Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Pneumococcal Strain and rBCG Strain
2.2. Neonatal Mice Immunization
2.3. Lethal Pneumococcal Challenge
2.4. Antibodies Measurement
2.5. Antibody Binding to Pneumococcal Surface
2.6. Spleen and Lungs Cell Culture
2.7. Bronchoalveolar Lavage Fluid (BALF) Collection and Cytokine Analysis
2.8. Statistical Analysis
3. Results
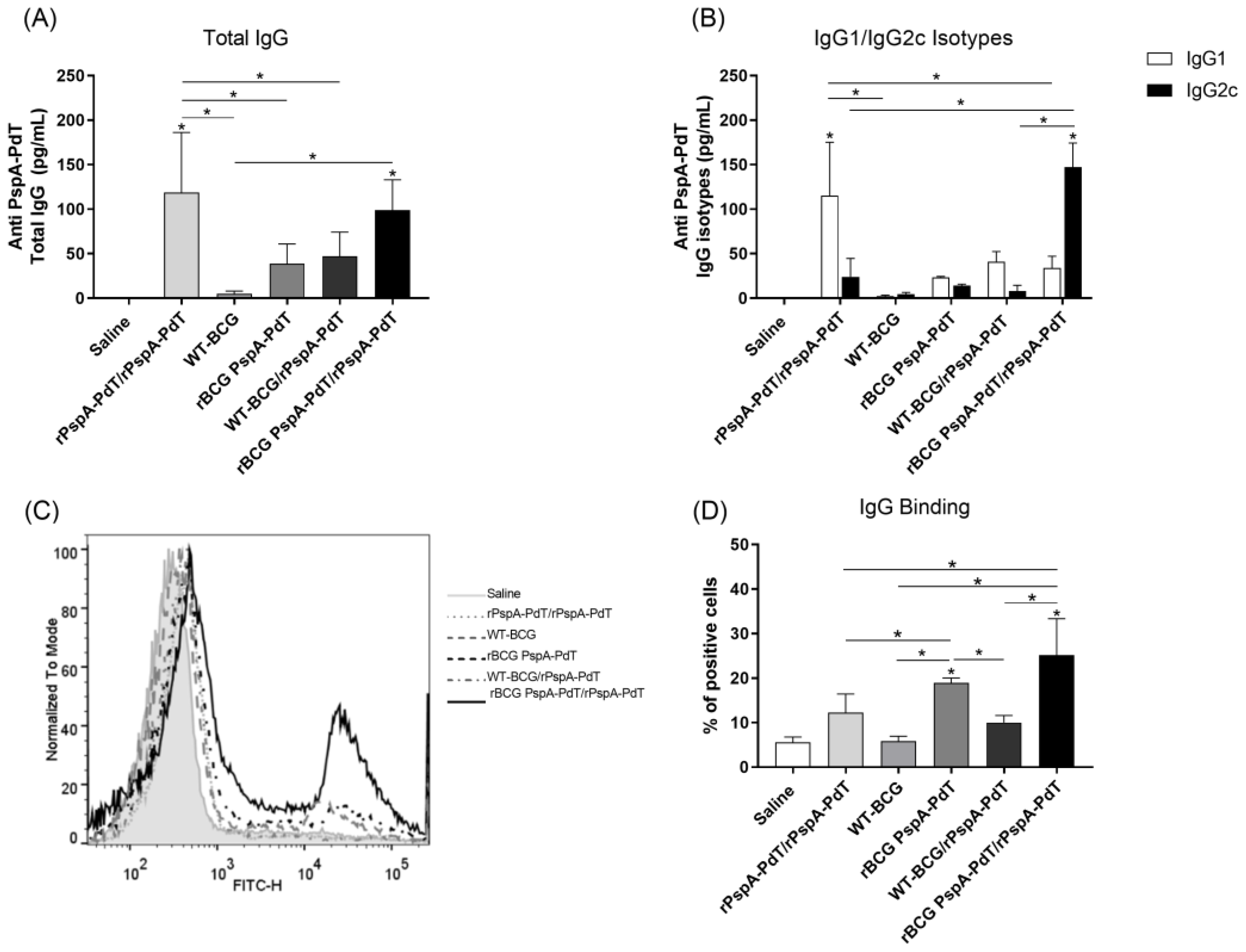
