Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines
Abstract
1. Introduction
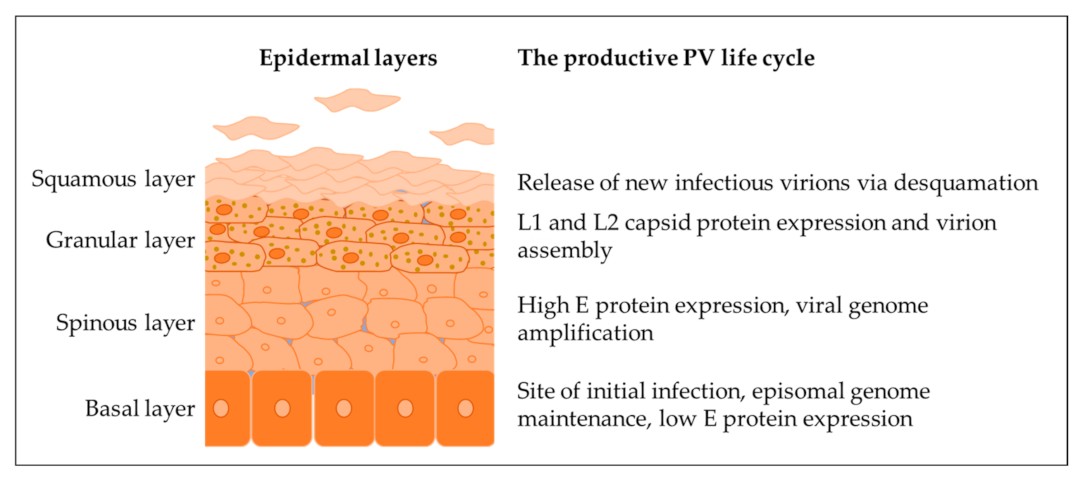
2. Association of Bovine Papillomaviruses with Sarcoid Disease
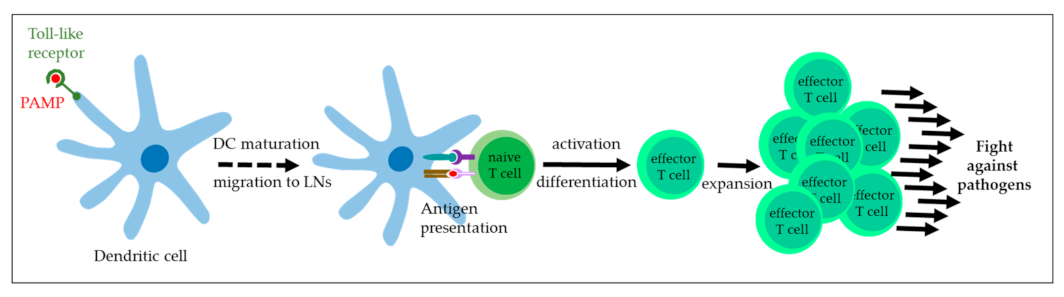
3. BPV Immune Escape in the Equid Host
4. Immunotherapy of Sarcoids
4.1. Toll-like Receptor Agonists
4.2. Immunostimulatory Cytokines
4.3. Recombinant Virus-like Particles
4.4. Autologous Vaccination
4.5. Influenza Virus Vector-Mediated Immunotherapy
5. Future and New Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knottenbelt, D.C. A suggested clinical classification for the equine sarcoid. Clin. Tech. Equine Pract. 2005, 4, 278–295. [Google Scholar] [CrossRef]
- Nasir, L.; Reid, S.W.J. Bovine papillomaviruses and equine sarcoids. In Papillomavirus Research: From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; Volume 1, pp. 389–397. [Google Scholar]
- Scott, D.W.; Miller, W.H., Jr. Sarcoid. Equine Dermatol. 2003, 1, 719–731. [Google Scholar]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Campo, M.S. Introduction. In Papillomavirus Research: From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; pp. 1–2. [Google Scholar]
- Campo, M.S. Bovine papillomavirus: Old system, new lessons? In Papillomavirus Research: From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; pp. 373–387. [Google Scholar]
- Olthof, N.C.; Huebbers, C.U.; Kolligs, J.; Henfling, M.; Ramaekers, F.C.; Cornet, I.; van Lent-Albrechts, J.A.; Stegmann, A.P.; Silling, S.; Wieland, U.; et al. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int. J. Cancer 2015, 136, E207–E218. [Google Scholar] [CrossRef] [PubMed]
- Pett, M.; Coleman, N. Integration of high-risk human papillomavirus: A key event in cervical carcinogenesis? J. Pathol. 2007, 212, 356–367. [Google Scholar] [CrossRef]
- Walline, H.M.; Komarck, C.M.; McHugh, J.B.; Bellile, E.L.; Brenner, J.C.; Prince, M.E.; McKean, E.L.; Chepeha, D.B.; Wolf, G.T.; Worden, F.P.; et al. Genomic Integration of High-Risk HPV Alters Gene Expression in Oropharyngeal Squamous Cell Carcinoma. Mol. Cancer Res. 2016, 14, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Olson, C., Jr.; Cook, R.H. Cutaneous sarcoma-like lesions of the horse caused by the agent of bovine papilloma. Exp. Biol. Med. 1951, 77, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.; Ellsmore, V.A.; O’Brien, P.M.; Reid, S.W.; Love, S.; Campo, M.S.; Nasir, L. Association of bovine papillomavirus with the equine sarcoid. J. Gen. Virol. 2003, 84, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; de Alcantara, B.K.; Otonel, R.A.; Rodrigues, W.B.; Alfieri, A.F.; Alfieri, A.A. Bovine papillomavirus type 13 DNA in equine sarcoids. J. Clin. Microbiol. 2013, 51, 2167–2171. [Google Scholar] [CrossRef]
- Jindra, C.; Kamjunke, A.K.; Jones, S.; Brandt, S. Screening for bovine papillomavirus type 13 (BPV13) in a European population of sarcoid-bearing equids. Equine Vet. J. 2021, 54, 662–669. [Google Scholar] [CrossRef]
