Immune Persistence following Primary Immunization and the Immunogenicity and Safety of a Booster Dose of a Multidose Sabin Strain-Based Inactivated Polio Vaccine in Infants Aged 18 Months
Abstract
1. Introduction
2. Methods
2.1. Study Design
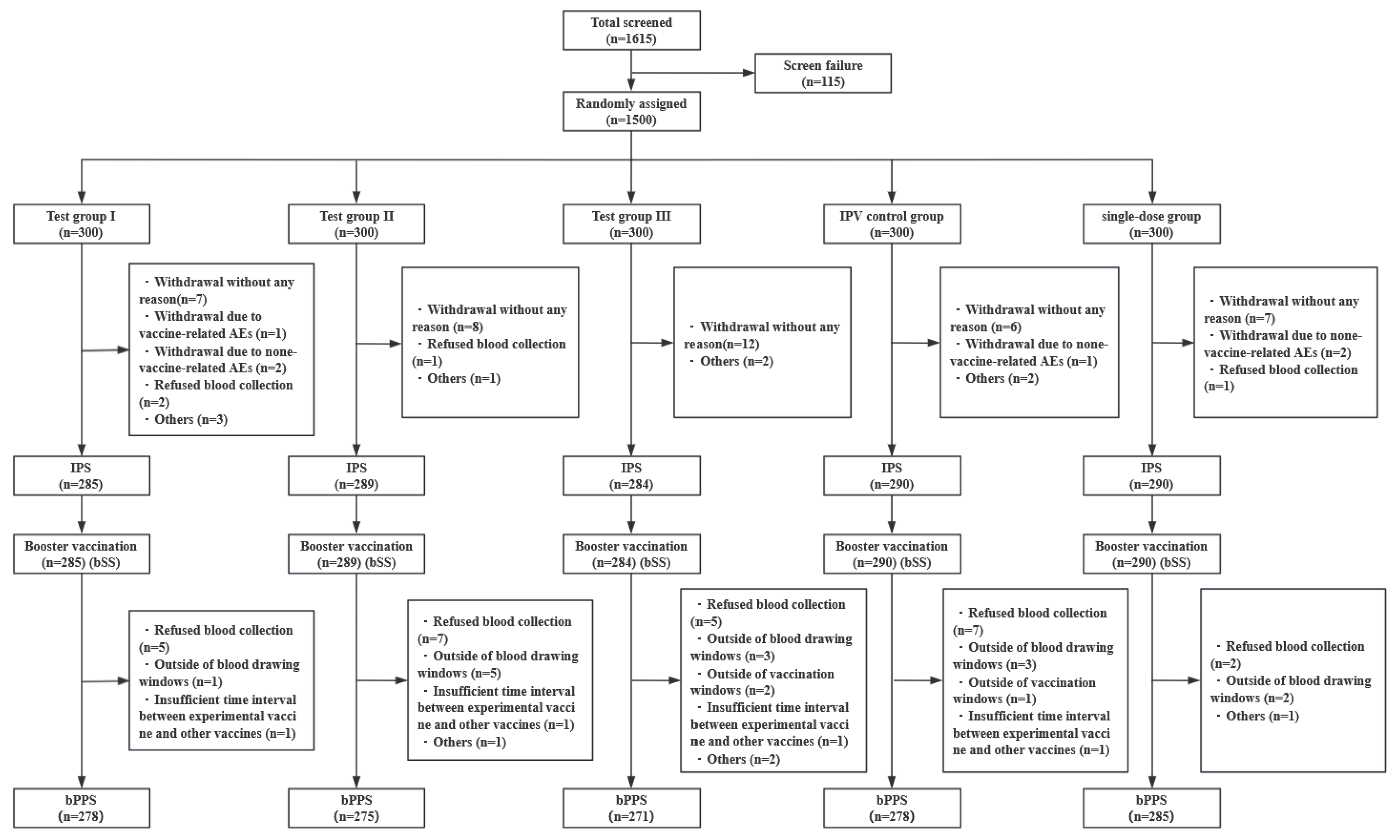
2.2. Participants
2.3. Randomization and Blinding
2.4. Vaccines
2.5. Procedures
2.6. Laboratory Testing
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Immune Persistence
3.3. Booster Immunogenicity
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Poliomyelitis. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 23 August 2023).
