Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of PTMSP
2.3. IR-pyrolyzed PAN Materials
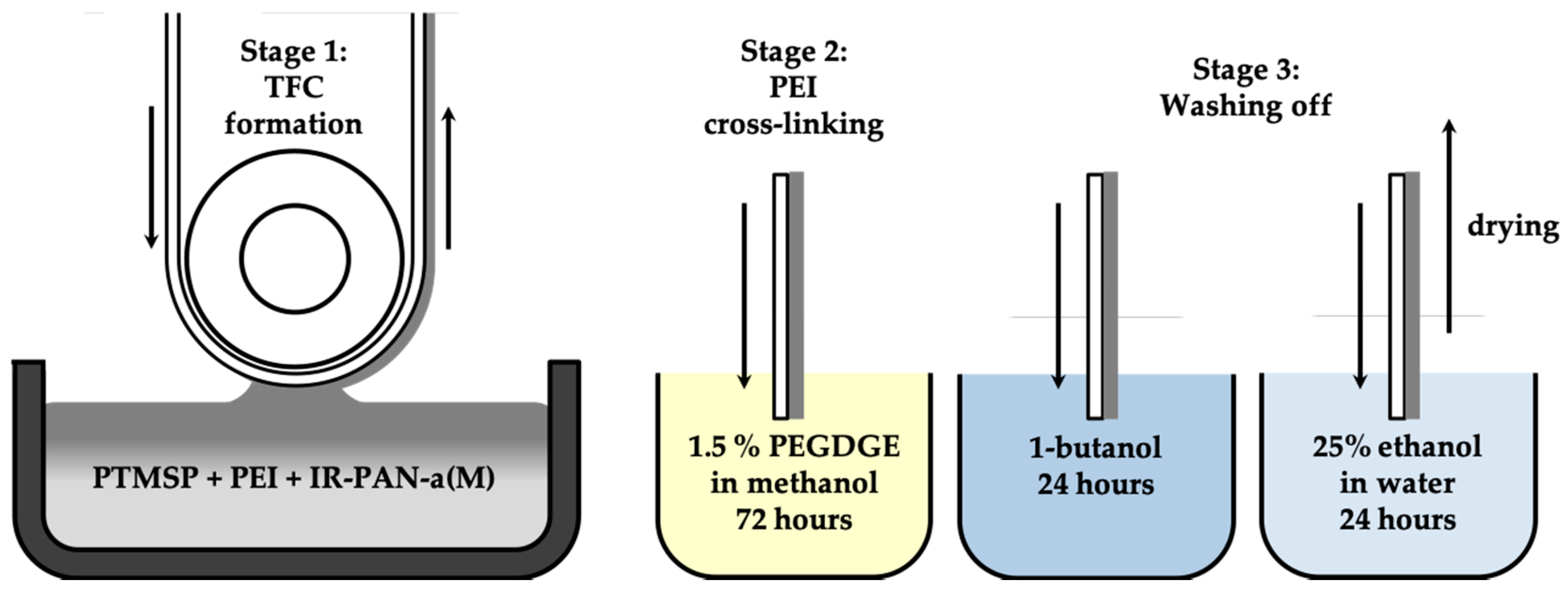
2.4. Membrane Fabrication
2.4.1. Dense Membranes
2.4.2. Thin-Film Composite Membranes
2.5. Gas Permeability Measurement and Aging
2.5.1. Dense Membranes
2.5.2. TFC Membranes
2.6. Scanning Electron Microscopy (SEM)
2.7. Particle Size Distribution
3. Results and Discussion
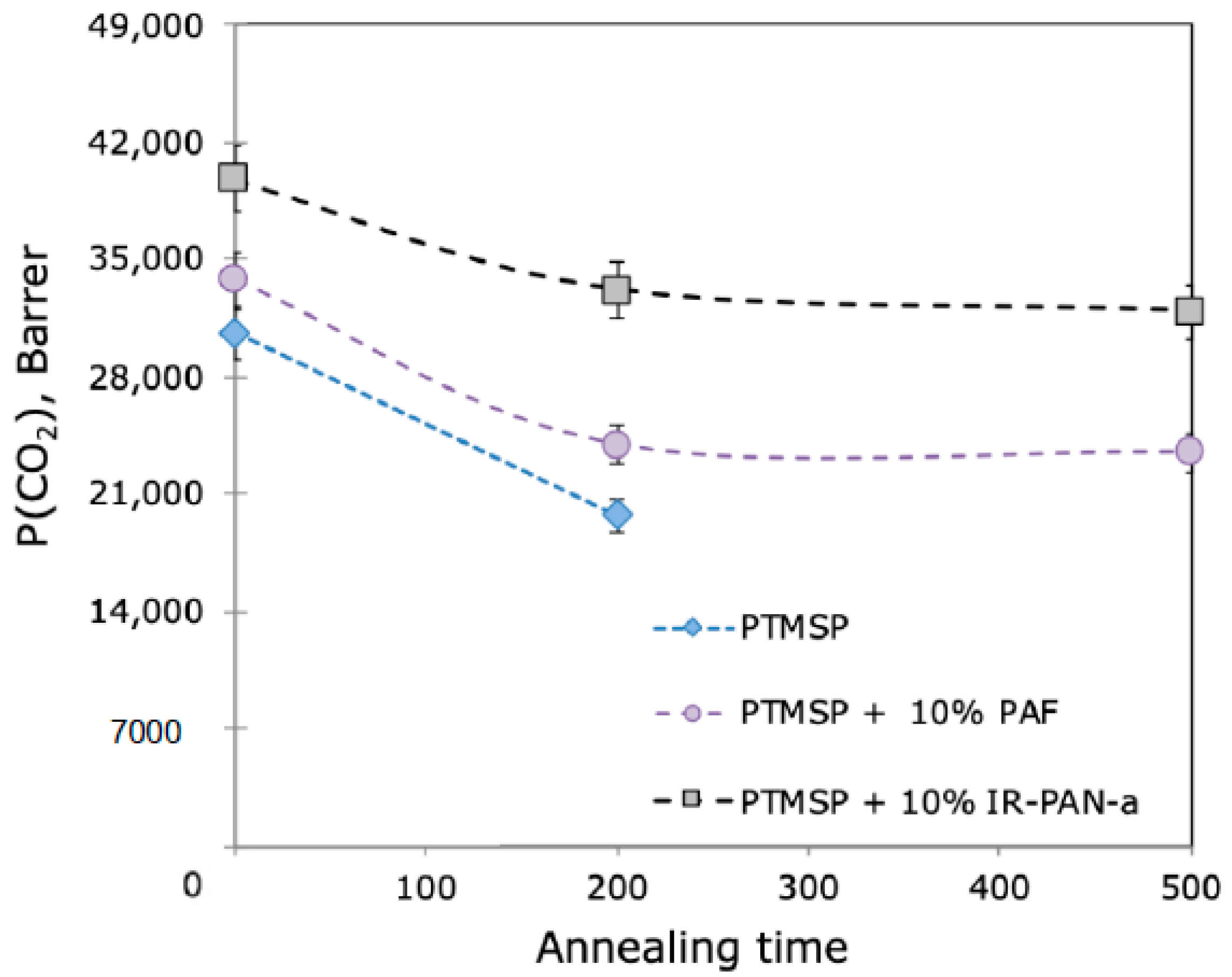
3.1. Dense Hybrid Membranes Aging
3.2. TFC Hybrid Membrane Aging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons, Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Hägg, M.B.; Deng, L. Membranes in gas separation. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications, 2nd ed.; Pabby, A.K., Rizvi, S.S.H., Sastve, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 143–180. [Google Scholar]
- Tasaka, S.; Inagaki, N.; Igawa, M. Effect of annealing on structure and permeability of poly[(l-trimethylsilyl)-l-propyne]. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 691–694. [Google Scholar] [CrossRef]
- Morlière, N.; Vallières, C.; Perrin, L.; Roizard, D. Impact of thermal ageing on sorption and diffusion properties of PTMSP. J. Membr. Sci. 2006, 270, 123–131. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.-B. Crosslinking and stabilization of nanoparticle filled PMP nanocomposite membranes for gas separations. J. Membr. Sci. 2009, 326, 285–292. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.-B. Crosslinking and stabilization of nanoparticle filled poly(1-trimethylsilyl-1-propyne) nanocomposite membranes for gas separations. J. Appl. Polym. Sci. 2009, 113, 3078–3088. [Google Scholar] [CrossRef]
