Transport Analysis of Anti-Wetting Composite Fibrous Membranes for Membrane Distillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospinning
2.2. Membrane Characterization
2.3. Membrane Distillation
3. Results and Discussion
3.1. Morphology of the Membrane
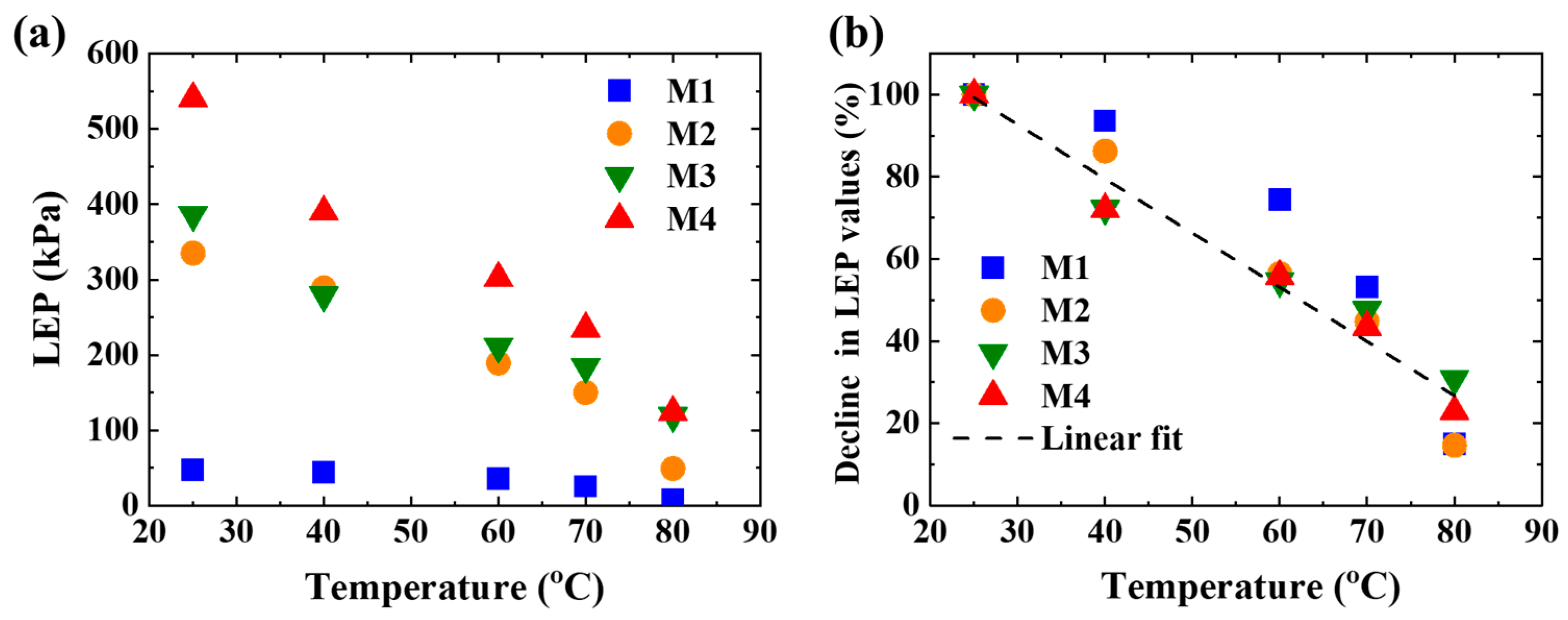
3.2. Wettability of the Membranes
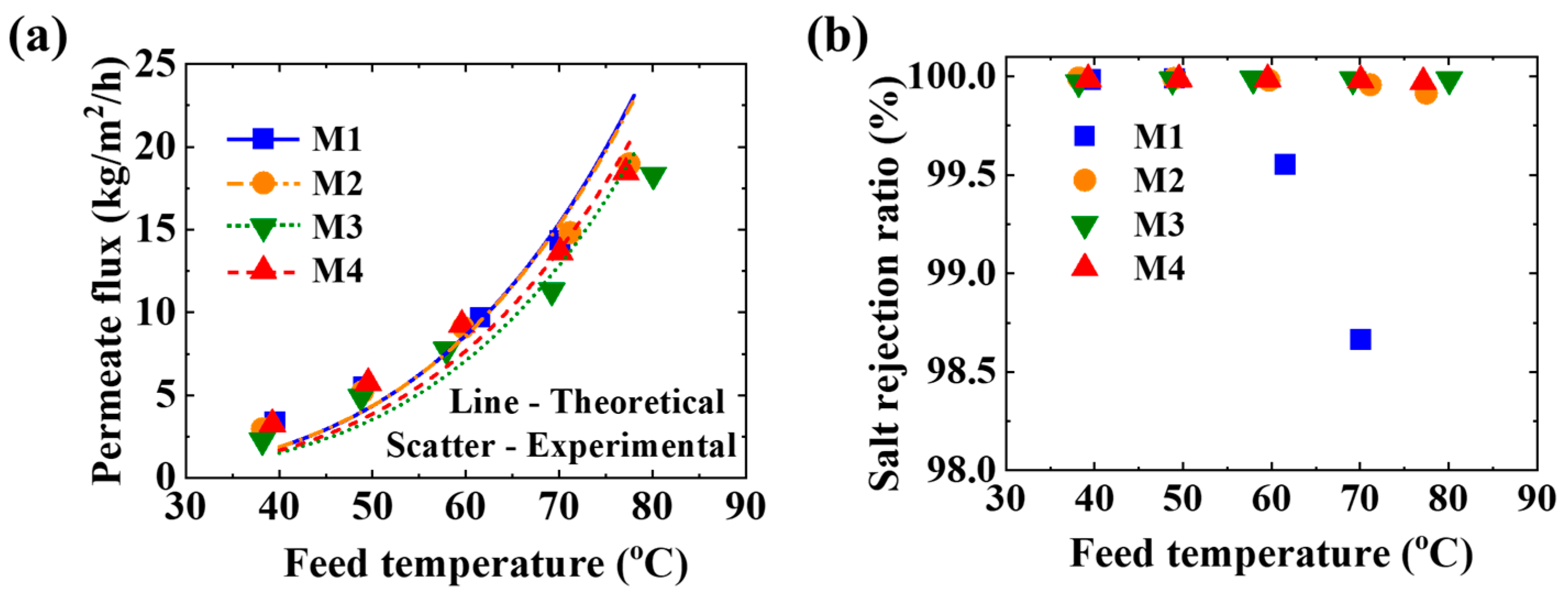
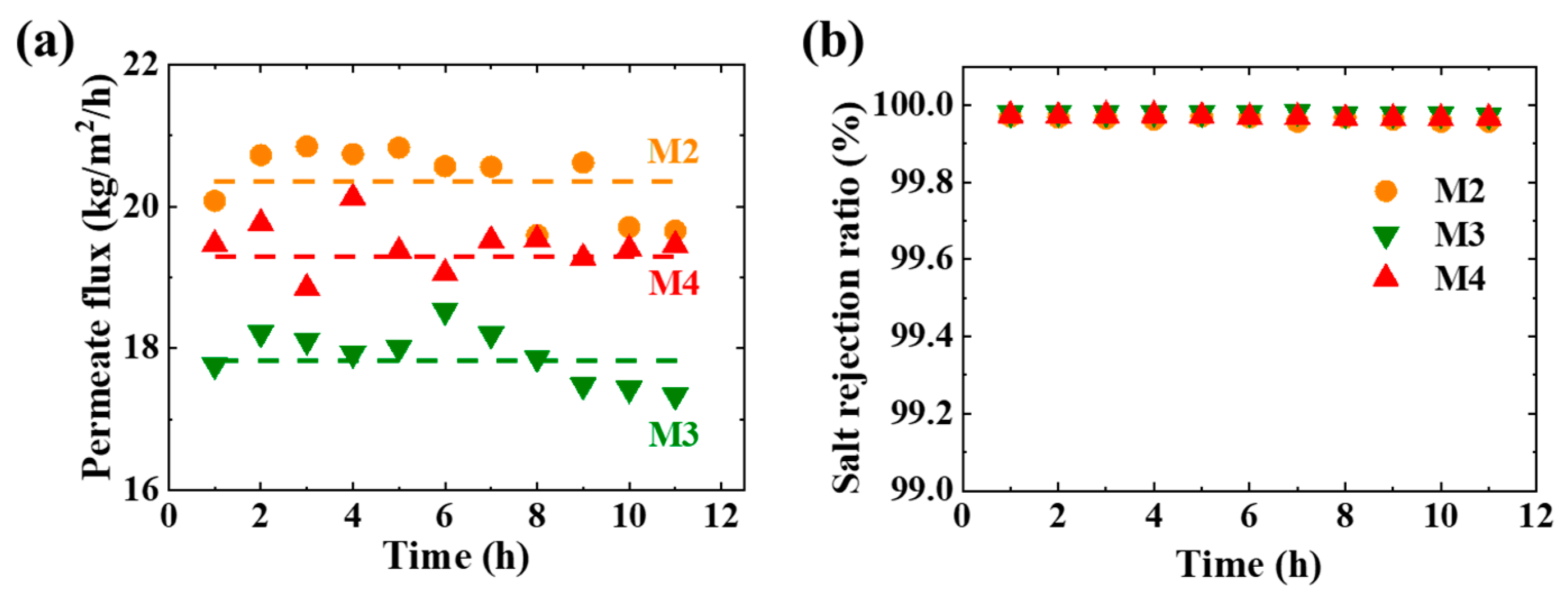
3.3. Membrane Distillation
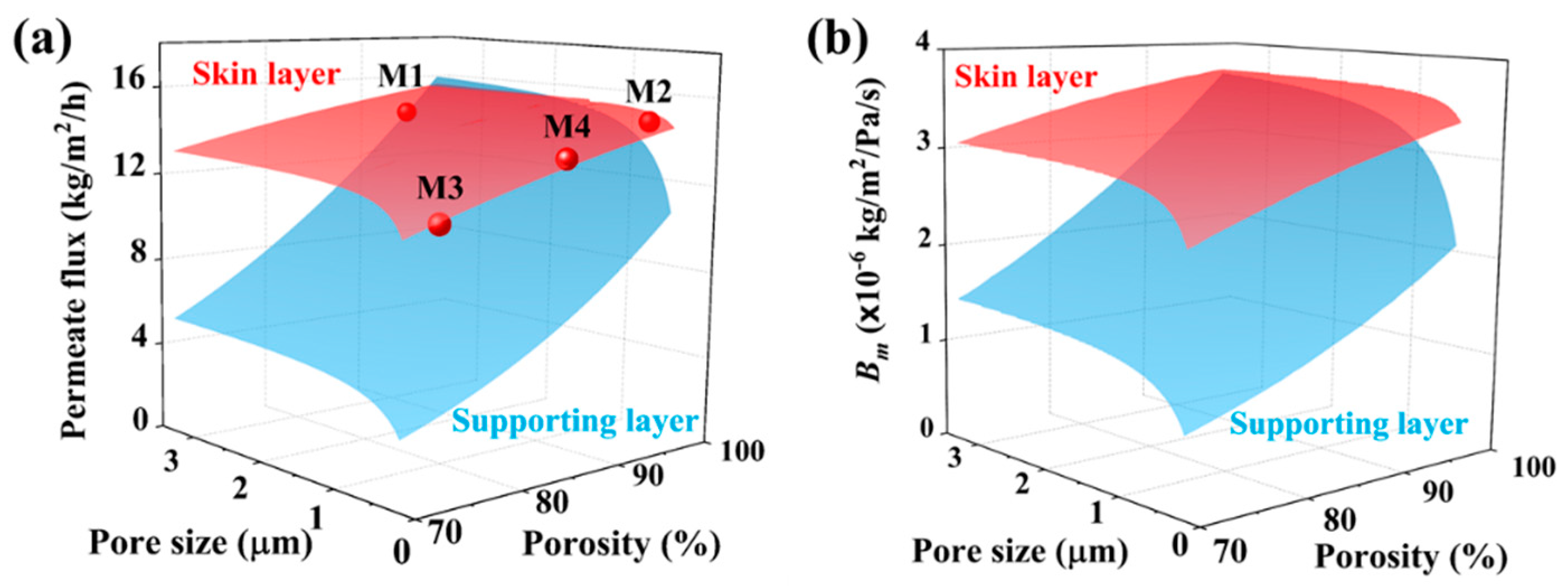
3.4. Permeation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| B | mass transfer coefficient (kg/m2/s/Pa) |
| C | coefficients related to temperature in mass transfer diffusion (kg/m2/s/Pa) |
| d | pore diameter (μm) |
| m | mass (g) |
| N | permeate flux obtained from the test (kg/m2/h) |
| P | pressure (Pa) |
| T | temperature (°C) |
| Greek Letters | |
| δ | thickness (μm) |
| ε | porosity (%) |
| ρ | density (g/cm3) |
| Index | |
| 1 | skin layer |
| 2 | supporting layer |
| a | air |
| c | coolant |
| f | feed |
| fm | interface between the membrane and feed |
| IPA | isopropanol |
| K | Knudsen diffusion |
| m | membrane |
| ma | interface between the membrane and air gap |
| M | Molecular diffusion |
| p | polymer |
| t | total CFM |
| wm | membrane wetted with the isopropanol |
References
- Schofield, R.-W.; Fane, A.G.; Fell, C.-J.-D. Heat and mass transfer in membrane distillation. J. Membr. Sci. 1987, 33, 299–313. [Google Scholar] [CrossRef]
- Kimura, S.; Nakao, S.-I.; Shimatani, S.-I. Transport phenomena in membrane distillation. J. Membr. Sci. 1987, 33, 285–298. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Lawson, K.-W.; Lloyd, D.-R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Jin, J.; Lin, S. Novel Janus membrane for membrane distillation with simultaneous fouling and wetting resistance. Environ. Sci. Technol. 2017, 51, 13304–13310. [Google Scholar] [CrossRef]
