The Long-Term Immunogenicity of mRNABNT162b Third Vaccine Dose in Solid Organ Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
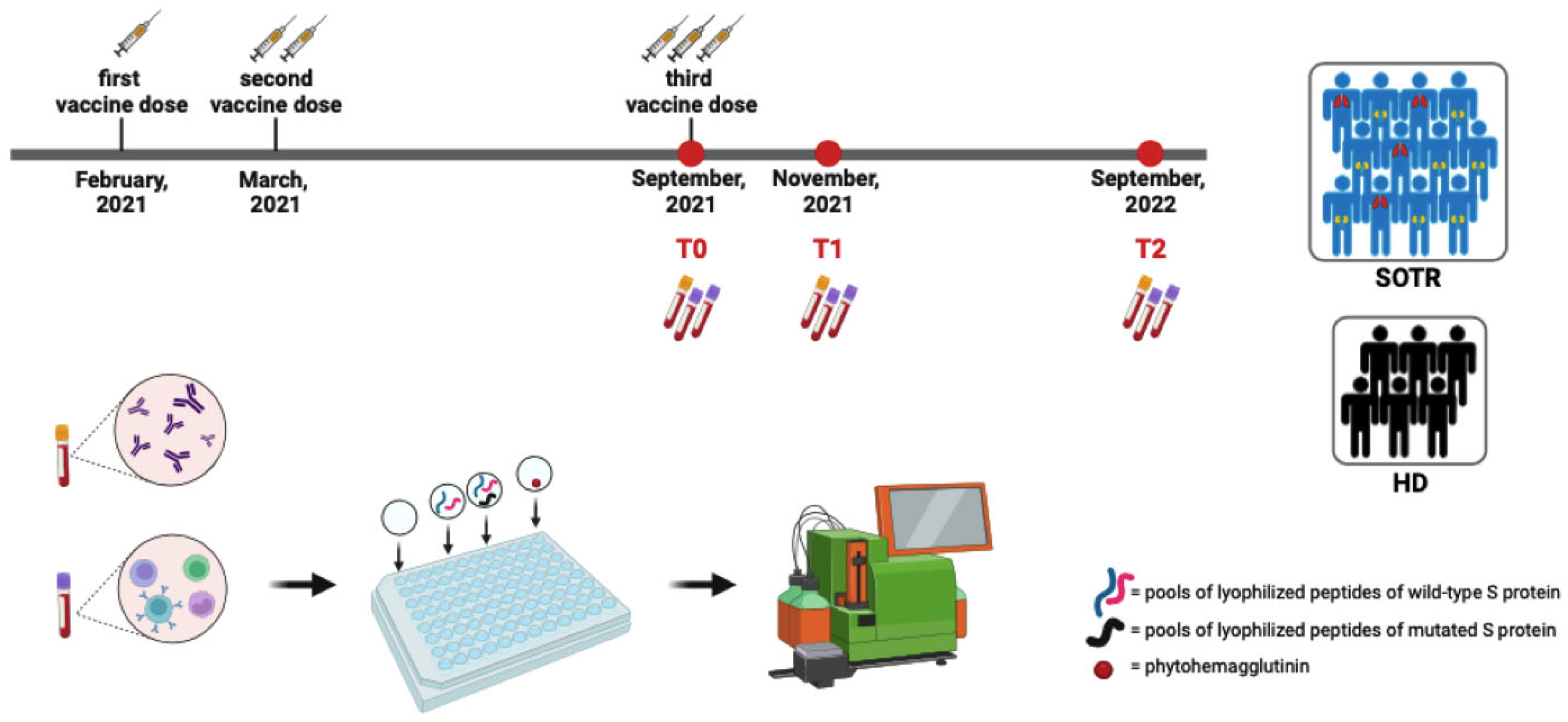
2.1. Study Population
2.2. Serological and Specific T-Cell Assessment
2.3. Statistical Analysis
3. Results
3.1. Study Population and Sample Collection
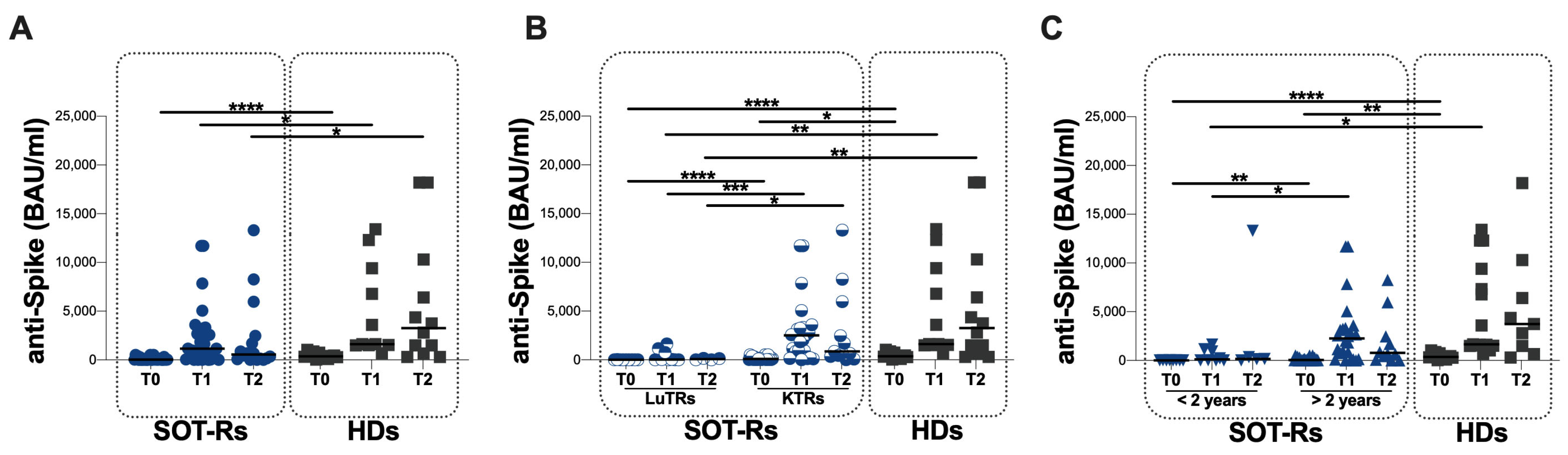
3.2. Specific Humoral Response in the Study Population
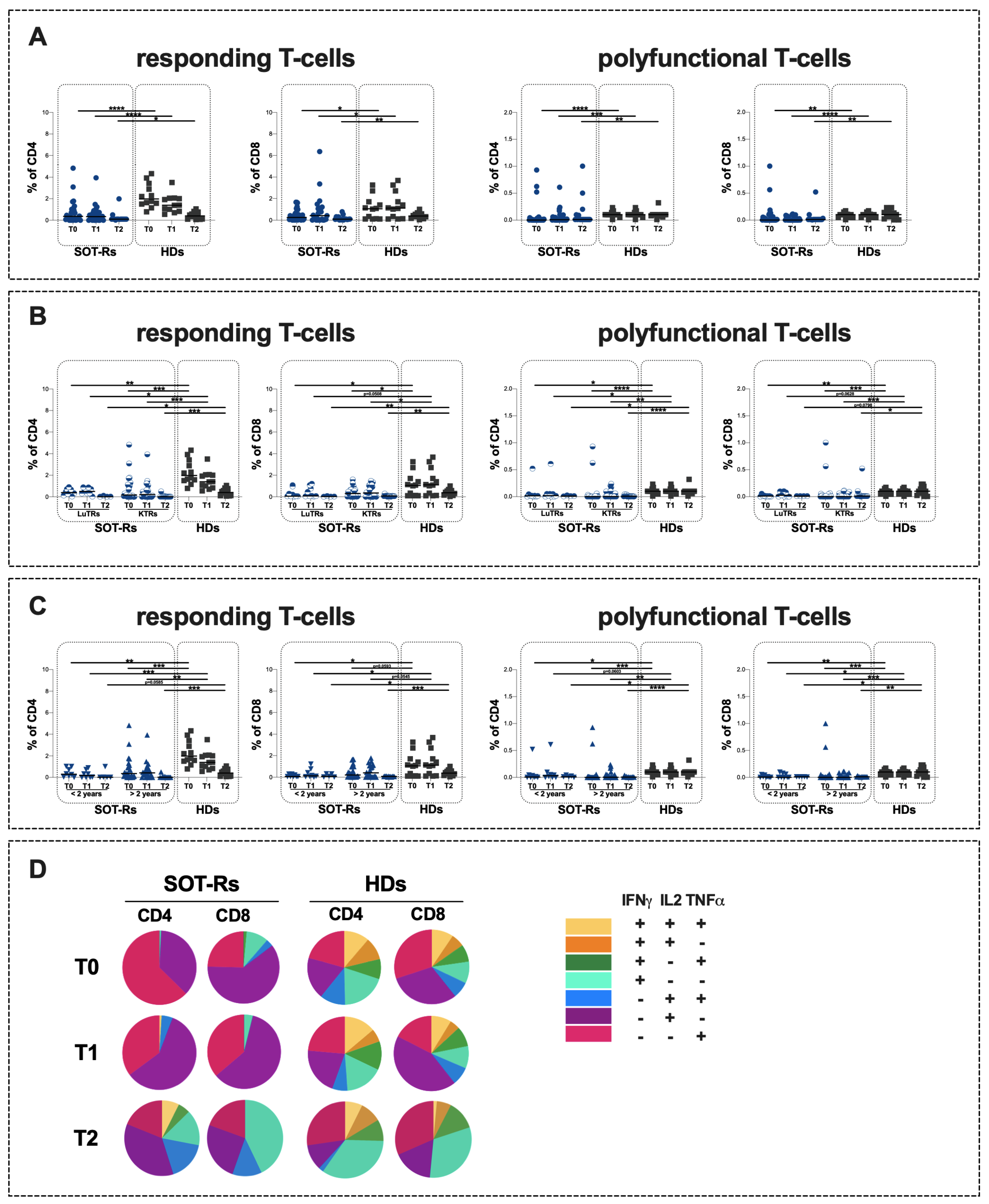
3.3. Specific T-Cell Response in Study Population
3.4. Evaluation of S-Specific Omicron B.1.1.529/BA.5 T-Cell Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of mRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; de Silva, T., I; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-Specific Immune Responses and Clinical Outcomes after COVID-19 Vaccination in Patients with Immune-Suppressive Disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, J.L.; Abdala, E.; Hoff, P.M.; Luiz, A.M.; Oliveira, D.S.; Saad, C.G.S.; Laurindo, I.M.M.; Viso, A.T.R.; Tayra, A.; Pierrotti, L.C.; et al. Immunogenicity and Reactogenicity of 2009 Influenza A (H1N1) Inactivated Monovalent Non-Adjuvanted Vaccine in Elderly and Immunocompromised Patients. PLoS ONE 2011, 6, e27214. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Guardiani, M.; Zingaropoli, M.A.; Cogliati Dezza, F.; Centofanti, A.; Carillo, C.; Tortellini, E.; Dominelli, F.; Napoli, A.; Del Borgo, C.; Gaeta, A.; et al. Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients. Vaccines 2022, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Tortellini, E.; Zingaropoli, M.A.; Mancarella, G.; Marocco, R.; Carraro, A.; Jamhour, M.; Barbato, C.; Guardiani, M.; Dominelli, F.; Pasculli, P.; et al. Quality of T-Cell Response to SARS-CoV-2 mRNA Vaccine in ART-Treated PLWH. Int. J. Mol. Sci. 2022, 23, 14988. [Google Scholar] [CrossRef]
- Dominelli, F.; Zingaropoli, M.A.; Tartaglia, M.; Tortellini, E.; Guardiani, M.; Perri, V.; Pasculli, P.; Ciccone, F.; Malimpensa, L.; Baione, V.; et al. Multiple Sclerosis-Disease Modifying Therapies Affect Humoral and T-Cell Response to mRNA COVID-19 Vaccine. Front. Immunol. 2022, 13, 1050183. [Google Scholar] [CrossRef]
- López-Medrano, F.; Aguado, J.M.; Lizasoain, M.; Folgueira, D.; Juan, R.S.; Díaz-Pedroche, C.; Lumbreras, C.; Morales, J.M.; Delgado, J.F.; Moreno-González, E. Clinical Implications of Respiratory Virus Infections in Solid Organ Transplant Recipients: A Prospective Study. Transplantation 2007, 84, 851–856. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D. The AST ID Community of Practice Vaccination of Solid Organ Transplant Candidates and Recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Martínez-Urbistondo, M.; Calderón-Parra, J.; Mills, P.; Muñoz-Serrano, A.; Arias-Milla, A.; Benítez, L.; Aguilar-Pérez, M.; Múñez-Rubio, E.; Ramos-Martínez, A.; et al. COVID-19 in Hospitalized Solid Organ Transplant Recipients in a Nationwide Registry Study. Int. J. Infect. Dis. 2023, 134, 154–159. [Google Scholar] [CrossRef]
- Chong, P.P.; Avery, R.K. A Comprehensive Review of Immunization Practices in Solid Organ Transplant and Hematopoietic Stem Cell Transplant Recipients. Clin. Ther. 2017, 39, 1581–1598. [Google Scholar] [CrossRef]
