Sero and Carriage Epidemiology of Pertussis in Urban and Rural Regions in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Area and Participants’ Enrollment
2.3. Data Collection and Laboratory Testing
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Prevalence of Recent Pertussis Infection in Nha Trang and Quang Ngai
3.2. Factors Associated with Prevalence of anti-PT IgG ≥ 62.5 IU/mL in Nha Trang and Quang Ngai Surveys
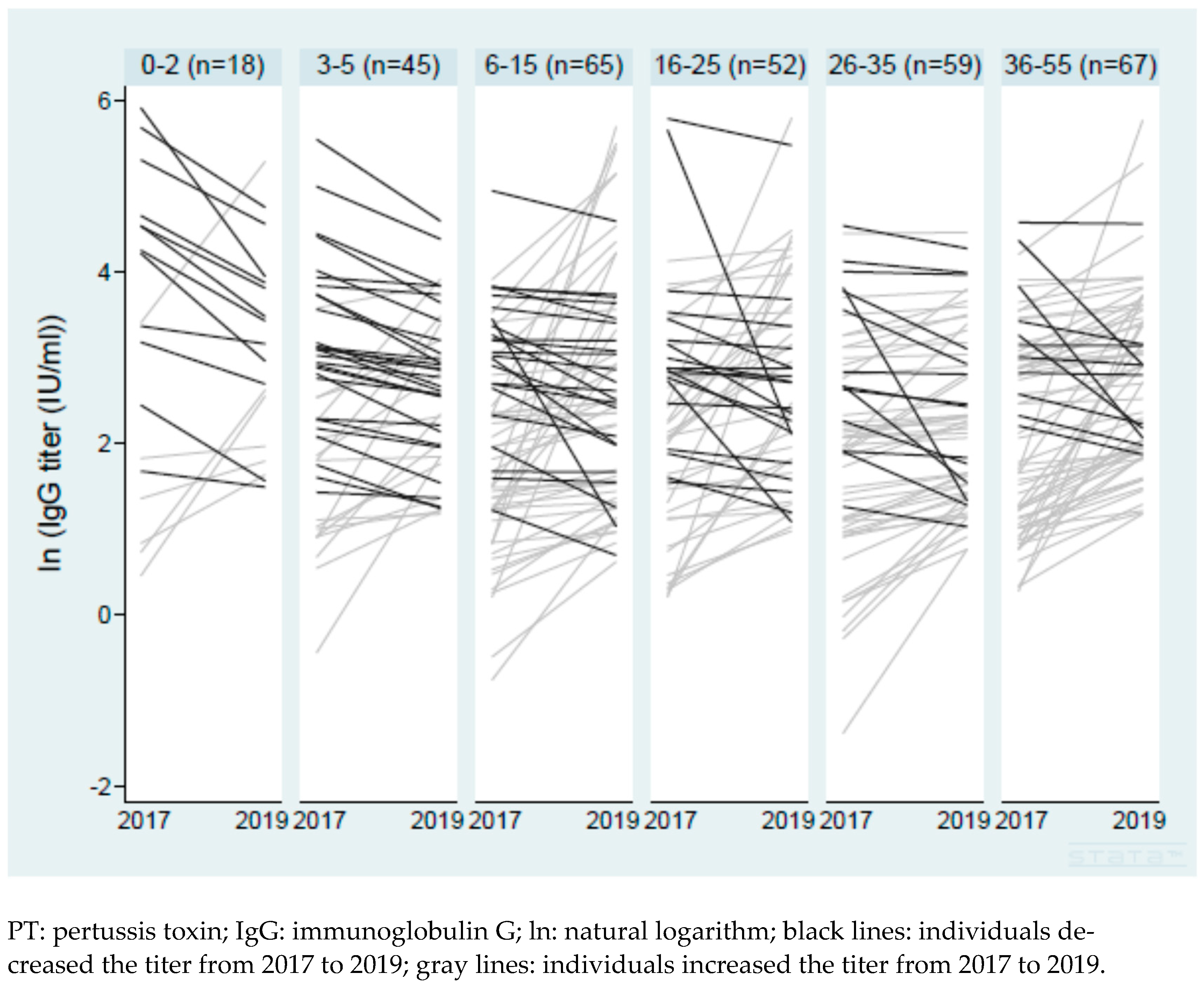
3.3. Change in Anti-PT IgG Titers in Paired Samples from 2017 to 2019
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeung, K.H.T.; Duclos, P.; Nelson, E.A.S.; Hutubessy, R.C.W. An update of the global burden of pertussis in children younger than 5 years: A modelling study. Lancet Infect. Dis. 2017, 17, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Rohani, P.; Drake, J.M. The decline and resurgence of pertussis in the US. Epidemics 2011, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.A. Changing Pertussis Epidemiology: Everything Old is New Again. J. Infect. Dis. 2014, 209, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Rohani, P.; Scarpino, S.V. (Eds.) Pertussis: Epidemiology, Immunology, and Evolution; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Thisyakorn, U.; Tantawichien, T.; Thisyakorn, C.; Buchy, P. Pertussis in the Association of Southeast Asian Nations: Epidemiology and challenges. Int. J. Infect. Dis. 2019, 87, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vietnam Ministry of Health. 25 years of Expanded Program of Immunization in Vietnam [in Vietnamese]. 2012; Unpublished work. [Google Scholar]
- World Health Organization. Immunization Data. Available online: https://immunizationdata.who.int/pages/coverage/dtp.html?CODE=VNM&ANTIGEN=DTPCV3&YEAR= (accessed on 11 December 2023).