- Lalia, B.-S.; Guillen, E.; Arafat, H.-A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Ali, M.I.; Summers, E.-K.; Arafat, H.-A.; Lienhard, V.J.-H. Effects of membrane properties on water production cost in small scale membrane distillation systems. Desalination 2012, 306, 60–71. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. Design and fabrication of lotus-root-like multi-bore hollow fiber membrane for direct contact membrane distillation. J. Membr. Sci. 2012, 421–422, 361–374. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.-C.; Matsuura, T.; Tabe, S.; Ismail, A.-F. Preparation and characterization of electro-spun nanofiber membranes and their possible applications in water treatment. Sep. Purif. Technol. 2013, 102, 118–135. [Google Scholar] [CrossRef]
- Khalifa, A.-E. Performances of air gap and water gap MD desalination modules. Water Pract. Technol. 2018, 13, 200–209. [Google Scholar] [CrossRef]
- He, K.; Hwang, H.-J.; Moon, I.S. Air gap membrane distillation on the different types of membrane. Korean J. Chem. Eng. 2011, 28, 770–777. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Mientka, A. Separation of HCl from HCl-H2SO4 solutions by membrane distillation. Desalination 2009, 240, 244–250. [Google Scholar] [CrossRef]
- Lalia, B.-S.; Guillen-Burrieza, E.; Arafat, H.-A.; Hashaikeh, R. Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 428, 104–115. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.-A.; Guo, J.; An, A.-K. Superhydrophobic (polyvinylidene fluoride-co-hexafluoropropylene)/(polystyrene) composite membrane via a novel hybrid electrospin-electrospray process. J. Membr. Sci. 2020, 611. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Zhao, Y.; Guo, F. Membrane desalination using surface fluorination treated electrospun polyacrylonitrile membranes with nonwoven structure and quasi-parallel fibrous structure. Desalination 2018, 429, 70–75. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.-K.; Rutledge, G.-C. Desalination by membrane distillation using electrospun polyamide fiber membranes with surface fluorination by chemical vapor deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225–8232. [Google Scholar] [CrossRef]
- Adnan, S.; Hoang, M.; Wang, H.; Xie, Z. Commercial PTFE membranes for membrane distillation application: Effect of microstructure and support material. Desalination 2012, 284, 297–308. [Google Scholar] [CrossRef]
- Khayet, M.; Wang, R. Mixed matrix polytetrafluoroethylene/polysulfone electrospun nanofibrous membranes for water desalination by membrane distillation. ACS Appl. Mater. Interfaces 2018, 10, 24275–24287. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Fouladivanda, M.; Karimi-Sabet, J.; Abbasi, F.; Moosavian, M.-A. Step-by-step improvement of mixed-matrix nanofiber membrane with functionalized graphene oxide for desalination via air-gap membrane distillation. Sep. Purif. Technol. 2021, 256, 117809. [Google Scholar] [CrossRef]
- Beauregard, N.; Al-Furaiji, M.; Dias, G.; Worthington, M.; Suresh, A.; Srivastava, R.; Burkey, D.-D.; McCutcheon, J.-R. Enhancing iCVD modification of electrospun membranes for membrane distillation using a 3D printed Scaffold. Polymers 2020, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.-D.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.-K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Air gap membrane distillation: A detailed study of high saline solution. Desalination 2017, 403, 179–186. [Google Scholar] [CrossRef]