- Kumar, D.; Unger, E.R.; Panicker, G.; Medvedev, P.; Wilson, L.; Humar, A. Immunogenicity of Quadrivalent Human Papillomavirus Vaccine in Organ Transplant Recipients. Am. J. Transplant. 2013, 13, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Walti, L.N.; Mugglin, C.; Mombelli, M.; Manuel, O.; Hirsch, H.H.; Khanna, N.; Mueller, N.J.; Berger, C.; Boggian, K.; Garzoni, C.; et al. Vaccine-Preventable Infections Among Solid Organ Transplant Recipients in Switzerland. JAMA Netw. Open 2023, 6, e2310687. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.-L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired Humoral Immunity to SARS-CoV-2 BNT162b2 Vaccine in Kidney Transplant Recipients and Dialysis Patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Ou, M.T.; Greenberg, R.S.; Teles, A.T.; Werbel, W.A.; Avery, R.K.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Safety of the First Dose of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation 2021, 105, e56–e57. [Google Scholar] [CrossRef] [PubMed]
- Natori, Y.; Shiotsuka, M.; Slomovic, J.; Hoschler, K.; Ferreira, V.; Ashton, P.; Rotstein, C.; Lilly, L.; Schiff, J.; Singer, L.; et al. A Double-Blind, Randomized Trial of High-Dose vs Standard-Dose Influenza Vaccine in Adult Solid-Organ Transplant Recipients. Clin. Infect. Dis. 2018, 66, 1698–1704. [Google Scholar] [CrossRef]
- Han, A.; Min, S.; Jo, E.-A.; Lee, H.; Kim, Y.C.; Han, S.S.; Kang, H.G.; Ahn, Y.H.; Oh, I.; Song, E.Y.; et al. Association Between Low Anti-Spike Antibody Levels After the Third Dose of SARS-CoV-2 Vaccination and Hospitalization Due to Symptomatic Breakthrough Infection in Kidney Transplant Recipients. Ann. Lab. Med. 2024, 44, 64–73. [Google Scholar] [CrossRef]