- Hoang, H.T.T.; Leuridan, E.; Maertens, K.; Nguyen, T.D.; Hens, N.; Vu, N.H.; Caboré, R.N.; Duong, H.T.; Huygen, K.; Van Damme, P.; et al. Pertussis vaccination during pregnancy in Vietnam: Results of a randomized controlled trial Pertussis vaccination during pregnancy. Vaccine 2016, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory Data Repository. Available online: https://apps.who.int/gho/data/view.main.1540_43?lang=en (accessed on 11 December 2023).
- Le Thi Thanh, X.; Nguyen Ngoc, A.; Dao Huu, T.; Le Hai, D.; Pham Duy, T.; Kim, A.D. Assessing the spatial and temporal analysis of pertussis transmission among Hanoi, Vietnam population in the period of 2015 to 2019. Cogent Public Health 2022, 9, 2135216. [Google Scholar] [CrossRef]
- de Melker, H.E.; Versteegh, F.G.A.; Schellekens, J.F.P.; Teunis, P.F.M.; Kretzschmar, M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J. Infect. 2006, 53, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Hoan, T.; Do, H.; Dao, T.; Le, L.; Le, T.T.T.; Doan, T.T.T.; Chau, T.; Dinh, H.; Iwaki, M.; et al. Seroepidemiology and Carriage of Diphtheria in Epidemic-Prone Area and Implications for Vaccination Policy, Vietnam. Emerg. Infect. Dis. 2023, 29, 70. [Google Scholar] [CrossRef]
- Khanh Hoa Health Service. Population of 27 communes in Nha Trang. 2018, Unpublished work.
- Kitamura, N.; Le, L.T.; Le, T.T.T.; Nguyen, H.-A.T.; Edwards, T.; Madaniyazi, L.; Bui, M.X.; Do, H.T.; Dang, D.-A.; Toizumi, M.; et al. The seroprevalence, waning rate, and protective duration of anti-diphtheria toxoid IgG antibody in Nha Trang, Vietnam. Int. J. Infect. Dis. 2022, 116, 273–280. [Google Scholar] [CrossRef] [PubMed]
- General Statistics Office. Completed Results of the 2019 Viet Nam Population and Housing Census. 2019. Available online: https://www.gso.gov.vn/en/data-and-statistics/2020/11/completed-results-of-the-2019-viet-nam-population-and-housing-census/ (accessed on 18 February 2024).