- Ahmed, F.-E.; Lalia, B.-S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, M.-C.; García-Fernández, L.; Contreras-Martínez, J. Dual-layered electrospun nanofibrous membranes for membrane distillation. Desalination 2018, 426, 174–184. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.-J.; Wright, C.-J.; Hilal, N. Comparison between dual-layer (superhydrophobic–hydrophobic) and single superhydrophobic layer electrospun membranes for heavy metal recovery by air-gap membrane distillation. Desalination 2018, 439, 31–45. [Google Scholar] [CrossRef]
- Tijing, L.-D.; Woo, Y.-C.; Johir, M.-A.-H.; Choi, J.-S.; Shon, H.-K. A novel dual-layer bicomponent electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Chem. Eng. J. 2014, 256, 155–159. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, J.; Li, N.; Ng, D.; Gray, S.-R.; Xie, Z. Antiwettability and performance stability of a composite hydrophobic/hydrophilic dual-layer membrane in wastewater treatment by membrane distillation. Ind. Eng. Chem. Res. 2018, 57, 9313–9322. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Wang, K.; Wang, J.; Lin, S. Composite membrane with electrospun multiscale-textured surface for robust oil-fouling resistance in membrane distillation. J. Membr. Sci. 2018, 546, 179–187. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, S.; Zhao, H.; Liu, Y. Distillation membrane constructed by TiO2 nanofiber followed by fluorination for excellent water desalination performance. Desalination 2017, 405, 51–58. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Wang, R.; Fane, A.-G. Electrospun superhydrophobic membranes with unique structures for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 16035–16048. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, Z.; Zhong, L.; Song, C.; Shi, W.; Cui, F.; Wang, W. Breathable and asymmetrically superwettable Janus membrane with robust oil-fouling resistance for durable membrane distillation. J. Membr. Sci. 2018, 563, 602–609. [Google Scholar] [CrossRef]