- Shao, L.; Samseth, J.; Hägg, M.-B. Crosslinking and stabilization of high fractional free volume polymers for gas separation. Int. J. Greenh. Gas Control. 2008, 2, 492–501. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of Graphene and Graphene Oxide Nanoplatelets on the Gas Permselectivity and Aging Behavior of Poly(trimethylsilyl propyne) (PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Jia, J.; Baker, G.L. Cross-linking of poly[1-(trimethylsilyl)-1-propyne] membranes using bis (aryl azides). J. Polym. Sci. Part B Polym. Phys. 1998, 36, 959–968. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Kanehashi, S.; Ozcelik, B.; Kentish, S.E.; Hill, A.J.; Hill, M.R. Tailoring Physical Aging in Super Glassy Polymers with Functionalized Porous Aromatic Frameworks for CO2 Capture. Chem. Mater. 2015, 27, 4756–4762. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.Y.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-Separation Membranes Loaded with Porous Aromatic Frameworks that Improve with Age. Angew. Chem. 2015, 127, 2707–2711. [Google Scholar] [CrossRef]
- Kitchin, M.; Teo, J.; Sumby, C.J.; Thornton, A.W.; Konstas, K.; Lau, C.H.; Doonan, C.J.; Hill, M.R. AIMs: A new strategy to control physical aging and gas transport in mixed-matrix membranes. J. Mater. Chem. A 2015, 3, 15241–15247. [Google Scholar] [CrossRef]
- Volkov, A.V.; Bakhtin, D.S.; Kulikov, L.A.; Terenina, M.V.; Golubev, G.S.; Bondarenko, G.N.; Legkov, S.A.; Shandryuk, G.A.; Volkov, V.V.; Khotimskiy, V.S.; et al. Stabilization of gas transport properties of PTMSP with porous aromatic framework: Effect of annealing. J. Membr. Sci. 2016, 517, 80–90. [Google Scholar] [CrossRef]
- Yin, H.; Chapala, P.; Bermeshev, M.; Schönhals, A.; Böhning, M. Molecular Mobility and Physical Aging of a Highly Permeable Glassy Polynorbornene as Revealed by Dielectric Spectroscopy. ACS Macro Lett. 2017, 6, 813–818. [Google Scholar] [CrossRef]