- Son, S.; Thamlikitkul, V.; Chokephaibulkit, K.; Perera, J.; Jayatilleke, K.; Hsueh, P.R.; Lu, C.Y.; Balaji, V.; Moriuchi, H.; Nakashima, Y.; et al. Prospective multinational serosurveillance study of Bordetella pertussis infection among 10- to 18-year-old Asian children and adolescents. Clin. Microbiol. Infect. 2019, 25, 250.e1–250.e27. [Google Scholar] [CrossRef]
- Kamachi, K.; Yoshino, S.; Katsukawa, C.; Otsuka, N.; Hiramatsu, Y.; Shibayama, K. Laboratory-based surveillance of pertussis using multitarget real-time PCR in Japan: Evidence for Bordetella pertussis infection in preteens and teens. New Microbes New Infect. 2015, 8, 70–74. [Google Scholar] [CrossRef][Green Version]
- Jõgi, P.; Oona, M.; Toompere, K.; Leedo, S.; Epstein, J.; Lutsar, I. Seroprevalence of IgG antibodies to pertussis toxin in children and adolescents in Estonia. Vaccine 2014, 32, 5311–5315. [Google Scholar] [CrossRef]
- Pebody, R.G.; Gay, N.J.; Giammanco, A.; Baron, S.; Schellekens, J.; Tischer, A.; Olander, R.M.; Andrews, N.J.; Edmunds, W.J.; Lecoeur, H.; et al. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiol. Infect. 2005, 133, 159–171. [Google Scholar] [CrossRef] [PubMed]
- de Greeff, S.C.; de Melker, H.E.; van Gageldonk, P.G.M.; Schellekens, J.F.P.; van der Klis, F.R.M.; Mollema, L.; Mooi, F.R.; Berbers, G.A.M. Seroprevalence of Pertussis in the Netherlands: Evidence for increased circulation of Bordetella pertussis. PLoS ONE 2010, 5, e14183. [Google Scholar] [CrossRef]
- Campbell, P.; McIntyre, P.; Quinn, H.; Hueston, L.; Gilbert, G.L.; McVernon, J. Increased population prevalence of low pertussis toxin antibody levels in young children preceding a record pertussis epidemic in Australia. PLoS ONE 2012, 7, e35874. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Ikematsu, H.; Kawai, N.; Yajima, S. A cross sectional survey measuring sero-incidence of pertussis infection among Japanese junior and senior high school students in 2013 and 2014. Vaccine 2017, 35, 3859–3864. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Endo, A.; Le, L.T.; Nguyen, T.B.; Do, H.T.; Toizumi, M.; Yoshida, L.M.; Mori, Y.; Rose, S.; Efstratiou, A.; et al. Evaluation and validation of a commercial ELISA versus the in vitro toxin neutralization assay for determination of diphtheria anti-toxin in human serum. J. Med. Microbiol. 2023, 72, 001721. [Google Scholar] [CrossRef] [PubMed]
- Magpantay, F.M.; Rohani, P. Dynamics of Pertussis Transmission in the United States. Am. J. Epidemiol. 2015, 181, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Broutin, H.; Viboud, C.; Grenfell, B.T.; Miller, M.A.; Rohani, P. Impact of vaccination and birth rate on the epidemiology of pertussis: A comparative study in 64 countries. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Macdonald-Laurs, E.; Ganeshalingham, A.; Lillie, J.; McSharry, B.; Segedin, E.R.; Best, E.; Pillai, A.; Harnden, A.; Gilchrist, C.A.; Grant, C.C. Increasing Incidence of Life-threatening Pertussis: A Retrospective Cohort Study in New Zealand. Pediatr. Infect. Dis. J. 2017, 36, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Pollock, T.M.; Miller, E.; Lobb, J. Severity of whooping cough in England before and after the decline in pertussis immunisation. Arch. Dis. Child. 1984, 59, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.A.; Savic, M.; Kandeil, W. Pertussis in High Risk Groups: An Overview of the Past Quarter Century. Hum. Vaccines Immunother. 2020, 16, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Kurosky, S.K.; Mullooly, J.P.; Chun, C.; Weinmann, S. A Ten-Year Case-Control Study of Passive Smoke Exposure as a Risk Factor for Pertussis in Children. Perm. J. 2015, 19, 59–63. [Google Scholar] [CrossRef]
- Vanker, A.; Nduru, P.M.; Barnett, W.; Dube, F.S.; Sly, P.D.; Gie, R.P.; Nicol, M.P.; Zar, H.J. Indoor air pollution and tobacco smoke exposure: Impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019, 5, 00052-2018. [Google Scholar] [CrossRef] [PubMed]
- El Ahmer, O.R.; Essery, S.D.; Saadi, A.T.; Raza, M.W.; Ogilvie, M.M.; Weir, D.M.; Blackwell, C.C. The effect of cigarette smoke on adherence of respiratory pathogens to buccal epithelial cells. FEMS Immunol. Med. Microbiol. 1999, 23, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Best Practices for Healthcare Professionals on the Use of Polymerase Chain Reaction (PCR) for Diagnosing Pertussis. Available online: https://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-pcr-bestpractices.html (accessed on 11 December 2023).