- Humoud, M.-S.; Roy, S.; Mitra, S. Enhanced performance of carbon nanotube immobilized membrane for the treatment of high salinity produced water via direct contact membrane distillation. Membranes 2020, 10, 325. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water desalination using carbon-nanotube-enhanced membrane distillation. ACS Appl. Mater. Interfaces 2011, 3, 110–114. [Google Scholar] [CrossRef]
- Tan, Y.-Z.; Ang, E.-H.; Chew, J.-W. Metallic spacers to enhance membrane distillation. J. Membr. Sci. 2019, 572, 171–183. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.-G. Engineering superhydrophobic surface on poly(vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 440, 77–87. [Google Scholar] [CrossRef]
- An, X.; Xu, G.; Xie, B.; Hu, Y. Structural tailoring of hierarchical fibrous composite membranes to balance mass transfer and heat transfer for state-of-the-art desalination performance in membrane distillation. J. Mater. Chem. A 2019, 7, 2376–2384. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Surface segregation of fluorinated modifying macromolecule for hydrophobic/hydrophilic membrane preparation and application in air gap and direct contact membrane distillation. J. Membr. Sci. 2012, 417–418, 163–173. [Google Scholar] [CrossRef]
- Bonyadi, S.; Chung, T.-S. Flux enhancement in membrane distillation by fabrication of dual layer hydrophilic-hydrophobic hollow fiber membranes. J. Membr. Sci. 2007, 306, 134–146. [Google Scholar] [CrossRef]
- Woo, Y.-C.; Tijing, L.-D.; Park, M.-J.; Yao, M.; Choi, J.-S.; Lee, S.; Kim, S.-H.; An, K.-J.; Shon, H.-K. Electrospun dual-layer nonwoven membrane for desalination by air gap membrane distillation. Desalination 2017, 403, 187–198. [Google Scholar] [CrossRef]