- Kelman, S.D.; Rowe, B.W.; Bielawski, C.W.; Pas, S.J.; Hill, A.J.; Paul, D.; Freeman, B.D. Crosslinking poly[1-(trimethylsilyl)-1-propyne] and its effect on physical stability. J. Membr. Sci. 2008, 320, 123–134. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Starannikova, L.E.; Nikiforov, R.Y.; Bezgin, D.A.; Ponomarev, I.I.; Volkova, Y.A.; Blagodatskikh, I.V.; Yampolskii, Y.P. The Synthesis and Gas Transport Properties of PIM-1 Polybenzodioxane Modified with Benzanilide. Membr. Membr. Technol. 2020, 2, 203–209. [Google Scholar] [CrossRef]
- Starannikova, L.; Khodzhaeva, V.; Yampolskii, Y. Mechanism of aging of poly[1-(trimethylsilyl)-1-propyne] and its effect on gas permeability. J. Membr. Sci. 2004, 244, 183–191. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Konstas, K.; Doherty, C.M.; Wood, C.D.; Mulet, X.; Xie, Z.; Ng, D.; Hill, M.R.; Shao, L.; Lau, C.H. Hyper-Cross-Linked Additives that Impede Aging and Enhance Permeability in Thin Polyacetylene Films for Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 14401–14408. [Google Scholar] [CrossRef]
- Hou, R.; Smith, S.J.D.; Wood, C.D.; Mulder, R.J.; Lau, C.H.; Wang, H.; Hill, M.R. Solvation Effects on the Permeation and Aging Performance of PIM-1-Based MMMs for Gas Separation. ACS Appl. Mater. Interfaces 2019, 11, 6502–6511. [Google Scholar] [CrossRef]
- Putintseva, M.N.; Yushkin, A.A.; Bondarenko, G.N.; Kirk, R.A.; Budd, P.M.; Volkov, A.V. Crosslinking of Polybenzodioxane PIM-1 for Improving Its Stability in Aromatic Hydrocarbons. Polym. Sci. Ser. B 2019, 61, 795–805. [Google Scholar] [CrossRef]