- Waters, V.; Jamieson, F.; Richardson, S.E.; Finkelstein, M.; Wormsbecker, A.; Halperin, S.A. Outbreak of atypical pertussis detected by polymerase chain reaction in immunized preschool-aged children. Pediatr. Infect. Dis. J. 2009, 28, 582–587. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Schmidt-Schläpfer, G.; Just, M.; Matter, H.C.; Nikkari, S.; Viljanen, M.K.; Mertsola, J. Impact of polymerase chain reaction on clinical pertussis research: Finnish and Swiss experiences. J. Infect. Dis. 1996, 174, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Macintyre, C.R.; McIntyre, P.B.; Gilbert, G.L.; Staff, M.; Hanlon, M.; Heron, L.G.; Cagney, M.; Bennett, C. A boarding school outbreak of pertussis in adolescents: Value of laboratory diagnostic methods. Epidemiol. Infect. 2005, 133, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Klement, E.; Uliel, L.; Engel, I.; Hasin, T.; Yavzori, M.; Orr, N.; Davidovitz, N.; Lahat, N.; Srugo, I.; Zangvil, E.; et al. An outbreak of pertussis among young Israeli soldiers. Epidemiol. Infect. 2003, 131, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Macina, D.; Evans, K.E. Bordetella pertussis in School-Age Children, Adolescents, and Adults: A Systematic Review of Epidemiology, Burden, and Mortality in Asia. Infect. Dis. Ther. 2021, 10, 1115–1140. [Google Scholar] [CrossRef] [PubMed]
- Tondella, M.L.; Carlone, G.M.; Messonnier, N.; Quinn, C.P.; Meade, B.D.; Burns, D.L.; Cherry, J.D.; Guiso, N.; Hewlett, E.L.; Edwards, K.M.; et al. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19–20 July 2007. Vaccine 2009, 27, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pang, J.; Zhang, Y.; Ding, Y.; Chen, N.; Zhang, N.; He, Q. Seroprevalence of Pertussis in Adults at Childbearing Age Pre- and Post-COVID-19 in Beijing, China. Vaccines 2022, 10, 872. [Google Scholar] [CrossRef] [PubMed]
| Nha Trang in 2017 | Quang Ngai in 2019 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | N | ≥62.5 IU/ml | Prevalence of ≥62.5 IU/Ml (95% CI) | ≥125 IU/mL | Prevalence of ≥125 IU/mL (95% CI) | GMC (IU/mL) (95% CI) | N | ≥62.5 IU/mL | Prevalence of ≥62.5 IU/mL (95% CI) | ≥125 IU/mL | Prevalence of ≥125 IU/mL (95% CI) | GMC (IU/mL) (95% CI) |
| 0−2 | 27 | 13 | 48.1 (30.3−66.4) | 4 | 14.8 (5.7−33.5) | 27.6 (13.8−54.9) | 87 | 25 | 28.7 (20.2−39.1) | 10 | 11.5 (6.3−20.1) | 24.9 (19.0−32.7) |
| 3−5 | 73 | 7 | 9.6 (4.6−18.8) | 4 | 5.5 (2.1−13.7) | 15.3 (11.6−20.2) | 182 | 18 | 9.9 (6.3−15.2) | 8 | 4.4 (2.2−8.5) | 13.3 (11.4−15.6) |
| 6−15 | 107 | 6 | 5.6 (2.5−11.9) | 2 | 1.9 (0.5−7.2) | 9.3 (7.4−11.7) | 299 | 34 | 11.4 (8.2−15.5) | 11 | 3.7 (2.0−6.5) | 15.7 (14.0−17.7) |
| 16−25 | 105 | 7 | 6.7 (3.2−13.3) | 3 | 2.9 (0.9−8.5) | 10.4 (8.2−13.3) | 158 | 12 | 7.6 (4.4−12.9) | 3 | 1.9 (0.6−5.7) | 14.5 (12.5−16.9) |
| 26−35 | 94 | 2 | 2.1 (0.5−8.1) | 0 | NA | 8.5 (6.8−10.8) | 292 | 21 | 7.2 (4.7−10.8) | 6 | 2.1 (0.9−4.5) | 15.2 (13.7−16.8) |
| 36−55 | 104 | 4 | 3.8 (1.4−9.8) | 0 | NA | 9.6 (7.9−11.9) | 198 | 19 | 9.6 (6.2−14.6) | 6 | 3.0 (1.4−6.6) | 15.9 (14.1−18.1) |
| Characteristics | Nha Trang in 2017 Number (%) | Quang Ngai in 2019 Number (%) |