- Global Polio Eradication Initiative. The Vaccines. Available online: https://polioeradication.org/polio-today/polio-prevention/the-vaccines/ (accessed on 23 August 2023).
- World Health Organization. Polio Eradication Strategy 2022–2026: Executive Summary. Available online: https://iris.who.int/handle/10665/341938 (accessed on 18 January 2024).
- World Health Organization. Does Polio Still Exist? Is It Curable? Available online: http://www.who.int/features/qa/07/en/ (accessed on 26 August 2023).
- World Health Organization. Polio Vaccines: WHO Position Paper—June 2022. Available online: https://www.who.int/publications/i/item/WHO-WER9725-277-300 (accessed on 14 September 2023).
- Yu, W.-Z.; Wen, N.; Zhang, Y.; Wang, H.-B.; Fan, C.-X.; Zhu, S.-L.; Xu, W.-B.; Liang, X.-F.; Luo, H.-M.; Li, L. Poliomyelitis eradication in China: 1953–2012. J. Infect. Dis. 2014, 210 (Suppl. S1), S268–S274. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Vaccine-Derived Polioviruses. Available online: https://polioeradication.org/polio-today/polio-prevention/the-virus/vaccine-derived-polio-viruses/ (accessed on 27 August 2023).
- Ming, L.C.; Hussain, Z.; Yeoh, S.F.; Koh, D.; Lee, K.S. Circulating vaccine-derived poliovirus: A menace to the end game of polio eradication. Glob. Health 2020, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Circulating Vaccine-Derived Poliovirus Type 2—Global Update. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/circulating-vaccine-derived-poliovirus-type-2-global-update (accessed on 29 August 2023).
- Pallansch, M.A. Ending Use of Oral Poliovirus Vaccine—A Difficult Move in the Polio Endgame. N. Engl. J. Med. 2018, 379, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, H.; Sein, C.; Hamidi, A.; Bakker, W.A.M.; Sutter, R.W. Development of inactivated poliovirus vaccine from Sabin strains: A progress report. Biol. J. Int. Assoc. Biol. Stand. 2016, 44, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, H.; Sutter, R.W.; Jafari, H.S.; Takane, M.; Aylward, R.B. Affordable inactivated poliovirus vaccine: Strategies and progress. J. Infect. Dis. 2014, 210 (Suppl. S1), S459–S464. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine 2016, 34, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Bakker, W.A.M.; Thomassen, Y.E.; Oever, A.G.v.t.; Westdijk, J.; Oijen, M.G.C.T.v.; Sundermann, L.C.; Veld, P.v.t.; Sleeman, E.; Nimwegen, F.W.v.; Hamidi, A.; et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine 2011, 29, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Z.; Zhu, W.B. The role of Sabin inactivated poliovirus vaccine in the final phase of global polio eradication. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2016, 50, 1032–1035. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, D.; Han, W.; Xie, Z.; Jiang, Z.; Huang, L.; Wang, J.; Zhang, W.; Xu, L.; Tan, J.; et al. Safety, immunogenicity, and lot-to-lot consistency of a multidose Sabin strain-based inactivated polio vaccine: A phase III, randomized, blinded, positive-control clinical trial in infants aged 2 months. Int. J. Infect. Dis. IJID 2023, 130, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210 (Suppl. S1), S283–S293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations for Routine Immunization—Summary Tables. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/who-recommendations-for-routine-immunization---summary-tables (accessed on 15 September 2023).