- Saharia, K.K.; Anjan, S.; Streit, J.; Beekmann, S.E.; Polgreen, P.M.; Kuehnert, M.; Segev, D.L.; Baddley, J.W.; Miller, R.A. EIN COVID-19 Study Team Clinical Characteristics of COVID-19 in Solid Organ Transplant Recipients Following COVID-19 Vaccination: A Multicenter Case Series. Transpl. Infect. Dis. 2022, 24, e13774. [Google Scholar] [CrossRef]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef]
- Andre, M.; Lau, L.-S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Akkaoui, J.; Ales, E.; Brown, H.; Shil, R.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef]
- Del Mastro, A.; Picascia, S.; D’Apice, L.; Trovato, M.; Barba, P.; Di Biase, I.; Di Biase, S.; Laccetti, M.; Belli, A.; Amato, G.; et al. Booster Dose of SARS-CoV-2 mRNA Vaccine in Kidney Transplanted Patients Induces Wuhan-Hu-1 Specific Neutralizing Antibodies and T Cell Activation but Lower Response against Omicron Variant. Viruses 2023, 15, 1132. [Google Scholar] [CrossRef] [PubMed]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 Variants of Concern Partially Escape Humoral but Not T-Cell Responses in COVID-19 Convalescent Donors and Vaccinees. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, D.; Mei, B.; Du, J.; Liu, X.; Xie, H.; Liu, L.; Su, S.; Mai, G. Immunogenicity of COVID-19 Vaccines in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2023, 29, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Governo Italiano. Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 20 April 2022).