3.1. Prime/Boost Immunization Using rBCG PspA-PdT and rPspA-PdT Promotes IgG1/IgG2c Antibody Isotype Class Shift and Memory B Cells in Neonatal Mouse Model
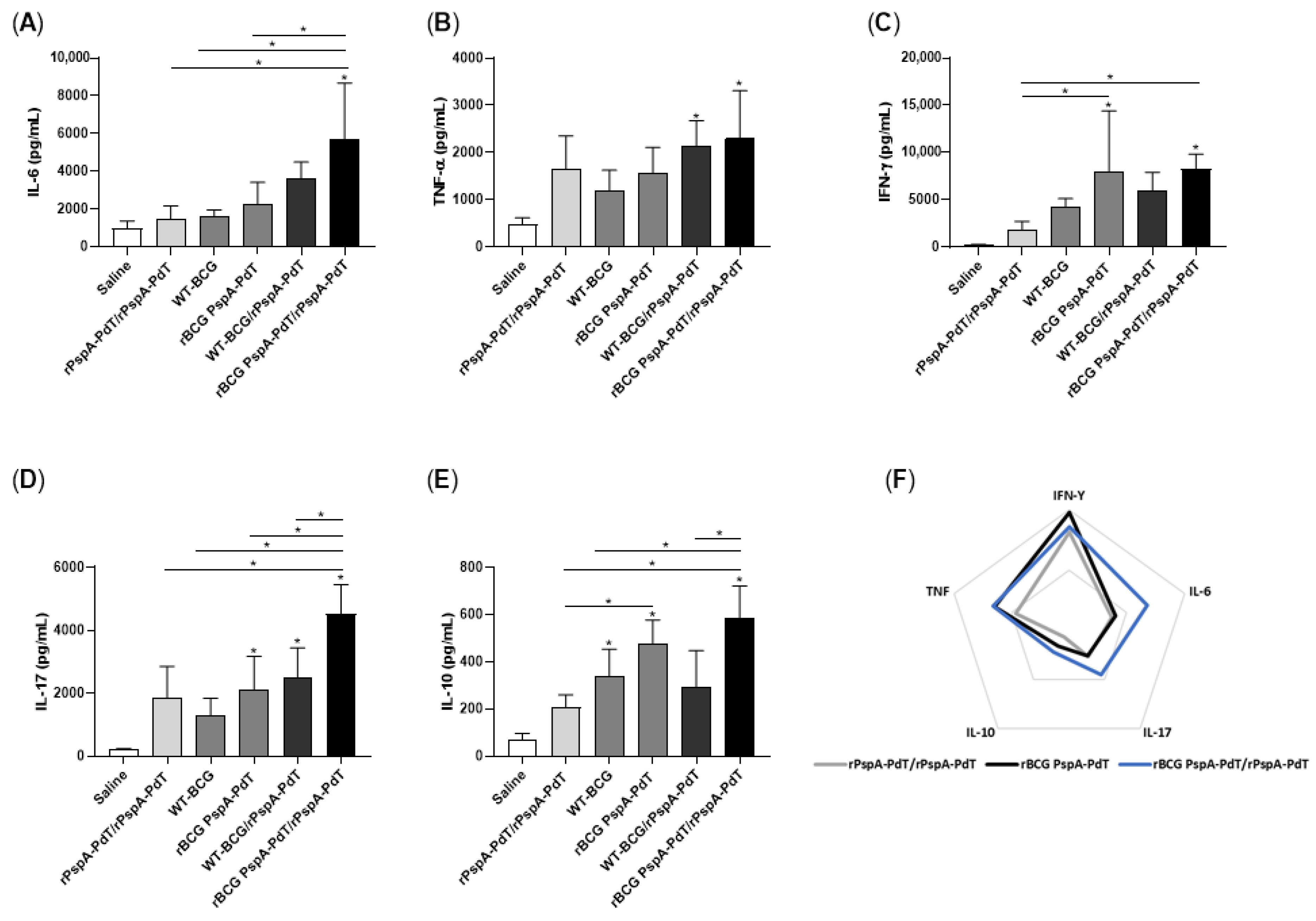
3.2. Prime/Boost Immunization Using rBCG PspA-PdT and rPspA-PdT Induces Inflammatory Cytokine and Memory T Cells in Neonatal Mouse
3.3. rBCG PspA-PdT/rPspA-PdT Protects Neonatal Mice against Lethal Pneumococcal Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Pneumonia in Children. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 1 September 2023).