- Qiu, J.; Zheng, J.-M.; Peinemann, K.-V. Gas Transport Properties of Poly(trimethylsilylpropyne) and Ethylcellulose Filled with Different Molecular Weight Trimethylsilylsaccharides: Impact on Fractional Free Volume and Chain Mobility. Macromolecules 2007, 40, 3213–3222. [Google Scholar] [CrossRef]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.-A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Y.; Liao, K.-S.; Chung, T.-S. Highly permeable and aging resistant 3D architecture from polymers of intrinsic microporosity incorporated with beta-cyclodextrin. J. Membr. Sci. 2017, 523, 92–102. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of thin-film composite membranes based on PTMSP loaded with porous aromatic frameworks. J. Membr. Sci. 2018, 554, 211–220. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Bakhtin, D.S.; Polevaya, V.G.; Balynin, A.V.; Maksimov, A.L.; Volkov, A.V. Friedel-Crafts Synthesis of New Porous Aromatic Frameworks for Stabilizing Gas Transport Properties of Highly Permeable Glassy Polymers. Russ. J. Appl. Chem. 2019, 92, 199–207. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Maksimov, A.L.; Volkov, A.V. Composite Membranes Based on the Poly(1-trimethylsylyl-1-propine): Influence of the Porous Aromatic Frameworks Produced from the Friedel–Crafts Reaction and Introduced into the Polymer Matrix. Russ. J. Appl. Chem. 2020, 93, 252–257. [Google Scholar] [CrossRef]
- Stanovsky, P.; Zitkova, A.; Karaszova, M.; Šyc, M.; Jansen, J.C.; Gándara, B.C.; McKeown, N.; Izák, P. Flue gas purification with membranes based on the polymer of intrinsic microporosity PIM-TMN-Trip. Sep. Purif. Technol. 2020, 242, 116814. [Google Scholar] [CrossRef]