- Zingaropoli, M.A.; Iannetta, M.; Pontecorvo, S.; Anzivino, E.; Prezioso, C.; Rodio, D.M.; Morreale, M.; D’Abramo, A.; Oliva, A.; Lichtner, M.; et al. JC Virus-DNA Detection Is Associated with CD8 Effector Accumulation in Peripheral Blood of Patients with Multiple Sclerosis under Natalizumab Treatment, Independently from JC Virus Serostatus. Biomed. Res. Int. 2018, 2018, 5297980. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Receiving Disease Modifying Therapies: Immunological Patterns and Clinical Implications. Front. Immunol. 2021, 12, 796482. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low Immunogenicity to SARS-CoV-2 Vaccination among Liver Transplant Recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Ierullo, M.; Mavandadnejad, F.; Kurtesi, A.; Hu, Q.; Hardy, W.R.; Hall, V.G.; Pinzon, N.; Yotis, D.; Gingras, A.-C.; et al. Omicron BA.4/5 Neutralization and T-Cell Responses in Organ Transplant Recipients After Booster Messenger RNA Vaccine: A Multicenter Cohort Study. Clin. Infect. Dis. 2023, 77, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Willauer, A.N.; Rouster, S.D.; Meeds, H.L.; Jennings, C.L.; Abdel-Hameed, E.A.; Daria, D.E.; Stambrook, E.P.; Shata, M.T.M.; Sherman, K.E. Humoral and T-Cell–Mediated Immunity to SARS-CoV-2 Vaccination in Patients with Liver Disease and Transplant Recipients. Hepatol. Commun. 2023, 7, e0100. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Newton, J.N.; Diamond, I.; Studley, R.; Taylor, N.; Bell, J.I.; Farrar, J.; Kolenchery, J.; et al. Protection against SARS-CoV-2 Omicron BA.4/5 Variant Following Booster Vaccination or Breakthrough Infection in the UK. Nat. Commun. 2023, 14, 2799. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Beretta, M.; Lepore, M.; Abete, R.; Benatti, S.V.; Grassini, M.V.; Camagni, S.; Chiodini, G.; Vargiu, S.; Vittori, C.; et al. Vaccination Recommendations in Solid Organ Transplant Adult Candidates and Recipients. Vaccines 2023, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Messika, J.; Eloy, P.; Roux, A.; Hirschi, S.; Nieves, A.; Le Pavec, J.; Sénéchal, A.; Saint Raymond, C.; Carlier, N.; Demant, X.; et al. COVID-19 in Lung Transplant Recipients. Transplantation 2021, 105, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Vafea, M.T.; Haidar, G. COVID-19 Prevention in Solid Organ Transplant Recipients. Infect. Dis. Clin. N. Am. 2023, 37, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Castilletti, C.; Goletti, D.; Meschi, S.; Sacchi, A.; Matusali, G.; Bordoni, V.; Petrone, L.; Lapa, D.; Notari, S.; et al. Coordinate Induction of Humoral and Spike Specific T-Cell Response in a Cohort of Italian Health Care Workers Receiving BNT162b2 mRNA Vaccine. Microorganisms 2021, 9, 1315. [Google Scholar] [CrossRef]
- Lin, L.; Finak, G.; Ushey, K.; Seshadri, C.; Hawn, T.R.; Frahm, N.; Scriba, T.J.; Mahomed, H.; Hanekom, W.; Bart, P.-A.; et al. COMPASS Identifies T-Cell Subsets Correlated with Clinical Outcomes. Nat. Biotechnol. 2015, 33, 610–616. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Hamm, S.R.; Heftdal, L.D.; Pérez-Alós, L.; Møller, D.L.; Perch, M.; Madsen, J.R.; Hald, A.; Hansen, C.B.; Armenteros, J.J.A.; et al. Humoral and T-Cell Response 12 Months after the First BNT162b2 Vaccination in Solid Organ Transplant Recipients and Controls: Kinetics, Associated Factors, and Role of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 1075423. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Jeong, S.D.; Noh, J.Y.; Kim, D.-U.; Jung, S.; Song, J.Y.; Jeong, H.W.; Park, S.-H.; Shin, E.-C. BNT162b2-Induced Memory T Cells Respond to the Omicron Variant with Preserved Polyfunctionality. Nat. Microbiol. 2022, 7, 909–917. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Van Elslande, J.; Gruwier, L.; Godderis, L.; Vermeersch, P. Estimated Half-Life of SARS-CoV-2 Anti-Spike Antibodies More Than Double the Half-Life of Anti-Nucleocapsid Antibodies in Healthcare Workers. Clin. Infect. Dis. 2021, 73, 2366–2368. [Google Scholar] [CrossRef] [PubMed]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Vande Weygaerde, Y.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal Follow-up of IgG Anti-Nucleocapsid Antibodies in SARS-CoV-2 Infected Patients up to Eight Months after Infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- Gupta, R.K.; Topol, E.J. COVID-19 Vaccine Breakthrough Infections. Science 2021, 374, 1561–1562. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Kwan, A.T.; Rodríguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 Breakthrough Infections and Reinfections during the Omicron Wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Yu, T.; Yin, A.; Pisanic, N.; Demko, Z.O.; Antar, A.A.R.; Cox, A.L.; Heaney, C.D.; Manabe, Y.C.; Klein, S.L. Reconsideration of Antinucleocapsid IgG Antibody as a Marker of SARS-CoV-2 Infection Postvaccination for Mild COVID-19 Patients. Open Forum Infect. Dis. 2023, 10, ofac677. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.; Gigan, M.; Vermorel, A.; Charrier, M.; Guirle, L.; Jambon, F.; Lacapère, A.; Ménard, C.; Moreau, K.; Neau-Cransac, M.; et al. COVID-19 Morbidity Decreases with Tixagevimab-Cilgavimab Preexposure Prophylaxis in Kidney Transplant Recipient Nonresponders or Low-Vaccine Responders. Kidney Int. 2022, 102, 936–938. [Google Scholar] [CrossRef]
- Gottlieb, J.; Simon, S.; Barton, J.; Barnikel, M.; Bachmann, M.; Klingenberg, M.-S.; Veit, T.; Kneidinger, N. Efficacy of Pre-Exposure Prophylaxis to Prevent SARS-CoV-2 Infection after Lung Transplantation: A Two Center Cohort Study during the Omicron Era. Infection 2023, 51, 1481–1489. [Google Scholar] [CrossRef]
- Jordan, S.C.; Joung, S.Y.; Wang, M.; Tran, T.A.; Bravo, M.; Masoom, H.; Chang, C.; Mendez, M.; Sun, N.; Patel, J.; et al. Assessing the Post Hoc Effectiveness of Tixagevimab-Cilgavimab for Prevention of SARS-CoV-2 Infections in Solid Organ Transplant Recipients. Transpl. Infect. Dis. 2023, 26, e14182. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Drenko, P.; Kacer, M.; Kielberger, L.; Vlas, T.; Topolcan, O.; Kucera, R.; Reischig, T. Safety and Efficacy of One and Two Booster Doses of SARS-CoV-2 mRNA Vaccines in Kidney Transplant Recipients: A Randomized Clinical Trial. Transpl. Infect. Dis. 2023, 25, e14150. [Google Scholar] [CrossRef]

| SOT-Rs (n = 32) | LuT-Rs (n = 9) | KT-Rs (n = 23) | HDs (n = 12) | |
|---|---|---|---|---|
| Age, years | 56 (48–61) | 56 (46–62) | 55 (49–61) | 50 (47–58) |
| Male/female | 22/10 | 7/2 | 15/8 | 7/5 |
| Time elapsed since transplant, months | 76 (42–162) | 20 (13–96) | 76 (60–181) | |