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of Disease Caused by Streptococcus Pneumoniae in Children Younger than 5 Years: Global Estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Oligbu, G.; Hsia, Y.; Folgori, L.; Collins, S.; Ladhani, S. Pneumococcal Conjugate Vaccine Failure in Children: A Systematic Review of the Literature. Vaccine 2016, 34, 6126–6132. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Kagucia, E.W.; Loo, J.D.; Link-Gelles, R.; Puhan, M.A.; Cherian, T.; Levine, O.S.; Whitney, C.G.; O’Brien, K.L.; Moore, M.R.; et al. Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites. PLoS Med. 2013, 10, e1001517. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Willis, J.; Moore, H.C.; Giele, C.; Murphy, D.; Keil, A.D.; Harrison, C.; Bayley, K.; Watson, M.; Richmond, P.; et al. The Changing Epidemiology of Invasive Pneumococcal Disease in Aboriginal and Non-Aboriginal Western Australians from 1997 through 2007 and Emergence of Nonvaccine Serotypes. Clin. Infect. Dis. 2010, 50, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Diekema, D.J.; Heilmann, K.P.; Dohrn, C.L.; Riahi, F.; Doern, G.V. Changes in Pneumococcal Serotypes and Antimicrobial Resistance after Introduction of the 13-Valent Conjugate Vaccine in the United States. Antimicrob. Agents Chemother. 2014, 58, 6484–6489. [Google Scholar] [CrossRef]
- Keller, L.E.; Robinson, D.A.; McDaniel, L.S. Nonencapsulated Streptococcus Pneumoniae: Emergence and Pathogenesis. MBio 2016, 7, e01792-15. [Google Scholar] [CrossRef]
- Hsu, H.E.; Shutt, K.A.; Moore, M.R.; Beall, B.W.; Bennett, N.M.; Craig, A.S.; Farley, M.M.; Jorgensen, J.H.; Lexau, C.A.; Petit, S.; et al. Effect of Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis. N. Engl. J. Med. 2009, 360, 244–256. [Google Scholar] [CrossRef]
- Hansen, J.; Black, S.; Shinefield, H.; Cherian, T.; Benson, J.; Fireman, B.; Lewis, E.; Ray, P.; Lee, J. Effectiveness of Heptavalent Pneumococcal Conjugate Vaccine in Children Younger than 5 Years of Age for Prevention of Pneumonia: Updated Analysis Using World Health Organization Standardized Interpretation of Chest Radiographs. Pediatr. Infect. Dis. J. 2006, 25, 779–781. [Google Scholar] [CrossRef]
- Ghaffar, F.; Barton, T.; Lozano, J.; Muniz, L.S.; Hicks, P.; Gan, V.; Ahmad, N.; McCracken, G.H. Effect of the 7-Valent Pneumococcal Conjugate Vaccine on Nasopharyngeal Colonization by Streptococcus Pneumoniae in the First 2 Years of Life. Clin. Infect. Dis. 2004, 39, 930–938. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Tai, S.S. Streptococcus Pneumoniae Protein Vaccine Candidates: Properties, Activities and Animal Studies. Crit. Rev. Microbiol. 2006, 32, 139–153. [Google Scholar] [CrossRef]
- Darrieux, M.; Goulart, C.; Briles, D.; Leite, L.C.D.C. Current Status and Perspectives on Protein-Based Pneumococcal Vaccines. Crit. Rev. Microbiol. 2015, 41, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Briles, D.E.; Paton, J.C.; Mukerji, R.; Swiatlo, E.; Crain, M.J. Pneumococcal Vaccines. Microbiol. Spectr. 2019, 7, 6. [Google Scholar] [CrossRef]
- Namie Miyaji, E.; Leonor Sarno Oliveira, M.; Carvalho, E.; Lee Ho, P. Serotype-Independent Pneumococcal Vaccines. Cell. Mol. Life Sci. 2013, 70, 3303–3326. [Google Scholar] [CrossRef]