|---|---|---|
| n = 483 | n = 1129 | |
| Demographics | ||
| Age group | ||
| 3–5 | 73 (15.1) | 182 (16.1) |
| 6–15 | 107 (22.2) | 299 (26.5) |
| 16–25 | 105 (21.7) | 158 (14.0) |
| 26–35 | 94 (19.5) | 292 (25.9) |
| 36–55 | 104 (21.5) | 198 (17.5) |
| Sex | ||
| Male | 207 (42.9) | 558 (49.4) |
| Female | 276 (57.1) | 571 (50.6) |
| Clinical information: Recent symptoms and medication | ||
| Respiratory symptom | in proceeding two weeks | in proceeding one month, n = 1128 |
| Yes | 270 (23.9) | |
| No | 858 (76.1) | |
| Cough | ||
| Yes | 60 (12.4) | |
| No | 423 (87.6) | |
| Runny nose | ||
| Yes | 58 (12) | |
| No | 425 (88) | |
| Difficulty breathing | ||
| Yes | 2 (0.4) | |
| No | 481 (99.6) | |
| Took antibiotics | ||
| Yes | 28 (5.8) | 211 (18.9) |
| No | 455 (94.2) | 907 (81.1) |
| Clinical information: History and underlying condition | ||
| Ever diagnosed with Pertussis | n = 291 (asked in 2019) | n = 1057 |
| Yes | 1 (0.3) | 3 (0.3) |
| No | 290 (99.7) | 1054 (99.7) |
| Ever had a persistent cough | n = 291 (asked in 2019) | n = 1073 |
| Yes | 8 (2.8) | 10 (0.9) |
| No | 283 (97.3) | 1063 (99.1) |
| DPT history orally reported | ||
| At least one dose | 204 (42.2) | |
| No history or unknown | 279 (57.8) | |
| DPT vaccine history confirmed | n = 95 (confirmed in 2019) | n = 291 |
| At least one dose | 90 (94.7) | 275 (94.5) |
| No DPT history | 5 (5.3) | 16 (5.5) |
| Going to nursery or school | ||
| Yes | 214 (44.3) | 382 (33.8) |
| No | 269 (55.7) | 748 (66.2) |
| Chronic disease | ||
| Yes | 18 (3.7) | 159 (13.7) |
| No | 465 (96.3) | 970 (86.3) |
| Smoking | ||
| Yes | 28 (5.8) | 155 (13.7) |
| No | 455 (94.2) | 974 (86.3) |
| Travel history | ||
| Traveled to another province in Vietnam since 2012 | n = 291 (asked in 2019) | n = 1128 |
| Yes | 39 (13.4) | 105 (9.3) |
| No | 252 (86.6) | 1023 (90.7) |
| Traveled to another country since 2012 | n = 291 (asked in 2019) | n = 1128 |
| Yes | 6 (2.1) | 7 (0.6) |
| No | 285 (97.9) | 1121 (99.4) |
| Household | ||
| Family with >4 members | ||
| ≥5 | 262 (54.2) | 569 (50.4) |
| 1–4 | 221 (45.8) | 561 (49.7) |
| Family has child(ren) aged <12 years | ||
| Yes | 258 (53.4) | 995 (88.1) |
| No | 225 (46.6) | 135 (12) |
| Family has smoker(s) | n = 1127 | |
| Yes | 253 (52.4) | 659 (58.5) |
| No | 230 (47.6) | 468 (41.5) |
| Family has a member with long-lasting cough (>3 months) | ||
| Yes | 3 (0.6) | |
| No | 480 (99.4) | |
| House size (m2) | n = 482 | n = 490 |
| Less than 80 | 278 (60.0) | 438 (89.4) |
| 81 or more | 204 (40.0) | 52 (10.6) |
| Nha Trang in 2017 | Quang Ngai in 2019 | |||||
|---|---|---|---|---|---|---|
| Characteristics | All Number (%) | IgG ≥ 62.5 IU/mL N (%) | Adjusted Odds Ratio * | All Number (%) | IgG ≥ 62.5 IU/mL N (%) | Adjusted Odds Ratio * |
| n = 483 | n = 26 | n = 1129 | n = 104 | |||
| Demographics | ||||||
| Age group | ||||||
| 3–5 | 73 (15.1) | 7 (9.6) | 2.43 (0.68–8.70) ** | 182 (16.1) | 18 (9.9) | 1.06 (0.54–2.10) ** |
| 6–15 | 107 (22.2) | 6 (5.6) | 1.32 (0.36–4.89) ** | 299 (26.5) | 34 (11.4) | 1.23 (0.68-2.23) ** |
| 16–25 | 105 (21.7) | 7 (6.7) | 1.70 (0.48–6.02) ** | 158 (14.0) | 12 (7.6) | 0.74 (0.35–1.57) ** |