- Platt, L.R.; Estívariz, C.F.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014, 210 (Suppl. S1), S380–S389. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, A.; Bakker, W.A.M. Innovative IPV from attenuated Sabin poliovirus or newly designed alternative seed strains. Pharm. Pat. Anal. 2012, 1, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bird, C.; Holland, D.; Joshi, S.B.; Volkin, D.B. Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Hum. Vaccines Immunother. 2022, 18, 2154100. [Google Scholar] [CrossRef]
- Li, R.; Li, C.G.; Li, Y.; Liu, Y.; Zhao, H.; Chen, X.; Kuriyakose, S.; Meeren, O.V.D.; Hardt, K.; Hezareh, M.; et al. Primary and booster vaccination with an inactivated poliovirus vaccine (IPV) is immunogenic and well-tolerated in infants and toddlers in China. Vaccine 2016, 34, 1436–1443. [Google Scholar] [CrossRef]
- Li, R.C.; Li, F.X.; Li, Y.P.; Hou, Q.M.; Li, C.G.; Li, Y.N.; Chen, F.S.; Hu, X.Z.; Su, W.B.; Zhang, S.M.; et al. Antibody persistence at 18–20 months of age and safety and immunogenicity of a booster dose of a combined DTaP-IPV//PRP∼T vaccine compared to separate vaccines (DTaP, PRP∼T and IPV) following primary vaccination of healthy infants in the People’s Republic of China. Vaccine 2011, 29, 9337–9344. [Google Scholar] [CrossRef]
- Liao, G.; Li, R.; Li, C.; Sun, M.; Jiang, S.; Li, Y.; Mo, Z.; Xia, J.; Xie, Z.; Che, Y.; et al. Phase 3 Trial of a Sabin Strain-Based Inactivated Poliovirus Vaccine. J. Infect. Dis. 2016, 214, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Han, W.; Jiang, D.; Jiang, Z.; Zhu, T.; Xu, W.; Hu, Y.; Zeng, G. Cross-neutralization Capacity of Immune Serum from Different Dosage of Sabin Inactivated Poliovirus Vaccine Immunization against Multiple Individual Polioviruses. Expert Rev. Vaccines 2021, 20, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Li, Y.; Yu, D.; Song, Y.; Liu, S.; Xue, F.; Shan, Y.; Meng, W.; Pan, H. Immunogenicity and immune persistence in 4-year-old children completing four doses of Sabin strain or wild strain inactivated poliovirus vaccine: A phase IV, open-labeled, parallel-controlled observational study. Vaccine 2023, 41, 3467–3471. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ying, Z.; Cai, W.; Wang, J.; Zhou, J.; Yang, H.; Gao, J.; Zhao, Z.; Liu, J.; Ouyang, S.; et al. Immune persistence of an inactivated poliovirus vaccine derived from the Sabin strain: A 10-year follow-up of a phase 3 study. EClinicalMedicine 2023, 64, 102151. [Google Scholar] [CrossRef] [PubMed]
| Characteristics (Stage One) (bSS) | Infants Aged 2 Months (n = 24) | |||
|---|---|---|---|---|
| Age at enrollment (days), mean (SD) | 2.47 (0.26) | |||
| Male n (%) | 10 (41.67) | |||
| Axillary temperature (°C), mean (SD) | 36.65 (0.24) | |||
| Height (cm), mean (SD) | 61.02 (2.23) | |||
| Weight (kg), (mean (SD) | 6.52 (0.68) | |||