- Chew, N.-G.-P.; Zhang, Y.; Goh, K.; Ho, J.-S.; Xu, R.; Wang, R. Hierarchically structured Janus membrane surfaces for enhanced membrane distillation performance. ACS Appl. Mater. Interfaces 2019, 11, 25524–25534. [Google Scholar] [CrossRef] [PubMed]
- Shaulsky, E.; Nejati, S.; Boo, C.; Perreault, F.; Osuji, C.-O.; Elimelech, M. Post-fabrication modification of electrospun nanofiber mats with polymer coating for membrane distillation applications. J. Membr. Sci. 2017, 530, 158–165. [Google Scholar] [CrossRef]
- Cai, J.; Guo, F. Study of mass transfer coefficient in membrane desalination. Desalination 2017, 407, 46–51. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Cong, S.; Guo, F. Janus nanofibrous membranes for desalination by air gap membrane distillation. ACS Appl. Polym. Mater. 2019, 1, 3443–3451. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon: Exposition et Application; Victor Dalmont: Paris, France, 1856. [Google Scholar]
- Bindels, M.; Brand, N.; Nelemans, B. Modeling of semibatch air gap membrane distillation. Desalination 2018, 430, 98–106. [Google Scholar] [CrossRef]
- Cong, S.; Liu, X.; Guo, F. Membrane distillation using surface modified multi-layer porous ceramics. Int. J. Heat Mass Transf. 2019, 129, 764–772. [Google Scholar] [CrossRef]
- Knudsen, M. Die Gesetze der molekularströmung und der inneren reibungsströmung der gase durch röhren. Ann. Phys. 1909, 333, 75–130. [Google Scholar] [CrossRef]
- Fick, A. Ueber Diffusion. Ann. Phys. 1855, 170, 59–86. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Koll, J.; Lilleodden, E.-T.; Elbahri, M. The solvent induced interfiber adhesion and its influence on the mechanical and filtration properties of polyethersulfone electrospun nanofibrous microfiltration membranes. Sep. Purif. Technol. 2012, 98, 456–463. [Google Scholar] [CrossRef]