| 26–35 | 94 (19.5) | 2 (2.1) | 0.55 (0.10–3.07) ** | 292 (25.9) | 21 (7.2) | 0.72 (0.38–1.38) ** |
| 36–55 | 104 (21.5) | 4 (3.9) | Reference | 198 (17.5) | 19 (9.6) | Reference |
| Sex | ||||||
| Male | 207 (42.9) | 15 (7.3) | Reference | 558 (49.4) | 43 (7.7) | Reference |
| Female | 276 (57.1) | 11 (4.0) | 0.59 (0.26–1.33) *** | 571 (50.6) | 61 (10.7) | 1.49 (0.99–2.25) *** |
| Clinical information: Recent symptoms and medication | ||||||
| Respiratory symptom | in proceeding two weeks | in proceeding one month, n = 1128 | ||||
| Yes | 270 (23.9) | 28 (10.4) | 0.84 (0.53–1.33) | |||
| No | 858 (76.1) | 76 (8.9) | Reference | |||
| Cough | ||||||
| Yes | 60 (12.4) | 9 (15.0) | 3.70 (1.52–9.03) | |||
| No | 423 (87.6) | 17 (4.0) | Reference | |||
| Runny nose | ||||||
| Yes | 58 (12) | 4 (6.9) | 1.18 (0.38–3.61) | |||
| No | 425 (88) | 22 (5.2) | Reference | |||
| Difficulty breathing | ||||||
| Yes | 2 (0.4) | 0 (0) | NA | |||
| No | 481 (99.6) | 26 (5.4) | ||||
| Took antibiotics | in proceeding one month, n = 1118 | |||||
| Yes | 28 (5.8) | 5 (17.9) | 3.70 (1.24-11.03) | 211 (18.9) | 22 (10.4) | 0.84 (0.50–1.38) |
| No | 455 (94.2) | 21 (4.6) | Reference | 907 (81.1) | 80 (8.8) | Reference |
| Clinical information: History and underlying condition | ||||||
| Ever diagnosed with pertussis | n = 291 (asked in 2019) | n = 1057 | ||||
| Yes | 1 (0.3) | 0 (0.0) | NA | 3 (0.3) | 2 (66.7) | 17.43 (1.54–197.70) |
| No | 290 (99.7) | 12 (4.1) | 1054 (99.7) | 95 (9.0) | Reference | |
| Ever had a persistent cough | n = 291 (asked in 2019) | n = 1073 | ||||
| Yes | 8 (2.8) | 0 (0.0) | NA | 10 (0.9) | 3 (30.0) | 4.27 (1.07–17.00) |
| No | 283 (97.3) | 12 (4.2) | 1063 (99.1) | 98 (9.2) | Reference | |
| DPT history orally reported | ||||||
| At least one dose | 204 (42.2) | 12 (5.9) | 0.37 (0.10–1.39) | |||
| No history/unknown | 279 (57.8) | 14 (5.0) | Reference | |||
| DPT vaccine history confirmed | n = 95 (confirmed in 2019) | n = 291 | ||||
| At least one dose | 90 (94.7) | 5 (5.3) | NA | 275 (94.5) | 30 (10.9) | 0.90 (0.19–4.19) |
| No DPT history | 5 (5.3) | 0 (0.0) | 16 (5.5) | 2 (12.5) | Reference | |
| Going to nursery or school | ||||||
| Yes | 214 (44.3) | 18 (8.4) | 4.2 (0.82–21.49) | 382 (33.8) | 42 (11.0) | 1.07 (0.51–2.24) |
| No | 269 (55.7) | 8 (3.0) | Reference | 748 (66.2) | 62 (8.3) | Reference |
| Chronic disease | ||||||
| Yes | 18 (3.7) | 1 (5.6) | 0.81 (0.10–6.51) | 159 (13.7) | 15 (9.4) | 1.09 (0.61–1.97) |
| No | 465 (96.3) | 25 (5.4) | Reference | 970 (86.3) | 89 (9.2) | Reference |
| Smoking | ||||||
| Yes | 28 (5.8) | 2 (7.1) | 1.71 (0.31–9.39) | 155 (13.7) | 16 (10.3) | 2.21 (1.07–4.57) |
| No | 455 (94.2) | 24 (5.3) | Reference | 974 (86.3) | 88 (9.0) | Reference |
| Travel history | ||||||
| Traveled to another province in Vietnam since 2012 | n = 291 (asked in 2019) | n = 1128 | ||||
| Yes | 39 (13.4) | 2 (5.1) | 0.86 (0.18–4.21) | 105 (9.3) | 6 (5.7) | 1.29 (0.53–3.16) |
| No | 252 (86.6) | 10 (4.0) | Reference | 1023 (90.7) | 98 (9.6) | Reference |
| Traveled to another country since 2012 | n = 291 (asked in 2019) | n = 1128 | ||||