- Briles, D.E.; Hollingshead, S.K.; Nabors, G.S.; Paton, J.C.; Brooks-Walter, A. The Potential for Using Protein Vaccines to Protect against Otitis Media Caused by Streptococcus Pneumoniae. Vaccine 2000, 19, S87–S95. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Akeda, Y.; Takeuchi, D.; Ishii, K.J.; Ubukata, K.; Briles, D.E.; Tomono, K.; Oishi, K. Protective Properties of a Fusion Pneumococcal Surface Protein A (PspA) Vaccine against Pneumococcal Challenge by Five Different PspA Clades in Mice. Vaccine 2014, 32, 5607–5613. [Google Scholar] [CrossRef]
- Kono, M.; Hotomi, M.; Hollingshead, S.K.; Briles, D.E.; Yamanaka, N. Maternal Immunization with Pneumococcal Surface Protein A Protects against Pneumococcal Infections among Derived Offspring. PLoS ONE 2011, 6, e0027102. [Google Scholar] [CrossRef]
- Roche, H.; Håkansson, A.; Hollingshead, S.K.; Briles, D.E. Regions of PspA/EF3296 Best Able to Elicit Protection against Streptococcus Pneumoniae in a Murine Infection Model. Infect. Immun. 2003, 71, 1033–1041. [Google Scholar] [CrossRef]
- Tu, A.H.T.; Fulgham, R.L.; Mccrory, M.A.; Briles, D.E.; Szalai, A.J. Pneumococcal Surface Protein A Inhibits Complement Activation by Streptococcus Pneumoniae. Infect. Immun. 1999, 67, 4720–4724. [Google Scholar] [CrossRef]
- Ren, B.; Szalai, A.J.; Hollingshead, S.K.; Briles, D.E. Effects of PspA and Antibodies to PspA on Activation and Deposition of Complement on the Pneumococcal Surface. Infect. Immun. 2004, 72, 114–122. [Google Scholar] [CrossRef]
- Daniels, C.C.; Kim, K.H.; Burton, R.L.; Mirza, S.; Walker, M.; King, J.; Hale, Y.; Coan, P.; Rhee, D.K.; Nahm, M.H.; et al. Modified Opsonization, Phagocytosis, and Killing Assays to Measure Potentially Protective Antibodies against Pneumococcal Surface Protein A. Clin. Vaccine Immunol. 2013, 20, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Converso, T.R.; Goulart, C.; Rodriguez, D.; Darrieux, M.; Leite, L.C.C. Rational Selection of Broadly Cross-Reactive Family 2 PspA Molecules for Inclusion in Chimeric Pneumococcal Vaccines. Microb. Pathog. 2017, 109, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Shaper, M.; Hollingshead, S.K.; Benjamin, W.H.; Briles, D.E. PspA Protects Streptococcus Pneumoniae from Killing by Apolactoferrin, and Antibody to PspA Enhances Killing of Pneumococci by Apolactoferrin. Infect. Immun. 2004, 72, 5031–5040, Corrected in Infect. Immun. 2004, 72, 7379. [Google Scholar] [CrossRef]
- Hollingshead, S.K.; Becker, R.; Briles, D.E. Diversity of PspA: Mosaic Genes and Evidence for Past Recombination in Streptococcus Pneumoniae. Infect. Immun. 2000, 68, 5889–5900. [Google Scholar] [CrossRef]
- Baril, L.; Briles, D.E.; Crozier, P.; King, J.; Punar, M.; Hollingshead, S.K.; McCormick, J.B. Characterization of Antibodies to PspA and PsaA in Adults over 50 Years of Age with Invasive Pneumococcal Disease. Vaccine 2004, 23, 789–793. [Google Scholar] [CrossRef]
- Pimenta, F.C.; Ribeiro-Dias, F.; Brandileone, M.C.C.; Miyaji, E.N.; Leite, L.C.C.; Sgambatti De Andrade, A.L.S. Genetic Diversity of PspA Types among Nasopharyngeal Isolates Collected during an Ongoing Surveillance Study of Children in Brazil. J. Clin. Microbiol. 2006, 44, 2838. [Google Scholar] [CrossRef][Green Version]