- Soliman, S.; Sant, S.; Nichol, J.-W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. Part A 2011, 96 A, 566–574. [Google Scholar] [CrossRef]
- Cassie, A.-B.-D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Park, I.-J.; Lee, S.-B.; Choi, C.-K.; Kim, K.-J. Surface properties and structure of poly(perfluoroalkylethyl methacrylate). J. Colloid Interface Sci. 1996, 181, 284–288. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.-K.; Rutledge, G.-C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Li, K.; Hou, D.; Fu, C.; Wang, K.; Wang, J. Fabrication of PVDF nanofibrous hydrophobic composite membranes reinforced with fabric substrates via electrospinning for membrane distillation desalination. J. Environ. Sci. 2019, 75, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nano-fibers: Effects of polymer concentration and desalination by direct contact membrane distillation. J. Membr. Sci. 2014, 454, 133–143. [Google Scholar] [CrossRef]
- Bagherzadeh, R.; Najar, S.-S.; Latifi, M.; Tehran, M.-A.; Kong, L. A theoretical analysis and prediction of pore size and pore size distribution in electrospun multilayer nanofibrous materials. J. Biomed. Mater. Res. Part A 2013, 101 A, 2107–2117. [Google Scholar] [CrossRef]
- Hottel, H.-C.; Noble, J.-J.; Sarofim, A.-F.; Silcox, G.-D.; Wankat, P.-C.; Knaebel, K.-S. Perry’s Chemical Engineers’ Handbook: Heat and Mass Transfer; McGraw-Hill: New York, NY, USA, 2008; ISBN 0071542124. [Google Scholar]
- Cai, J.; Yin, H.; Guo, F. Transport analysis of material gap membrane distillation desalination processes. Desalination 2020, 481, 114361. [Google Scholar] [CrossRef]
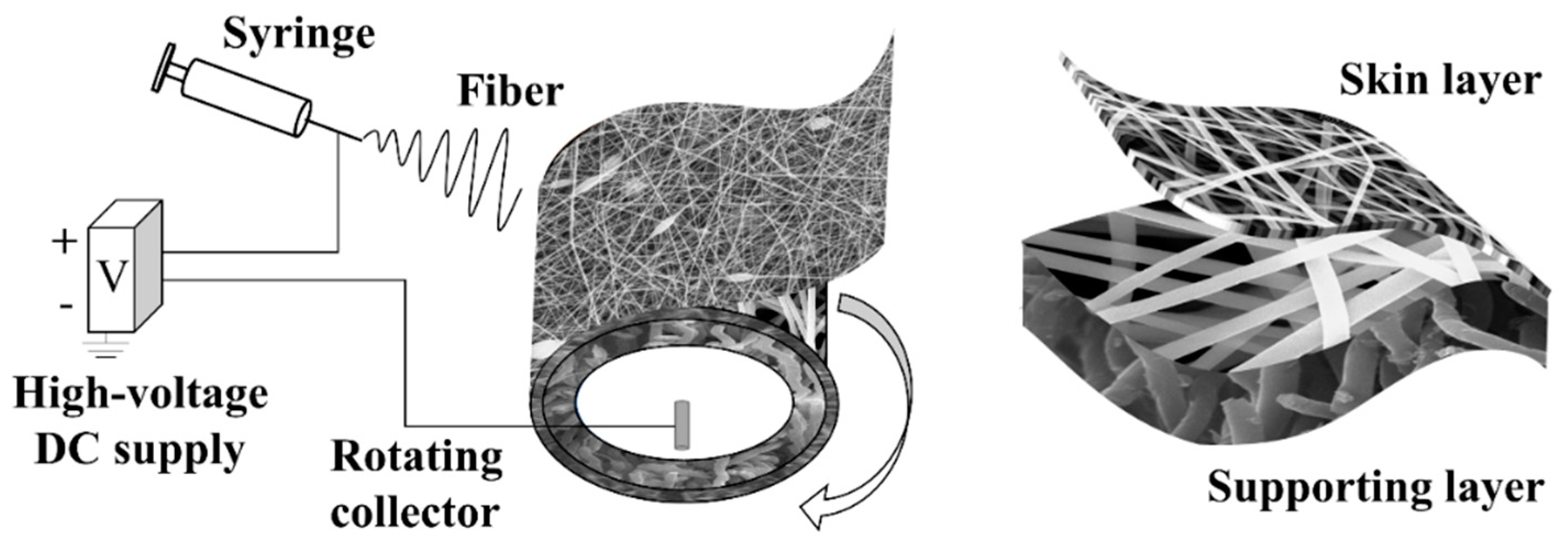