| Characteristics (Stage two) (bSS) | 5-dose sIPV (n = 858) | IPV (n = 290) | Sing-dose sIPV (n = 290) | p Value |
| Age at enrollment (days), mean ± SD) | 2.41 (0.27) | 2.39 (0.26) | 2.41 (0.25) | 0.5502 |
| Han ethnic n (%) | 854 (99.53) | 289 (99.66) | 290 (100.00) | 0.8266 |
| Male n (%) | 455 (53.03) | 141 (48.62) | 159 (54.83) | 0.2898 |
| Axillary temperature (°C), mean (SD) | 36.66 (0.24) | 36.66 (0.23) | 36.66 (0.23) | 0.9925 |
| Height (cm), mean (SD) | 60.52 (2.34) | 60.31 (2.49) | 60.64 (2.56) | 0.2433 |
| Weight (kg), mean (SD) | 6.36 (0.79) | 6.30 (0.83) | 6.41 (0.79) | 0.2278 |
| Variable | 5-Dose sIPV (n = 858) | IPV (n = 290) | Sing-Dose sIPV (n = 290) | p Value |
|---|---|---|---|---|
| Serotype 1 | ||||
| SPRs (≥1:8) (95%CI) | 100.00 (99.57, 100.00) | 100.00 (98.74, 100.00) | 99.66 (98.09, 99.99) | 0.4033 |
| GMTs (95%CI) | 642.57 (593.51, 695.69) | 193.05 (169.94, 219.30) | 701.65 (611.75, 804.77) | <0.0001 |
| GMIs (95%CI) | 0.23 (0.22, 0.25) | 0.35 (0.31, 0.40) | 0.23 (0.20, 0.26) | <0.0001 |
| Serotype 2 | ||||
| SPRs (≥1:8) (95%CI) | 99.88 (99.35, 100.00) | 98.97 (97.01, 99.79) | 100.00 (98.74, 100.00) | 0.0652 |
| GMTs (95%CI) | 312.63 (290.67, 336.25) | 142.34 (124.43, 162.83) | 327.51 (287.52, 373.07) | <0.0001 |
| GMIs (95%CI) | 0.67 (0.63, 0.72) | 0.73 (0.64, 0.84) | 0.63 (0.56, 0.72) | 0.2454 |
| Serotype 3 | ||||
| SPRs (≥1:8) (95%CI) | 99.53 (98.81, 99.87) | 97.23 (95.55, 99.24) | 99.66 (98.09, 99.99) | 0.0298 |
| GMTs (95%CI) | 325.81 (300.15, 353.67) | 130.54 (112.45, 151.53) | 365.23 (320.12, 416.69) | <0.0001 |
| GMIs (95%CI) | 0.16 (0.15, 0.18) | 0.13 (0.11, 0.15) | 0.16 (0.14, 0.19) | 0.0076 |
| Variable | 5-Dose sIPV (n = 824) | IPV (n = 278) | Sing-Dose sIPV (n = 285) | p Value a | p Value b |
|---|---|---|---|---|---|
| Serotype 1 | |||||
| SPRs (≥1:8) (95%CI) | 100.00 (99.55, 100.00) | 100.00 (98.68, 100.00) | 100.00 (98.71, 100.00) | / | / |
| SCRs (95%CI) | 91.26 (89.12, 93.10) | 92.45 (88.68, 95.26) | 89.47 (85.31, 92.78) | 0.5389 | 0.3661 |
| GMTs (95%CI) | 9962.89 (9530.88, 10,414.49) | 4086.46 (3686.81, 4529.43) | 10202.27 (9509.51, 10,945.49) | <0.0001 | 0.5877 |
| GMIs (95%CI) | 15.76 (14.54, 17.09) | 20.73 (17.76, 24.20) | 14.59 (12.78, 16.67) | 0.0012 | 0.3378 |
| Serotype 2 | |||||
| SPRs (≥1:8) (95%CI) | 100.00 (99.55, 100.00) | 100.00 (98.68, 100.00) | 100.00 (98.71, 100.00) | / | / |
| SCRs (95%CI) | 97.82 (96.57, 98.70) | 94.24 (90.82, 96.67) | 98.25 (95.95, 99.43) | 0.0029 | 0.6720 |
| GMTs (95%CI) | 10273.00 (9883.32, 10,678.04) | 4141.80 (3728.04, 4601.49) | 10859.77 (10,230.53, 11,527.72) | <0.0001 | 0.1436 |
| GMIs (95%CI) | 33.15 (30.68, 35.82) | 29.03 (24.87, 33.89) | 33.19 (29.13, 37.82) | 0.1050 | 0.9884 |
| Serotype 3 | |||||
| SPRs (≥1:8) (95%CI) | 100.00 (99.55, 100.00) | 100.00 (98.68, 100.00) | 100.00 (98.71, 100.00) | / | / |