- Goulart, C.; Darrieux, M.; Rodriguez, D.; Pimenta, F.C.; Brandileone, M.C.C.; de Andrade, A.L.S.S.; Leite, L.C.C. Selection of Family 1 PspA Molecules Capable of Inducing Broad-Ranging Cross-Reactivity by Complement Deposition and Opsonophagocytosis by Murine Peritoneal Cells. Vaccine 2011, 29, 1634–1642. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Dalziel, C.E. The Biology of Pneumolysin. Subcell. Biochem. 2014, 80, 145–160. [Google Scholar] [CrossRef]
- Marriott, H.; Mitchell, T.; Dockrell, D. Pneumolysin: A Double-Edged Sword during the Host-Pathogen Interaction. Curr. Mol. Med. 2008, 8, 497–509. [Google Scholar] [CrossRef]
- Calbo, E.; Garau, J. Factors Affecting the Development of Systemic Inflammatory Response Syndrome in Pneumococcal Infections. Curr. Opin. Infect. Dis. 2011, 24, 241–247. [Google Scholar] [CrossRef]
- Witzenrath, M.; Gutbier, B.; Hocke, A.C.; Schmeck, B.; Hippenstiel, S.; Berger, K.; Mitchell, T.J.; De Los Toyos, J.R.; Rosseau, S.; Suttorp, N.; et al. Role of Pneumolysin for the Development of Acute Lung Injury in Pneumococcal Pneumonia. Crit. Care Med. 2006, 34, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Ferrante, A. Inhibition of Human Polymorphonuclear Leukocyte Respiratory Burst, Bactericidal Activity, and Migration by Pneumolysin. Infect. Immun. 1983, 41, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Rowan Kelly, B.; Ferrante, A. Activation of Human Complement by the Pneumococcal Toxin Pneumolysin. Infect. Immun. 1984, 43, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Malley, R.; Henneke, P.; Morse, S.C.; Cieslewicz, M.J.; Lipsitch, M.; Thompson, C.M.; Kurt-Jones, E.; Paton, J.C.; Wessels, M.R.; Golenbock, D.T. Recognition of Pneumolysin by Toll-like Receptor 4 Confers Resistance to Pneumococcal Infection. Proc. Natl. Acad. Sci. USA 2003, 100, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- McNeela, E.A.; Burke, Á.; Neill, D.R.; Baxter, C.; Fernandes, V.E.; Ferreira, D.; Smeaton, S.; El-Rachkidy, R.; McLoughlin, R.M.; Mori, A.; et al. Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4. PLoS Pathog. 2010, 6, e1001191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Henneke, P.; Visintin, A.; Morse, S.C.; Martin, V.; Watkins, C.; Paton, J.C.; Wessels, M.R.; Golenbock, D.T.; Malley, R. The Apoptotic Response to Pneumolysin Is Toll-like Receptor 4 Dependent and Protects against Pneumococcal Disease. Infect. Immun. 2005, 73, 6479–6487. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, T.A.; Lingnau, K.; Nagy, E.; Jonsdottir, I. Novel Protein-Based Pneumococcal Vaccines Administered with the Th1-Promoting Adjuvant IC31 Induce Protective Immunity against Pneumococcal Disease in Neonatal Mice. Infect. Immun. 2012, 80, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, H.; Bjarnarson, S.; Del Giudice, G.; Moreau, M.; Siegrist, C.A.; Jonsdottir, I. Intranasal Immunization with Pneumococcal Conjugate Vaccines with LT-K63, a Nontoxic Mutant of Heat-Labile Enterotoxin, as Adjuvant Rapidly Induces Protective Immunity against Lethal Pneumococcal Infections in Neonatal Mice. Infect. Immun. 2002, 70, 1443–1452. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Dias, W.O.; Quintilio, W.; Christ, A.P.; Moraes, J.F.; Vancetto, M.D.C.; Ribeiro-dos-Santos, G.; Raw, I.; Leite, L.C.C. Neonatal Immunization with a Single Dose of Recombinant BCG Expressing Subunit S1 from Pertussis Toxin Induces Complete Protection against Bordetella Pertussis Intracerebral Challenge. Microbes Infect. 2008, 10, 198–202. [Google Scholar] [CrossRef]
