015, 111, 71–77. [Google Scholar] [CrossRef]
- Liang, L.; Wang, Q.; Wu, T.; Shen, J.; Kang, Y. Molecular Dynamics Simulation on Stability of Insulin on Graphene. Chin. J. Chem. Phys. 2013, 22, 627. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Shrader, C.D.; Bailey, K.M.; Konat, G.W.; Cilento, E.V.; Reilly, F.D. Insulin enhances proliferation and viability of human umbilical vein endothelial cells. Arch. Dermatol. Res. 2009, 301, 159–166. [Google Scholar] [CrossRef]



| Spinning Conditions | |
|---|---|
| Tube in orifice spinneret (mm) | OD: 1.3, ID: 0.7 |
| Dope temperature (°C) | 16 |
| Dope flow rate (cm3 h−1) | 150 |
| Bore composition (wt%) | 80/20 NMP/H2O |
| Bore flow rate (cm3 h−1) | 64 |
| Air gap height (cm) | 1.5 |
| Room temperature (°C) | 18.8 |
| Room humidity (%) | 87.7 |
| Quench bath composition (wt%) | 10/90 NMP/H2O |
| Quench bath temperature (°C) | 25 |
| Sequential Washing and Drying Procedure | |
| (1) Water baths | 1 (24 h) |
| (2) EtOH baths | 3 (20 min each) |
| (3) Ambient dry | 12 h |
| Parameter | PCL | PCL/G |
|---|---|---|
| Outer diameter, OD (µm) | 1474 ± 22 | 1789 ± 43 (*) |
| Inner diameter, ID (µm) | 908 ± 32 | 1080 ± 35 |
| HFs thickness (µm) | 273 ± 44 | 304 ± 74 |
| Selective layer thickness, (µm) 1 | 1.41 ± 0.12 | 0.60 ± 0.04 (*) |
| Surface pore size (µm) | 0.71 ± 0.04 | 0.89 ± 0.08 (*) |
| Bulk porosity, (%) | 84.75 ± 0.84 | 83.56 ± 0.44 |
| Parameter | PCL | PCL/G |
|---|---|---|
| Young Modulus (MPa) | 17.34 ± 0.79 | 16.62 ± 0.97 |
| Yield point (MPa) | 0.20 ± 0.03 | 0.24 ± 0.02 (*) |
| Ultimate tensile strength (MPa) | 1.65 ± 0.03 | 1.40 ± 0.13 (**) |
| Elongation at break (%) | 488.5 ± 46.4 | 327.1 ± 50.2 (**) |
| Membrane Material | Hydraulic Permeance (L m−2 h−1 bar−1) | References |
|---|---|---|
| PCL | 33 ± 7 | Present work |
| PCL/G | 119 ± 13 | Present work |
| PCL | 238 | Salerno et al. [16] |
| PAN | 146 | Morelli et al. [50] |
| PEEK-WC | 30 | Morelli et al. [51] |
| PLGA | 20 | Shearer et al. [52] |
| PLA | 120 | Moriya et al. [53] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantecón-Oria, M.; Diban, N.; Berciano, M.T.; Rivero, M.J.; David, O.; Lafarga, M.; Tapia, O.; Urtiaga, A. Hollow Fiber Membranes of PCL and PCL/Graphene as Scaffolds with Potential to Develop In Vitro Blood—Brain Barrier Models. Membranes 2020, 10, 161. https://doi.org/10.3390/membranes10080161
Mantecón-Oria M, Diban N, Berciano MT, Rivero MJ, David O, Lafarga M, Tapia O, Urtiaga A. Hollow Fiber Membranes of PCL and PCL/Graphene as Scaffolds with Potential to Develop In Vitro Blood—Brain Barrier Models. Membranes. 2020; 10(8):161. https://doi.org/10.3390/membranes10080161
Chicago/Turabian StyleMantecón-Oria, Marián, Nazely Diban, Maria T. Berciano, Maria J. Rivero, Oana David, Miguel Lafarga, Olga Tapia, and Ane Urtiaga. 2020. "Hollow Fiber Membranes of PCL and PCL/Graphene as Scaffolds with Potential to Develop In Vitro Blood—Brain Barrier Models" Membranes 10, no. 8: 161. https://doi.org/10.3390/membranes10080161
APA StyleMantecón-Oria, M., Diban, N., Berciano, M. T., Rivero, M. J., David, O., Lafarga, M., Tapia, O., & Urtiaga, A. (2020). Hollow Fiber Membranes of PCL and PCL/Graphene as Scaffolds with Potential to Develop In Vitro Blood—Brain Barrier Models. Membranes, 10(8), 161. https://doi.org/10.3390/membranes10080161