| Yes | 6 (2.1) | 1 (16.7) | 0.24 (0.02–2.42) | 7 (0.6) | 0 (0) | NA |
| No | 285 (97.9) | 11 (3.9) | Reference | 1121 (99.4) | 104 (9.3) | |
| Household | ||||||
| Family with >4 members | ||||||
| ≥5 | 262 (54.2) | 14 (5.3) | 0.98 (0.44–2.23) | 569 (50.4) | 55 (9.7) | 1.09 (0.73–1.64) |
| 1–4 | 221 (45.8) | 12 (5.4) | Reference | 561 (49.7) | 49 (8.7) | Reference |
| Family has child(ren) aged <12 years | ||||||
| Yes | 258 (53.4) | 13 (5.0) | 1.01 (0.44–2.34) | 995 (88.1) | 94 (9.5) | 1.28 (0.64–2.56) |
| No | 225 (46.6) | 13 (5.8) | Reference | 135 (12) | 10 (7.4) | Reference |
| Family has smoker(s) | n = 1127 | |||||
| Yes | 253 (52.4) | 15 (5.9) | 1.22 (0.54–2.76) | 659 (58.5) | 63 (9.6) | 0.91 (0.60–1.39) |
| No | 230 (47.6) | 11 (4.8) | Reference | 468 (41.5) | 41 (8.8) | Reference |
| Family has a member with long-lasting cough (>3 months) | ||||||
| Yes | 3 (0.6) | 0 (0) | NA | |||
| No | 480 (99.4) | 26 (5.4) | ||||
| House size (m2) | n = 482 | n = 490 | ||||
| Less than 80 | 278 (60.0) | 12 (4.3) | Reference | 438 (89.4) | 38 (8.7) | Reference |
| 81 or more | 204 (40.0) | 14 (6.9) | 1.73 (0.77–3.88) | 52 (10.6) | 5 (9.6) | 1.08 (0.40–2.89) |
| Characteristics | Number (%) | Ratio of IgG in 2019 to IgG in 2017 Geometric Mean (95% CI) | Adjusted Coefficient * |
|---|---|---|---|
| All | n = 306 | 1.45 (1.29–1.62) | Intercept: 0.95 (0.51–1.38) |
| Demographics | |||
| Age group (age in 2017) | |||
| 0–2 | 18 (5.9) | 0.86 (0.48–1.52) | −0.31 (−0.85–0.24) ** |
| 3–5 | 45 (14.7) | 1.11 (0.87–1.42) | −0.43 (−0.80–−0.06) ** |
| 6–15 | 65 (21.2) | 1.72 (1.27–2.33) | −0.09 (−0.43–0.24) ** |
| 16–25 | 52 (17.0) | 1.56 (1.11–2.18) | −0.15 (−0.51–0.21) ** |
| 26–35 | 59 (19.3) | 1.26 (1.06–1.51) | −0.33 (−0.67–0.01) ** |
| 36–55 | 67 (21.9) | 1.78 (1.41–2.25) | Reference |
| Sex | |||
| Male | 128 (41.8) | 1.59 (1.31–1.93) | Reference |
| Female | 178 (58.2) | 1.35 (1.17–1.56) | −0.20 (−0.42–0.03) *** |
| Baseline IgG | |||
| ≥62.5 IU/ml | 20 (6.5) | 0.45 (0.29–0.68) | −1.21 (−1.69–−0.73) **** |
| <62.5 IU/ml | 286 (93.5) | 1.57 (1.40–1.76) | Reference |
| Clinical information: Symptoms and medication in preceding two weeks in the 2017 survey | |||
| Cough | |||
| Yes | 46 (15.0) | 1.79 (1.14–2.81) | 0.44 (0.12–0.76) |
| No | 260 (85.0) | 1.39 (1.25–1.56) | Reference |
| Runny nose | |||
| Yes | 45 (14.7) | 1.44 (1.00–2.09) | 0.14 (−0.19–0.47) |
| No | 261 (85.3) | 1.45 (1.28–1.63) | Reference |
| Difficulty breathing | |||
| Yes | 1 (0.3) | 3.05 (NA) | 0.53 (−1.42–2.47) |
| No | 305 (99.7) | 1.44 (1.28–1.62) | Reference |
| Took antibiotics | |||
| Yes | 21 (6.9) | 1.43 (0.82–2.51) | 0.08 (−0.36–0.53) |
| No | 285 (93.1) | 1.45 (1.29–1.63) | Reference |
| Clinical information: History and underlying condition | |||
| Ever diagnosed with Pertussis | |||
| Yes | 1 (0.3) | 0.79 (NA) | −0.46 (−2.41–1.48) |
| No | 305 (99.7) | 1.45 (1.29–1.63) | Reference |
| Ever had a persistent cough | |||
| Yes | 8 (2.6) | 2.02 (0.78–5.24) | 0.29 (−0.41–0.99) |
| No | 298 (97.4) | 1.43 (1.27–1.61) | Reference |
| DPT history orally reported | |||