- Goulart, C.; da Silva, T.R.; Rodriguez, D.; Politano, W.R.; Leite, L.C.C.; Darrieux, M. Characterization of Protective Immune Responses Induced by Pneumococcal Surface Protein A in Fusion with Pneumolysin Derivatives. PLoS ONE 2013, 8, e0059605. [Google Scholar] [CrossRef]
- Goulart, C.; Rodriguez, D.; Kanno, A.I.; Lu, Y.J.; Malley, R.; Leite, L.C.C. Recombinant BCG Expressing a PspA-PdT Fusion Protein Protects Mice against Pneumococcal Lethal Challenge in a Prime-Boost Strategy. Vaccine 2017, 35, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.; Rodriguez, D.; Kanno, A.I.; Silva, J.L.S.C.; Leite, L.C.C. Early Pneumococcal Clearance in Mice Induced by Systemic Immunization with Recombinant BCG PspA-PdT Prime and Protein Boost Correlates with Cellular and Humoral Immune Response in Bronchoalveolar Fluids (BALF). Vaccine X 2019, 4, 100049. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.N.; Tettelin, H.; Orihuela, C.J. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front. Cell. Infect. Microbiol. 2020, 10, 613287. [Google Scholar] [CrossRef] [PubMed]
- Goettler, D.; Streng, A.; Kemmling, D.; Schoen, C.; von Kries, R.; Rose, M.A.; van der Linden, M.; Liese, J.G. Increase in Streptococcus Pneumoniae Serotype 3 Associated Parapneumonic Pleural Effusion/Empyema after the Introduction of PCV13 in Germany. Vaccine 2020, 38, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Briles, D.E.; King, J.D.; Gray, M.A.; Mcdaniel, L.S.; Swiatlo, E.; Benton, K.A. PspA, a Protection-Eliciting Pneumococcal Protein: Immunogenicity of Isolated Native PspA in Mice. Vaccine 1996, 14, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, B.P.; Lynch, J.M.; Briles, D.E.; Hollingshead, S.; Metzger, D.W. Intranasal Vaccination with Pneumococcal Surface Protein A and Interleukin-12 Augments Antibody-Mediated Opsonization and Protective Immunity against Streptococcus Pneumoniae Infection. Infect. Immun. 2001, 69, 6718–6724. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Oliveira, M.L.S.; Moreno, A.T.; Ho, P.L.; Briles, D.E.; Miyaji, E.N. Protection against Nasal Colonization with Streptococcus Pneumoniae by Parenteral Immunization with a DNA Vaccine Encoding PspA (Pneumococcal Surface Protein A). Microb. Pathog. 2010, 48, 205–213. [Google Scholar] [CrossRef]
- Lu, Y.J.; Gross, J.; Bogaert, D.; Finn, A.; Bagrade, L.; Zhang, Q.; Kolls, J.K.; Srivastava, A.; Lundgren, A.; Forte, S.; et al. Interleukin-17A Mediates Acquired Immunity to Pneumococcal Colonization. PLoS Pathog. 2008, 4, e1000159. [Google Scholar] [CrossRef]
- Malley, R.; Srivastava, A.; Lipsitch, M.; Thompson, C.M.; Watkins, C.; Tzianabos, A.; Anderson, P.W. Antibody-Independent, Interleukin-17A-Mediated, Cross-Serotype Immunity to Pneumococci in Mice Immunized Intranasally with the Cell Wall Polysaccharide. Infect. Immun. 2006, 74, 2187–2195. [Google Scholar] [CrossRef]
- Aujla, S.J.; Dubin, P.J.; Kolls, J.K. Th17 Cells and Mucosal Host Defense. Semin. Immunol. 2007, 19, 377–382. [Google Scholar] [CrossRef]
- Kerr, A.R.; Irvine, J.J.; Search, J.J.; Gingles, N.A.; Kadioglu, A.; Andrew, P.W.; McPheat, W.L.; Booth, C.G.; Mitchell, T.J. Role of Inflammatory Mediators in Resistance and Susceptibility to Pneumococcal Infection. Infect. Immun. 2002, 70, 1547–1557. [Google Scholar] [CrossRef]