| SCRs (95%CI) | 93.81 (91.94, 95.36) | 96.40 (93.48, 98.26) | 92.28 (88.55, 95.10) | 0.1025 | 0.3708 |
| GMTs (95%CI) | 7870.21 (7507.80, 8250.10) | 6844.97 (6253.64, 7492.22) | 8046.47 (7455.01, 8684.86) | 0.0046 | 0.6359 |
| GMIs (95%CI) | 24.50 (22.44, 26.75) | 51.32 (43.96, 59.92) | 22.24 (19.28, 25.64) | <0.0001 | 0.2655 |
| Stage one | Infants aged 18 months (n = 24) | |||
| Overall | 1 (4.17) | |||
| Solicited | 1 (4.17) | |||
| Local | 1 (4.17) | |||
| Systemic | 0 (0.00) | |||
| Unsolicited | 0 (0.00) | |||
| Stage two | 5-dose sIPV (n = 858) | IPV (n = 290) | Sing-dose sIPV (n = 290) | pValue |
| Overall | 77 (8.97) | 21 (7.24) | 27 (9.31) | 0.6237 |
| Solicited | 72 (8.39) | 20 (6.90) | 24 (8.28) | 0.7369 |
| Systemic | 66 (7.69) | 19 (6.55) | 24 (8.28) | 0.7390 |
| Fever | 61 (7.10) | 16 (5.52) | 23 (7.96) | 0.4845 |
| Vomiting | 3 (0.35) | 2 (0.69) | 3 (1.04) | 0.2519 |
| Irritability | 1 (0.12) | 1 (0.34) | 1 (0.34) | 0.3567 |
| Acute allergic reaction | 1 (0.12) | 0 (0.00) | 0 (0.00) | 1.0000 |
| Local | 6 (0.70) | 1 (0.34) | 0 (0.00) | 0.4480 |
| Redness | 5 (0.58) | 1 (0.34) | 0 (0.00) | 0.6352 |
| Tenderness | 1 (0.12) | 0 (0.00) | 0 (0.00) | 1.0000 |
| Unsolicited | 5 (0.58) | 1 (0.34) | 5 (1.73) | 0.1375 |
| Diarrhea | 5 (0.58) | 1 (0.34) | 5 (1.73) | 0.1230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Shao, M.; Wang, J.; Huang, L.; Tan, J.; Jiang, Z.; You, W.; Li, Y.; Yang, Y.; Li, J.; et al. Immune Persistence following Primary Immunization and the Immunogenicity and Safety of a Booster Dose of a Multidose Sabin Strain-Based Inactivated Polio Vaccine in Infants Aged 18 Months. Vaccines 2024, 12, 123. https://doi.org/10.3390/vaccines12020123
Feng G, Shao M, Wang J, Huang L, Tan J, Jiang Z, You W, Li Y, Yang Y, Li J, et al. Immune Persistence following Primary Immunization and the Immunogenicity and Safety of a Booster Dose of a Multidose Sabin Strain-Based Inactivated Polio Vaccine in Infants Aged 18 Months. Vaccines. 2024; 12(2):123. https://doi.org/10.3390/vaccines12020123
Chicago/Turabian StyleFeng, Guangwei, Ming Shao, Jianfeng Wang, Lili Huang, Jian Tan, Zhiwei Jiang, Wangyang You, Yurong Li, Yonghui Yang, Jing Li, and et al. 2024. "Immune Persistence following Primary Immunization and the Immunogenicity and Safety of a Booster Dose of a Multidose Sabin Strain-Based Inactivated Polio Vaccine in Infants Aged 18 Months" Vaccines 12, no. 2: 123. https://doi.org/10.3390/vaccines12020123
APA StyleFeng, G., Shao, M., Wang, J., Huang, L., Tan, J., Jiang, Z., You, W., Li, Y., Yang, Y., Li, J., & Wang, Y. (2024). Immune Persistence following Primary Immunization and the Immunogenicity and Safety of a Booster Dose of a Multidose Sabin Strain-Based Inactivated Polio Vaccine in Infants Aged 18 Months. Vaccines, 12(2), 123. https://doi.org/10.3390/vaccines12020123