| Immunosuppressive treatment | ||||
| Steroids | 26 | 9 | 17 | |
| Antimetabolites | 22 | 6 | 16 | |
| Calcineurin inhibitors | 32 | 9 | 23 | |
| mTOR inhibitors | 1 | 0 | 1 | |
| Laboratory data | ||||
| WBC (×109/L) | 7.2 (6.1–9.4) | 8.2 (5.4–10.0) | 7.2 (6.2–9.5) | |
| Neutrophils (×109/L) | 4.7 (3.4–6.4) | 5.1 (3.7–6.7) | 4.5 (3.4–6.4) | |
| Lymphocytes (×109/L) | 2.2 (1.4–2.4) | 1.4 (1.3–2.6) | 2.2 (1.7–2.5) | |
| PLT (×109/L) | 212 (156–244) | 243 (145–265) | 210 (159–240) | |
| Creatinine (mg/dL) | 1.6 (1.3–2.0) | 1.3 (1.3–2.1) | 1.7 (1.3–1.9) | |
| Azotemia (mg/dL) | 55 (40–79) | 54 (8–85) | 58 (42–78) | |
| Comorbidities | ||||
| Diabetes | 7 | 4 | 3 | |
| Arterial hypertension | 26 | 5 | 21 | |
| Dyslipidemia | 15 | 1 | 14 | |
| Cardiopathy | 4 | 2 | 2 |
| SOT-Rs (n = 32) | HDs (n = 12) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |
| anti-S antibody titers (BAU/mL) | 31 (23–149) | 1150 (101–3050) | 562 (80–2270) | 369 (189–701) | 1610 (1520–9400) | 3270 (869–9328) |
| responding T-cells | ||||||
| % of CD4+ | 0.36 (0.09–1.01) | 0.34 (0.00–0.72) | 0.10 (0.10–0.10) | 1.98 (1.52–3.29) | 1.39 (0.88–2.04) | 0.41 (0.11–0.72) |
| % of CD8+ | 0.25 (0.05–0.64) | 0.41 (0.04–1.10) | 0.09 (0.06–0.20) | 1.06 (0.11–1.63) | 1.12 (0.18–2.46) | 0.38 (0.20–0.57) |
| polyfunctional T-cells | ||||||
| % of CD4+ | 0.00 (0.00–0.02) | 0.01 (0.00–0.05) | 0.01 (0.01–0.09) | 0.10 (0.10–0.13) | 0.10 (0.10–0.13) | 0.10 (0.08–0.10) |
| % of CD8+ | 0.00 (0.00–0.05) | 0.00 (0.00–0.06) | 0.01 (0.01–0.01) | 0.10 (0.05–0.11) | 0.10 (0.05–0.11) | 0.10 (0.03–0.18) |
| LuT-Rs (n = 9) | KT-Rs (n = 23) | HDs (n = 12) | p Value * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | LuT-Rs vs. HD | KT-Rs vs. HD | |
| anti-S antibody titers (BAU/mL) | 4.8 (4.8–16.2) | 23 (7–956) | 70.3 (7–144) | 88.4 (31–289) | 2500 (862–3320) | 870 (399–7110) | 369 (189–701) | 1610 (1520–9400) | 3270 (869–9328) | T0: <0.0001 T1: 0.0004 T2: 0.0116 | T0: 0.0232 T1: ns T2: ns |
| responding T-cells | T0: 0.0025 T1: 0.0322 T2: 0.0360 | T0: 0.0003 T1: 0.0001 T2: 0.0002 | |||||||||
| % of CD4+ | 0.39 (0.20–0.69) | 0.50 (0.32–0.81) | 0.02 (0.01–0.08) | 0.17 (0.07–1.13) | 0.21 (0.0–0.65) | 0.01 (0.01–0.05) | 1.98 (1.52–3.29) | 1.39 (0.88–2.04) | 0.41 (0.11–0.72) | ||
| % of CD8+ | 0.07 (0.05–0.63) | 0.09 (0.05–0.93) | 0.03 (0.01–0.06) | 0.30 (0.02–0.65) | 0.35 (0.0–1.0) | 0.08 (0.06–0.10) | 1.06 (0.11–1.63) | 1.12 (0.18–2.46) | 0.38 (0.20–0.57) | T0: 0.0371 T1:0.0508 T2: 0.0011 | T0: 0.0492 T1: 0.0306 T2: 0.0021 |
| polyfunctional T-cells | T0: 0.0498 T1: 0.0415 T2: 0.0286 | T0: <0.0001 T1: 0.0025 T2: <0.0001 | |||||||||
| % of CD4+ | 0.02 (0.0–0.03) | 0.02 (0.0–0.05) | 0.01 (0.01–0.02) | 0.0 (0.0–0.01) | 0.0 (0.0–0.08) | 0.01 (0.0–0.01) | 0.10 (0.10–0.13) | 0.10 (0.10–0.13) | 0.10 (0.08–0.10) | ||
| % of CD8+ | 0.01 (0.0–0.02) | 0.03 (0.0–0.09) | 0.01 (0.01–0.01) | 0.0 (0.0–0.03) | 0.0 (0.0–0.04) | 0.01 (0.01–0.02) | 0.10 (0.05–0.11) | 0.10 (0.05–0.11) | 0.10 (0.03–0.18) | T0: 0.0052 T1: 0.0628 T2: 0.0798 | T0: 0.0003 T1: 0.0002 T2: 0.0470 |
| <2 Years since Tx (n = 8) | >2 Years since Tx (n = 24) | HDs (n = 12) | p Value * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | <2 Years since Tx vs. HD | >2 Years since Tx vs. HDs | |