- Dorkenoo, K.D.; Pfromm, P.H. Experimental evidence and theoretical analysis of physical aging in thin and thick amorphous glassy polymer films. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2239–2251. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Physical aging of thin glassy polymer films monitored by gas permeability. Polymer 2004, 45, 8377–8393. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Kryuchkov, S.S.; Zarubin, D.M.; Fukina, D.G.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Revealing the Surface Effect on Gas Transport and Mechanical Properties in Nonporous Polymeric Membranes in Terms of Surface Free Energy. Langmuir 2020, 36, 12911–12921. [Google Scholar] [CrossRef] [PubMed]
- Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Baranchikov, A.E.; Karpacheva, G.P. IR radiation assisted preparation of KOH-activated polymer-derived carbon for methylene blue adsorption. J. Environ. Chem. Eng. 2019, 7, 103514. [Google Scholar] [CrossRef]
- Efimov, M.N.; Sosenkin, V.E.; Volfkovich, Y.M.; Vasilev, A.A.; Muratov, D.G.; Baskakov, S.; Efimov, O.N.; Karpacheva, G. Electrochemical performance of polyacrylonitrile-derived activated carbon prepared via IR pyrolysis. Electrochem. Commun. 2018, 96, 98–102. [Google Scholar] [CrossRef]
- Lee, W.H.; Bae, J.Y.; Yushkin, A.; Efimov, M.; Jung, J.T.; Volkov, A.; Lee, Y.M. Energy and time efficient infrared (IR) irradiation treatment for preparing thermally rearranged (TR) and carbon molecular sieve (CMS) membranes for gas separation. J. Membr. Sci. 2020, 613, 118477. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Vasil’Ev, A.A.; Ivanov, V.I.; Bogdanova, Y.G.; Dolzhikova, V.D.; Karpacheva, G.P.; Bondarenko, G.N.; Volkov, A.V. Effect of IR Radiation on the Properties of Polyacrylonitrile and Membranes on Its Basis. Polym. Sci. Ser. A 2017, 59, 880–890. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Vasilev, A.A.; Bogdanova, Y.G.; Dolzhikova, V.D.; Karpacheva, G.P.; Volkov, A.V. Modification of polyacrylonitrile membranes by incoherent IR radiation. Pet. Chem. 2017, 57, 341–346. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Hou, R.; Konstas, K.; Akram, A.; Lau, C.H.; Hill, M.R. Control of Physical Aging in Super-Glassy Polymer Mixed Matrix Membranes. Acc. Chem. Res. 2020, 53, 1381–1388. [Google Scholar] [CrossRef]
- Golubev, G.S.; Borisov, I.L.; Litvinova, E.G.; Khotimsky, V.S.; Bakhtin, D.S.; Pastukhov, A.; Davankov, V.A.; Volkov, V.V. A novel hybrid material based on polytrimethylsilylpropyne and hypercrosslinked polystyrene for membrane gas separation and thermopervaporation. Pet. Chem. 2017, 57, 498–510. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Structure and properties of hypercrosslinked polystyrene—The first representative of a new class of polymer networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Malakhov, A.O.; Karpacheva, G.P.; Bondarenko, G.; Marbelia, L.; Vankelecom, I.F.J.; Volkov, A.V. Creation of highly stable porous polyacrylonitrile membranes using infrared heating. React. Funct. Polym. 2020, 104793. [Google Scholar] [CrossRef]
- Kossov, A.A.; Yushkin, A.A.; Khotimskiy, V.S.; Volkov, A.V. Study of accessible free volume and transport properties of TFPS-co-TMSP copolymer. Pet. Chem. 2015, 55, 783–790. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Borisov, I.L.; Bakhtin, D.S.; Rybakova, A.N.; Khotimskiy, V.S.; Molchanov, S.P.; Volkov, V.V. High-permeance crosslinked PTMSP thin-film composite membranes as supports for CO2 selective layer formation. Green Energy Environ. 2016, 1, 235–245. [Google Scholar] [CrossRef]