- Sun, K.; Salmon, S.L.; Lotz, S.A.; Metzger, D.W. Interleukin-12 Promotes Gamma Interferon-Dependent Neutrophil Recruitment in the Lung and Improves Protection against Respiratory Streptococcus Pneumoniae Infection. Infect. Immun. 2007, 75, 1196–1202. [Google Scholar] [CrossRef]
- Lima, F.A.; Ferreira, D.M.; Moreno, A.T.; Ferreira, P.C.D.; Palma, G.M.P.; Ferreira, J.M.C.; Raw, I.; Miyaji, E.N.; Ho, P.L.; Oliveira, M.L.S. Controlled Inflammatory Responses in the Lungs Are Associated with Protection Elicited by a Pneumococcal Surface Protein A-Based Vaccine against a Lethal Respiratory Challenge with Streptococcus Pneumoniae in Mice. Clin. Vaccine Immunol. 2012, 19, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Racedo, S.; Villena, J.; Medina, M.; Agüero, G.; Rodríguez, V.; Alvarez, S. Lactobacillus Casei Administration Reduces Lung Injuries in a Streptococcus Pneumoniae Infection in Mice. Microbes Infect. 2006, 8, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.A.; Etesami, N.S.; Shenoy, A.T.; Arafa, E.I.; Lyon de Ana, C.; Smith, N.M.S.; Martin, I.M.C.; Goltry, W.N.; Barron, A.M.S.; Browning, J.L.; et al. Lung-Resident Memory B Cells Protect against Bacterial Pneumonia. J. Clin. Investig. 2021, 131, 11. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.M.; Redhu, N.S.; Cheung, E.; Robertson, N.G.; Patik, I.; El Sayed, S.; Thompson, C.M.; Herd, M.; Lucas, K.B.; Conaway, E.; et al. Generation of Protective Pneumococcal-Specific Nasal Resident Memory CD4+ T Cells via Parenteral Immunization. Mucosal Immunol. 2020, 13, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.M.; Wasserman, G.A.; Coleman, F.T.; Hilliard, K.L.; Yamamoto, K.; Lipsitz, E.; Malley, R.; Dooms, H.; Jones, M.R.; Quinton, L.J.; et al. Regionally Compartmentalized Resident Memory T Cells Mediate Naturally Acquired Protection against Pneumococcal Pneumonia. Mucosal Immunol. 2018, 11, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.T.; Wasserman, G.A.; Arafa, E.I.; Wooten, A.K.; Smith, N.M.S.; Martin, I.M.C.; Jones, M.R.; Quinton, L.J.; Mizgerd, J.P. Lung CD4+ Resident Memory T Cells Remodel Epithelial Responses to Accelerate Neutrophil Recruitment during Pneumonia. Mucosal Immunol. 2020, 13, 334–343. [Google Scholar] [CrossRef]
- Converso, T.R.; Goulart, C.; Rodriguez, D.; Darrieux, M.; Leite, L.C.C. Systemic Immunization with RPotD Reduces Streptococcus Pneumoniae Nasopharyngeal Colonization in Mice. Vaccine 2017, 35, 149–155. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentini, M.M.; Rodriguez, D.; Kanno, A.I.; Goulart, C.; Darrieux, M.; de Cerqueira Leite, L.C. Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice. Vaccines 2024, 12, 122. https://doi.org/10.3390/vaccines12020122
Trentini MM, Rodriguez D, Kanno AI, Goulart C, Darrieux M, de Cerqueira Leite LC. Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice. Vaccines. 2024; 12(2):122. https://doi.org/10.3390/vaccines12020122
Chicago/Turabian StyleTrentini, Monalisa Martins, Dunia Rodriguez, Alex Issamu Kanno, Cibelly Goulart, Michelle Darrieux, and Luciana Cezar de Cerqueira Leite. 2024. "Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice" Vaccines 12, no. 2: 122. https://doi.org/10.3390/vaccines12020122
APA StyleTrentini, M. M., Rodriguez, D., Kanno, A. I., Goulart, C., Darrieux, M., & de Cerqueira Leite, L. C. (2024). Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice. Vaccines, 12(2), 122. https://doi.org/10.3390/vaccines12020122