| anti-S antibody titers (BAU/mL) | 16 (4.8–31) | 122 (11–1093) | 149 (70–6820) | 62 (31–287) | 2270 (286–3300) | 771 (163–5085) | 369 (189–701) | 1610 (1520–9400) | 3270 (869–9328) | T0: <0.0001 T1: 0.0020 T2: ns | T0: 0.0075 T1: ns T2: ns |
| responding T-cells | T0: 0.0020 T1: 0.0008 T2: 0.0545 | T0: 0.0004 T1: 0.0013 T2: 0.0007 | |||||||||
| % of CD4+ | 0.30 (0.06–0.94) | 0.17 (0.0–0.63) | 0.01 (0.01–0.51) | 0.35 (0.10–1.10) | 042 (0.08–0.75) | 0.01 (0.01–0.07) | 1.98 (1.52–3.29) | 1.39 (0.88–2.04) | 0.41 (0.11–0.72) | ||
| % of CD8+ | 0.06 (0.06–0.27) | 0.12 (0.05–0.67) | 0.08 (0.02–0.31) | 0.20 (0.02–0.98) | 0.40 (0.02–1.09) | 0.07 (0.06–0.09) | 1.06 (0.11–1.63) | 1.12 (0.18–2.46) | 0.38 (0.20–0.57) | T0: 0.0137 T1: 0.0416 T2: 0.0385 | T0: 0.0593 T1: 0.0545 T2: 0.0006 |
| polyfunctional T-cells | T0: 0.0212 T1: 0.0603 T2: 0.0211 | T0: 0.0001 T1: 0.0022 T2: <0.0001 | |||||||||
| % of CD4+ | 0.01 (0.0–0.04) | 0.03 (0.0–0.08) | 0.01 (0.01–0.03) | 0.0 (0.0–0.01) | 0.0 (0.0–0.05) | 0.01 (0.0–0.01) | 0.10 (0.10–0.13) | 0.10 (0.10–0.13) | 0.10 (0.08–0.10) | ||
| % of CD8+ | 0.01 (0.0–0.04) | 0.0 (0.0–0.07) | 0.01 (0.01–0.01) | 0.0 (0.0–0.02) | 0.0 (0.0–0.06) | 0.01 (0.01–0.01) | 0.10 (0.05–0.11) | 0.10 (0.05–0.11) | 0.10 (0.03–0.18) | T0: 0.0059 T1: 0.0120 T2: 0.0236 | T0: 0.0003 T1: 0.0006 T2: 0.0080 |
| SOT-Rs (n = 7) | HDs (n = 12) | |||
|---|---|---|---|---|
| wt | o | wt | o | |
| responding T-cells | ||||
| % of CD4+ | 0.01 (0.01–0.10) | 0.02 (0.01–0.06) | 0.41 (0.11–0.72) | 0.61 (0.33–0.78) |
| % of CD8+ | 0.08 (0.05–0.10) | 0.04 (0.02–0.06) | 0.38 (0.20–0.57) | 0.43 (0.10–1.10) |
| polyfunctional T-cells | ||||
| % of CD4+ | 0.01 (0.0–0.02) | 0.01 (0.0–0.02) | 0.10 (0.08–0.10) | 0.04 (0.01–0.11) |
| % of CD8+ | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.10 (0.03–0.18) | 0.10 (0.02–0.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingaropoli, M.A.; Guardiani, M.; Dominelli, F.; Tortellini, E.; Garofalo, M.; Cogliati Dezza, F.; Centofanti, A.; Carillo, C.; Napoli, A.; Venuta, F.; et al. The Long-Term Immunogenicity of mRNABNT162b Third Vaccine Dose in Solid Organ Transplant Recipients. Vaccines 2024, 12, 224. https://doi.org/10.3390/vaccines12030224
Zingaropoli MA, Guardiani M, Dominelli F, Tortellini E, Garofalo M, Cogliati Dezza F, Centofanti A, Carillo C, Napoli A, Venuta F, et al. The Long-Term Immunogenicity of mRNABNT162b Third Vaccine Dose in Solid Organ Transplant Recipients. Vaccines. 2024; 12(3):224. https://doi.org/10.3390/vaccines12030224
Chicago/Turabian StyleZingaropoli, Maria Antonella, Mariasilvia Guardiani, Federica Dominelli, Eeva Tortellini, Manuela Garofalo, Francesco Cogliati Dezza, Anastasia Centofanti, Carolina Carillo, Anna Napoli, Federico Venuta, and et al. 2024. "The Long-Term Immunogenicity of mRNABNT162b Third Vaccine Dose in Solid Organ Transplant Recipients" Vaccines 12, no. 3: 224. https://doi.org/10.3390/vaccines12030224
APA StyleZingaropoli, M. A., Guardiani, M., Dominelli, F., Tortellini, E., Garofalo, M., Cogliati Dezza, F., Centofanti, A., Carillo, C., Napoli, A., Venuta, F., Mastroianni, C. M., Pretagostini, R., Lichtner, M., Ciardi, M. R., & Russo, G. (2024). The Long-Term Immunogenicity of mRNABNT162b Third Vaccine Dose in Solid Organ Transplant Recipients. Vaccines, 12(3), 224. https://doi.org/10.3390/vaccines12030224