- Shishatskii, A.M.; Yampol’Skii, Y.P.; Peinemann, K.-V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Huang, C.-M.; Hsu, M.-Y.; Chen, H. Preparation of high-surface-area PAN-based activated carbon by solution-blowing process for CO2 adsorption. Sep. Purif. Technol. 2011, 82, 19–27. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Knyazeva, A.A.; Volkov, V.V.; Volkov, A.V. Polyalkylmethylsiloxanes composite membranes for hydrocarbon/methane separation: Eight component mixed-gas permeation properties. Sep. Purif. Technol. 2020, 241, 116696. [Google Scholar] [CrossRef]
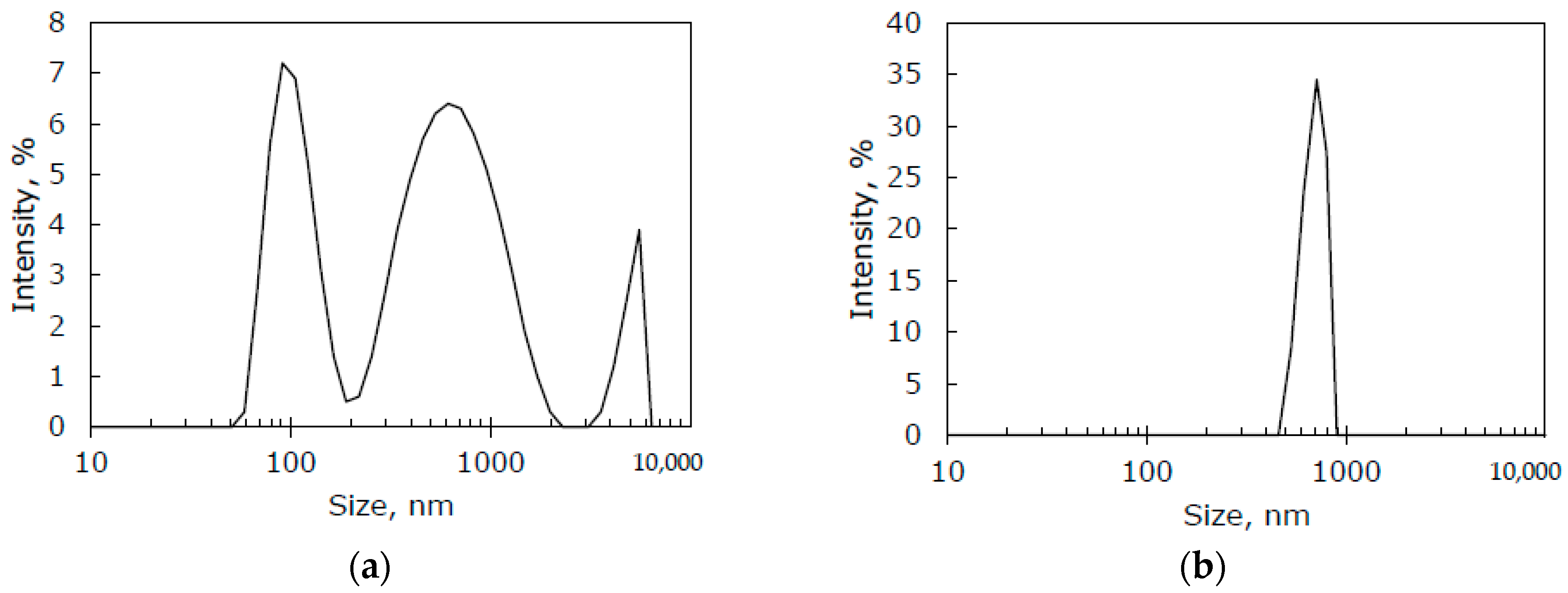
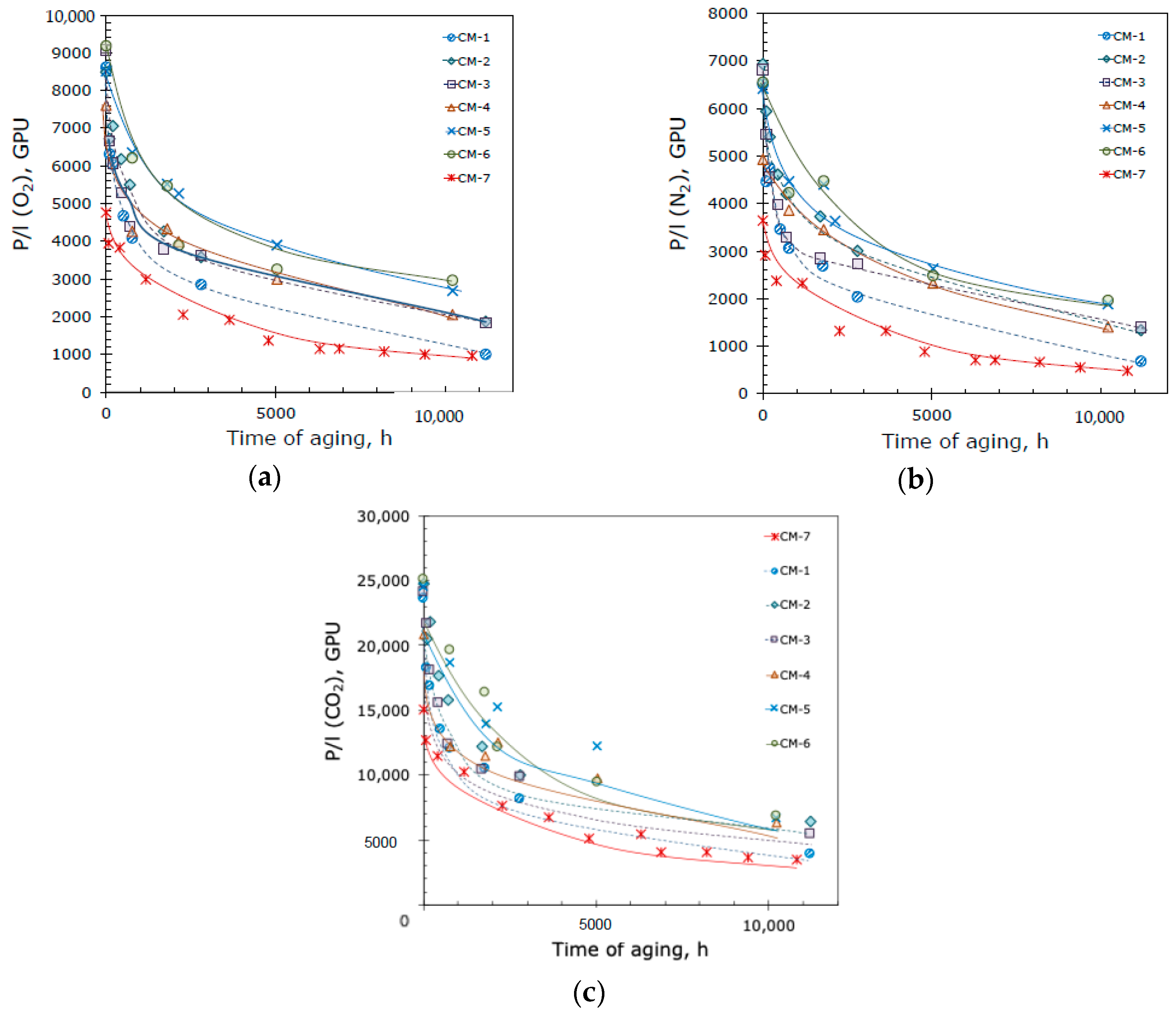
| Membrane Name | Crosslinking | Additive | Additive Content, wt% |
|---|---|---|---|
| CM-1 | PEI + PEGDGE | IR-PAN-a | 10 |
| CM-2 | PEI + PEGDGE | IR-PAN-a | 20 |
| CM-3 | PEI + PEGDGE | IR-PAN-a | 30 |
| CM-4 | PEI + PEGDGE | IR-PAN-aM | 10 |
| CM-5 | PEI + PEGDGE | IR-PAN-aM | 20 |
| CM-6 | PEI + PEGDGE | IR-PAN-aM | 30 |
| CM-7 | PEI + PEGDGE | - | - |
| Sample | Annealing Time at 100 °C, h | Thickness, µm | P, Barrer | α | Relative Change, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/N2 | O2/N2 | N2 | O2 | CO2 | |||
| PTMSP | 0 | 28.6 | 5500 | 8100 | 30,600 | 5.6 | 1.5 | 0 | 0 | 0 |
| 200 | 3200 | 5000 | 19,800 | 6.1 | 1.6 | 41 | 38 | 35 | ||
| 500 | - | - | - | - | - | - | - | - | ||
| PTMSP + 10 wt% IR-PAN-a | 0 | 38.0 | 7700 | 10,500 | 40,000 | 5.2 | 1.4 | 0 | 0 | 0 |
| 200 | 5600 | 8600 | 33,200 | 5.9 | 1.5 | 27 | 18 | 17 | ||
| 500 | 5500 | 8200 | 32,000 | 5.8 | 1.5 | 29 | 22 | 20 | ||
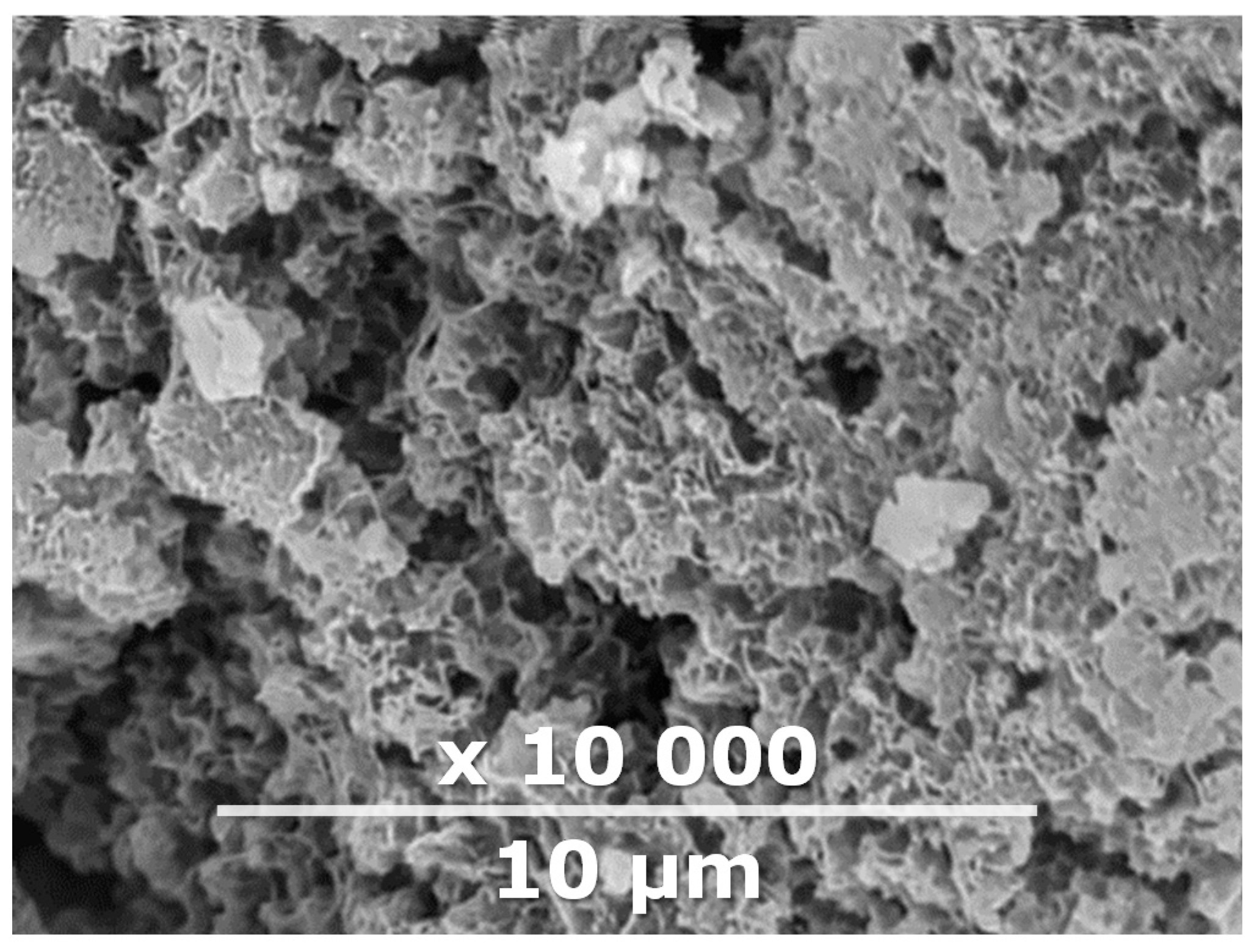
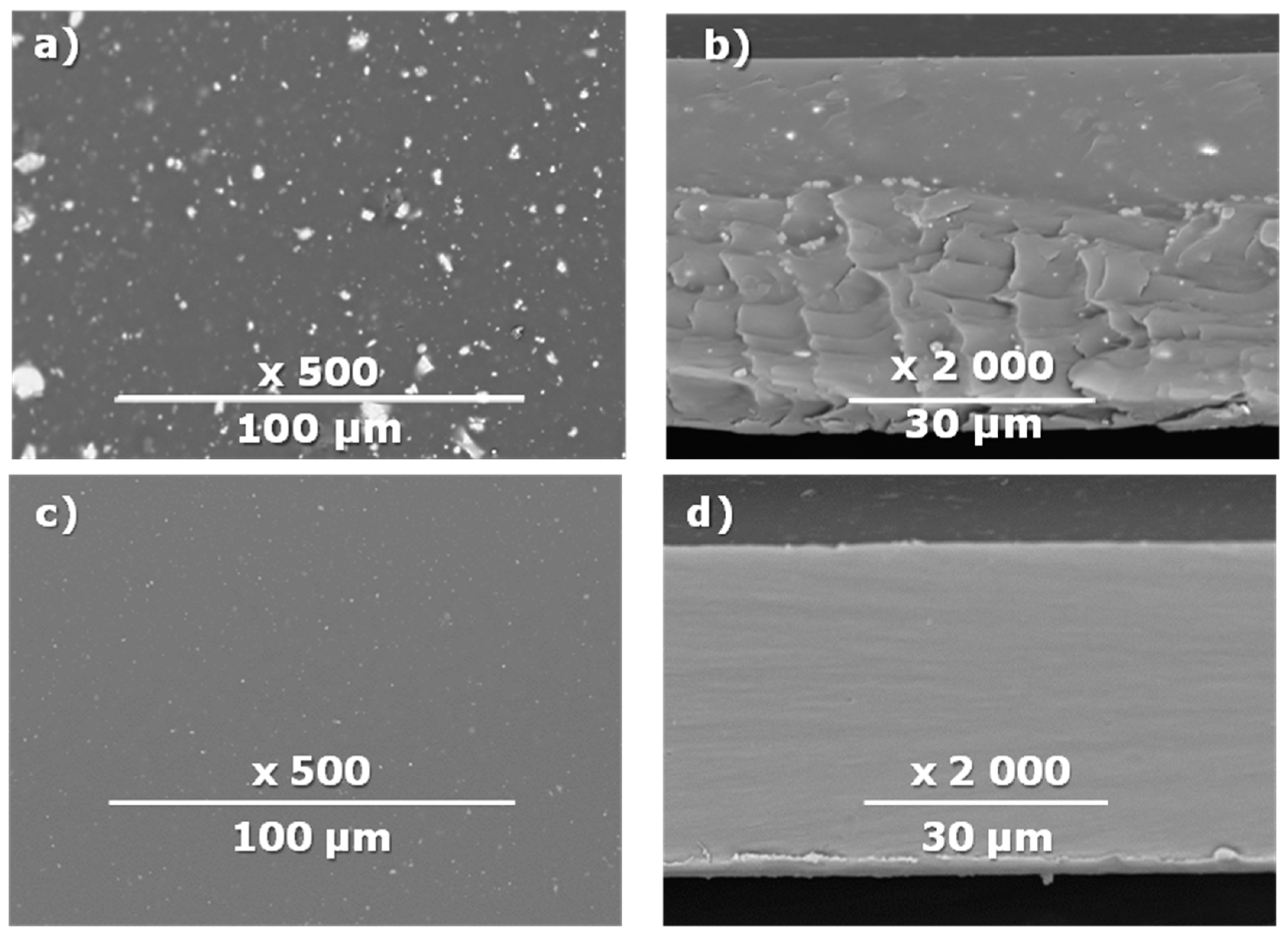
| Composite Membrane Designation | Crosslinking Agents | Filler | Filler Content, % wt | SEM | Selective Layer Thickness, µm |
|---|---|---|---|---|---|
| CM-1 | PEI+ PEGDGE | IR-PAN-a | 10 | 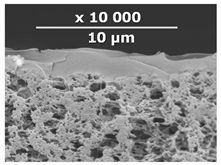 | 1.8 |
| CM-2 | PEI+ PEGDGE | IR-PAN-a | 20 | 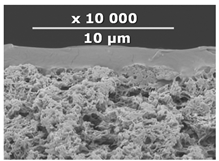 | 1.7 |