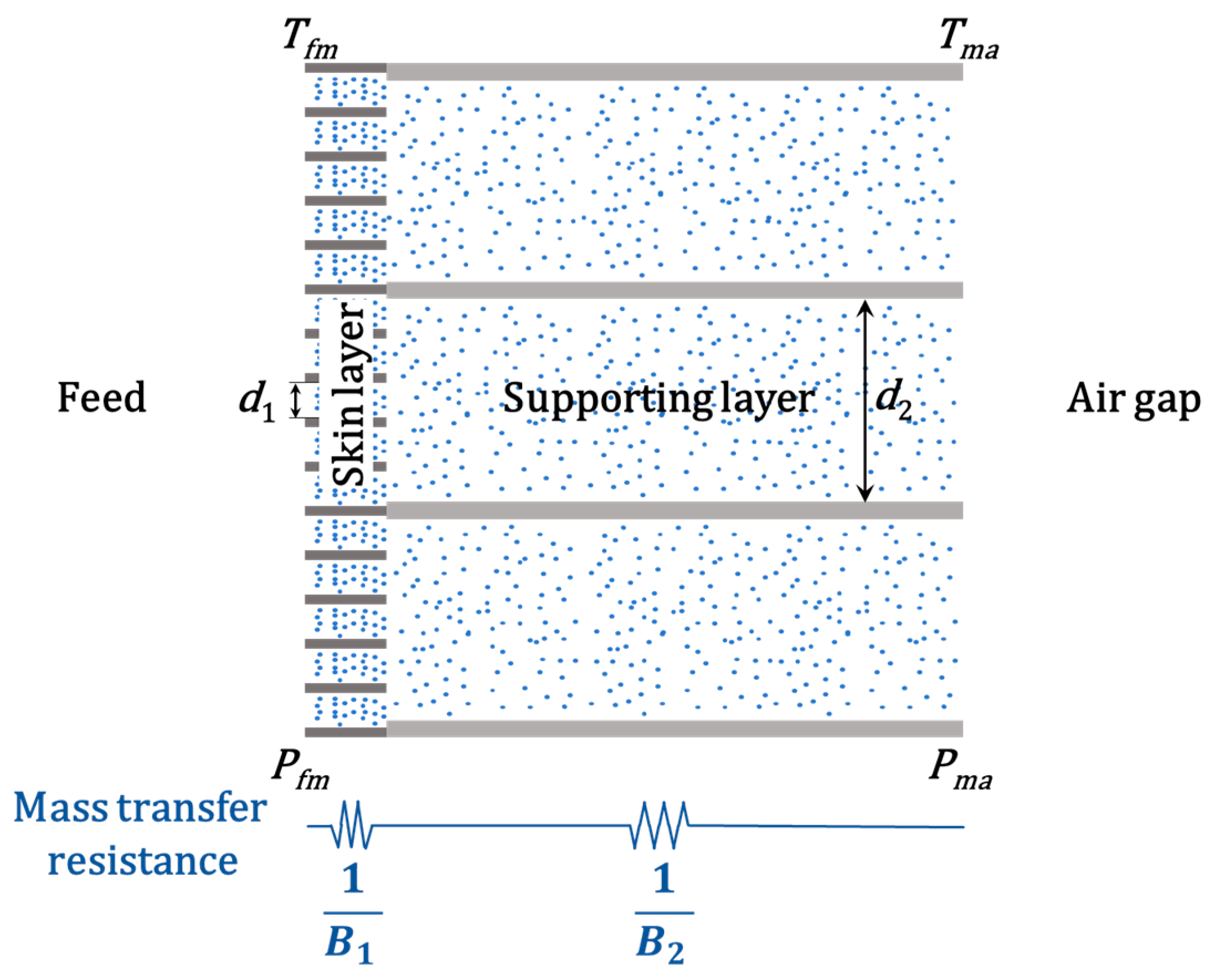
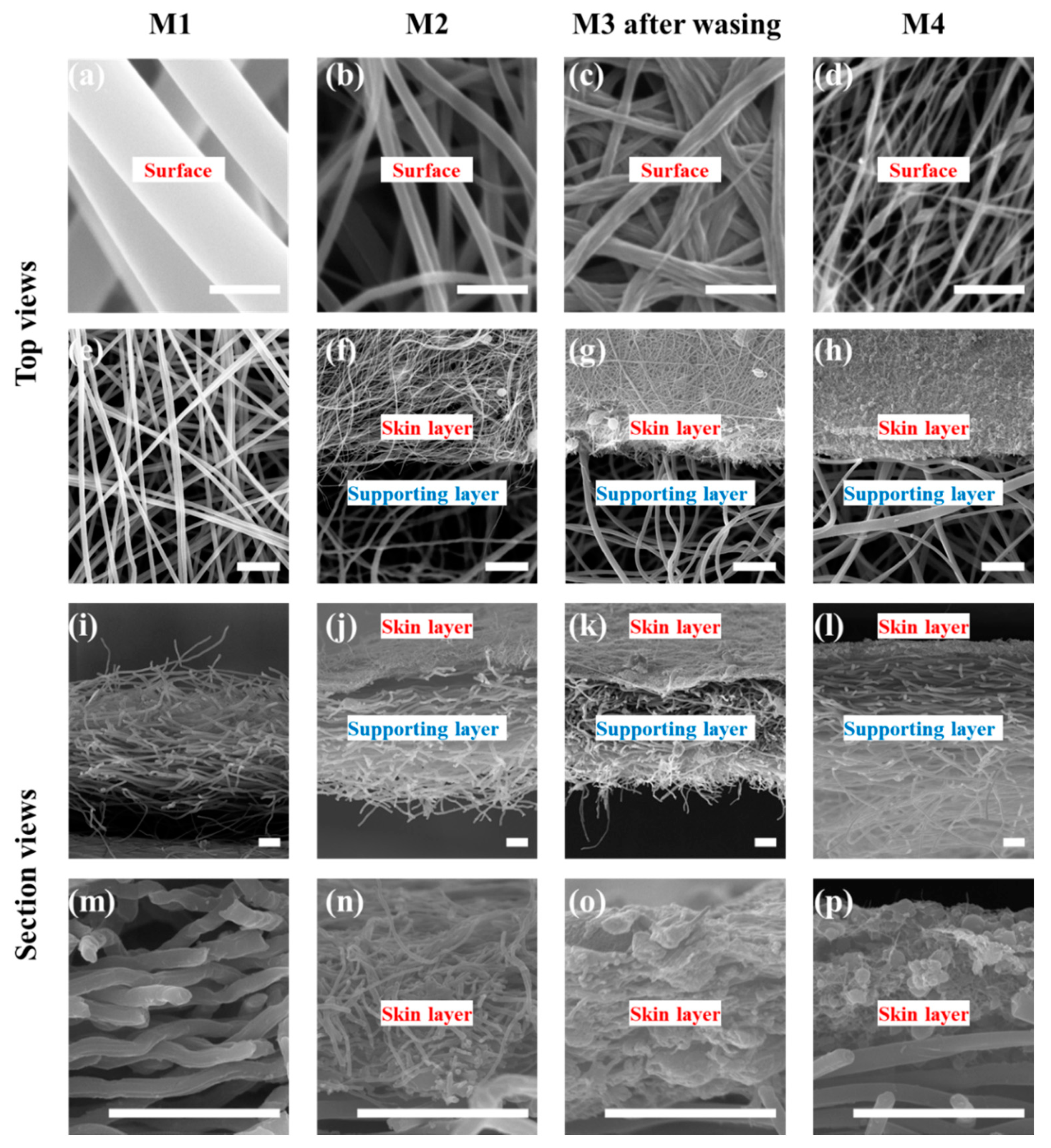
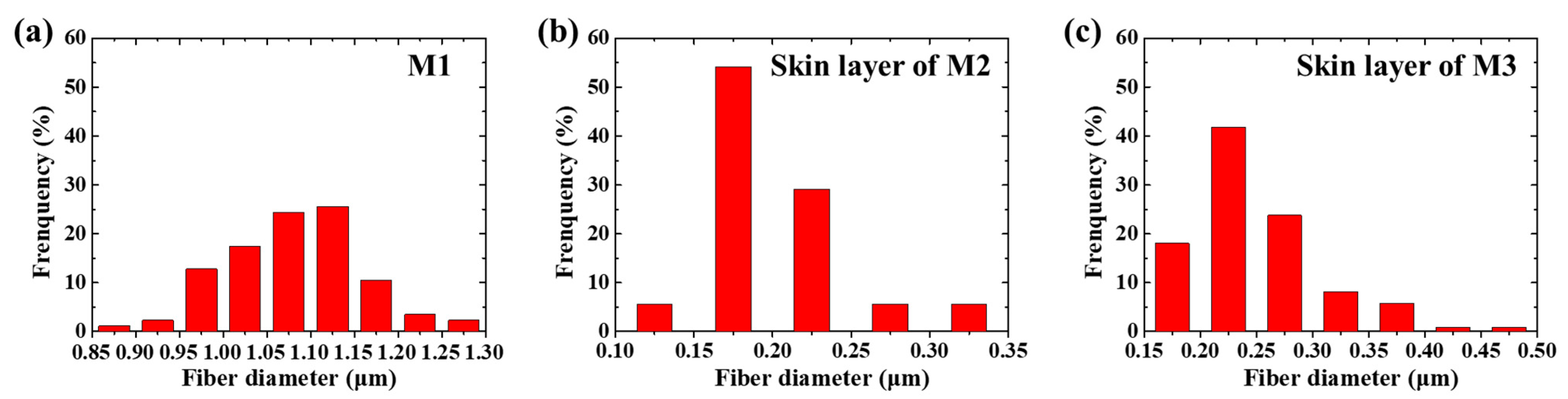

| Materials | Flow Rate (mL/min) | Voltage (kV) | Rotating Speed (rpm) | Fiber Diameter (μm) |
|---|---|---|---|---|
| PAN (19 wt.%) | 0.02 | +16/−6 | 40 | 1.1 ± 0.1 |
| PAN (11.5 wt.%) | 0.02 | +16/−6 | 40 | 0.2 ± 0.05 |
| PAN/PVP (5.5 wt.%/6 wt.%) | 0.02 | +16/−6 | 40 | 0.25 ± 0.05 |
| PAN (7 wt.%) | 0.02 | +12/−6 | 40 | 0.05 ± 0.02/0.6 ± 0.4 * |
| Code of Sample Membranes | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| Supporting layer | PAN (19 wt.%) | PAN (19 wt.%) | ||
| Skin layer | PAN (11.5 wt.%) | PAN/PVP (5.5 wt.%/6 wt.%) | PAN (7 wt.%) | |
| δ1 (μm) | ~7 | ~7 | ~9 | ~7 |
| δt (μm) | 60 ± 2 | 64 ± 3 | 58 ± 5 | 62 ± 4 |
| Contact angle (o) | 151 ± 3 | 153 ± 3 | 155 ± 5 | 160 ± 3 |
| LEP (kPa) (25 °C) | 47 | 335 | 386 | 541 |
| εt (%) | 93 ± 1 | 94 ± 1 | 90 ± 1 | 92 ± 1 |
| ε1 (%) | ~93 | ~97 | ~75 | ~86 |
| d1 (μm) | 3.22 | 0.49 | 0.42 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Liu, Z.; Guo, F. Transport Analysis of Anti-Wetting Composite Fibrous Membranes for Membrane Distillation. Membranes 2021, 11, 14. https://doi.org/10.3390/membranes11010014
Cai J, Liu Z, Guo F. Transport Analysis of Anti-Wetting Composite Fibrous Membranes for Membrane Distillation. Membranes. 2021; 11(1):14. https://doi.org/10.3390/membranes11010014
Chicago/Turabian StyleCai, Jingcheng, Zeman Liu, and Fei Guo. 2021. "Transport Analysis of Anti-Wetting Composite Fibrous Membranes for Membrane Distillation" Membranes 11, no. 1: 14. https://doi.org/10.3390/membranes11010014
APA StyleCai, J., Liu, Z., & Guo, F. (2021). Transport Analysis of Anti-Wetting Composite Fibrous Membranes for Membrane Distillation. Membranes, 11(1), 14. https://doi.org/10.3390/membranes11010014