| At least one dose | 146 (47.7) | 1.36 (1.13–1.63) | 0.05 (−0.30–0.40) |
| No history or unknown | 160 (52.3) | 1.53 (1.32–1.78) | Reference |
| DPT vaccine history confirmed | |||
| At least one dose | 104 (94.6) | 1.23 (1.01–1.50) | −0.97 (−1.83–−0.12) |
| No history | 6 (5.5) | 3.71 (0.58–23.87) | Reference |
| Going to nursery or school | |||
| Yes | 140 (45.8) | 1.35 (1.13–1.62) | −0.26 (−0.68–0.16) |
| No | 166 (54.3) | 1.53 (1.32–1.78) | Reference |
| Chronic disease | |||
| Yes | 10 (3.3) | 1.52 (0.90–2.58) | −0.04 (−0.67–0.59) |
| No | 296 (96.7) | 1.44 (1.28–1.63) | Reference |
| Smoking | |||
| Yes | 19 (6.2) | 2.06 (1.29–3.29) | 0.27 (−0.24–0.78) |
| No | 287 (93.8) | 1.41 (1.25–1.59) | Reference |
| Travel history | |||
| Traveled to another province in Vietnam since 2012 | |||
| Yes | 41 (13.4) | 1.22 (0.98–1.51) | 0.23 (−0.10–0.56) |
| No | 265 (86.6) | 1.48 (1.30–1.69) | Reference |
| Traveled to another country since 2012 | |||
| Yes | 7 (2.3) | 1.13 (0.57–2.22) | 0.03 (−0.72–0.78) |
| No | 299 (97.7) | 1.45 (1.29–1.64) | Reference |
| Household | |||
| Family with >4 members | |||
| ≥5 | 170 (55.6) | 1.38 (1.19–1.60) | −0.00 (−0.23–0.22) |
| 1–4 | 136 (44.4) | 1.53 (1.27–1.84) | Reference |
| Family has child(ren) aged <12 years | |||
| Yes | 170 (55.6) | 1.39 (1.21–1.61) | 0.01 (−0.23–0.26) |
| No | 136 (44.4) | 1.52 (1.25–1.83) | Reference |
| Family has smoker(s) | |||
| Yes | 162 (52.9) | 1.35 (1.16–1.57) | −0.09 (−0.31–0.14) |
| No | 144 (47.1) | 1.56 (1.30–1.87) | Reference |
| Family has a member with long-lasting cough (>3 months) | |||
| Yes | 2 (0.7) | 3.04 (0.00–2062.88) | 0.47 (−0.91–1.86) |
| No | 304 (99.4) | 1.44 (1.28–1.62) | Reference |
| House size (m2) (n = 482) | |||
| 15–80 | 173 (56.7) | 1.35 (1.17–1.57) | Reference |
| 81–600 | 132 (43.3) | 1.58 (1.31–1.90) | 0.13 (−0.09–0.36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toizumi, M.; Le, L.T.; Nguyen, H.A.T.; Le, T.T.T.; Kitamura, N.; Bui, L.X.; Ho, N.M.; Do, H.T.; Kamachi, K.; Otsuka, N.; et al. Sero and Carriage Epidemiology of Pertussis in Urban and Rural Regions in Vietnam. Vaccines 2024, 12, 225. https://doi.org/10.3390/vaccines12030225
Toizumi M, Le LT, Nguyen HAT, Le TTT, Kitamura N, Bui LX, Ho NM, Do HT, Kamachi K, Otsuka N, et al. Sero and Carriage Epidemiology of Pertussis in Urban and Rural Regions in Vietnam. Vaccines. 2024; 12(3):225. https://doi.org/10.3390/vaccines12030225
Chicago/Turabian StyleToizumi, Michiko, Lien Thuy Le, Hien Anh Thi Nguyen, Thao Thi Thu Le, Noriko Kitamura, Liem Xuan Bui, Nen Minh Ho, Hung Thai Do, Kazunari Kamachi, Nao Otsuka, and et al. 2024. "Sero and Carriage Epidemiology of Pertussis in Urban and Rural Regions in Vietnam" Vaccines 12, no. 3: 225. https://doi.org/10.3390/vaccines12030225
APA StyleToizumi, M., Le, L. T., Nguyen, H. A. T., Le, T. T. T., Kitamura, N., Bui, L. X., Ho, N. M., Do, H. T., Kamachi, K., Otsuka, N., Bui, M. X., Dang, D. A., & Yoshida, L.-M. (2024). Sero and Carriage Epidemiology of Pertussis in Urban and Rural Regions in Vietnam. Vaccines, 12(3), 225. https://doi.org/10.3390/vaccines12030225