| CM-3 | PEI+ PEGDGE | IR-PAN-a | 30 | 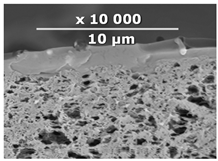 | 1.8 |
| CM-4 | PEI+ PEGDGE | IR-PAN-aM | 10 |  | 1.0 |
| CM-5 | PEI+ PEGDGE | IR-PAN-aM | 20 | 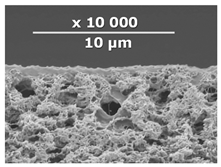 | 0.8 |
| CM-6 | PEI+ PEGDGE | IR-PAN-aM | 30 | 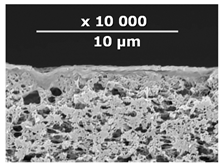 | 1.0 |
| CM-7 | PEI+ PEGDGE | - | - | 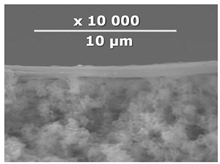 | 1.2 |
| Membrane | P′, % | α′ |
|---|---|---|
| CM-1 | 16.6 | 1.6 |
| CM-2 | 25.9 | 1.3 |
| CM-3 | 22.4 | 1.1 |
| CM-4 | 30.2 | 1.1 |
| CM-5 | 27.2 | 0.9 |
| CM-6 | 27.2 | 0.9 |
| CM-7 | 23.2 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtin, D.; Bazhenov, S.; Polevaya, V.; Grushevenko, E.; Makaev, S.; Karpacheva, G.; Volkov, V.; Volkov, A. Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile. Membranes 2020, 10, 419. https://doi.org/10.3390/membranes10120419
Bakhtin D, Bazhenov S, Polevaya V, Grushevenko E, Makaev S, Karpacheva G, Volkov V, Volkov A. Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile. Membranes. 2020; 10(12):419. https://doi.org/10.3390/membranes10120419
Chicago/Turabian StyleBakhtin, Danila, Stepan Bazhenov, Victoria Polevaya, Evgenia Grushevenko, Sergey Makaev, Galina Karpacheva, Vladimir Volkov, and Alexey Volkov. 2020. "Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile" Membranes 10, no. 12: 419. https://doi.org/10.3390/membranes10120419
APA StyleBakhtin, D., Bazhenov, S., Polevaya, V., Grushevenko, E., Makaev, S., Karpacheva, G., Volkov, V., & Volkov, A. (2020). Aging of Thin-Film Composite Membranes Based on Crosslinked PTMSP/PEI Loaded with Highly Porous Carbon Nanoparticles of Infrared Pyrolyzed Polyacrylonitrile. Membranes, 10(12), 419. https://doi.org/10.3390/membranes10120419