- Amtmann, E.; Muller, H.; Sauer, G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J. Virol. 1980, 35, 962–964. [Google Scholar] [CrossRef]
- Bogaert, L.; Martens, A.; Kast, W.M.; Van Marck, E.; De Cock, H. Bovine papillomavirus DNA can be detected in keratinocytes of equine sarcoid tumors. Vet. Microbiol. 2010, 146, 269–275. [Google Scholar] [CrossRef]
- Brandt, S.; Haralambus, R.; Shafti-Keramat, S.; Steinborn, R.; Stanek, C.; Kirnbauer, R. A subset of equine sarcoids harbours BPV-1 DNA in a complex with L1 major capsid protein. Virology 2008, 375, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Armstrong, E.L.; Gofton, R.G.; Mason, J.; De Toit, N.; Day, M.J. Characterisation of early and late bovine papillomavirus protein expression in equine sarcoids. Vet. Microbiol. 2013, 162, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, L.; Martens, A.; Van Poucke, M.; Ducatelle, R.; De Cock, H.; Dewulf, J.; De Baere, C.; Peelman, L.; Gasthuys, F. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses. Vet. Microbiol. 2008, 129, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.A.; Theon, A.P.; Madewell, B.R.; Griffey, S.M.; Hitchcock, M.E. Bovine papillomavirus DNA in neoplastic and nonneoplastic tissues obtained from horses with and without sarcoids in the western United States. Am. J. Vet. Res. 2001, 62, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; De Moor, A.; Ducatelle, R. PCR detection of bovine papilloma virus DNA in superficial swabs and scrapings from equine sarcoids. Vet. J. 2001, 161, 280–286. [Google Scholar] [CrossRef]
- Trenfield, K.; Spradbrow, P.B.; Vanselow, B. Sequences of papillomavirus DNA in equine sarcoids. Equine Vet. J. 1985, 17, 449–452. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. Sarcoid. In Pascoe’s Principles and Practice of Equine Dermatology; Knottenbelt, D.C., Ed.; Saunders Elsevier: London, UK, 2009; pp. 387–407. [Google Scholar]
- Knottenbelt, D.C. The Equine Sarcoid: Why Are There so Many Treatment Options? Vet. Clin. N. Am. Equine Pract. 2019, 35, 243–262. [Google Scholar] [CrossRef]
- Tarwid, J.N.; Fretz, P.B.; Clark, E.G. Equine sarcoids: A study with emphasis on pathological diagnosis. Compend. Contin. Educ. Pract. Vet. 1985, 7, 293–300. [Google Scholar]
- Chow, L.T.; Broker, T.R. Mechanisms and regulation of papillomavirus DNA replication. In Papillomavirus Research: From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; pp. 53–71. [Google Scholar]
- Hainisch, E.K.; Jindra, C.; Reicher, P.; Miglinci, L.; Brodesser, D.M.; Brandt, S. Bovine Papillomavirus Type 1 or 2 Virion-Infected Primary Fibroblasts Constitute a Near-Natural Equine Sarcoid Model. Viruses 2022, 14, 2658. [Google Scholar] [CrossRef]
- Yuan, Z.; Gault, E.A.; Campo, M.S.; Nasir, L. Different contribution of bovine papillomavirus type 1 oncoproteins to the transformation of equine fibroblasts. J. Gen. Virol. 2011, 92, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Gault, E.A.; Gobeil, P.; Nixon, C.; Campo, M.S.; Nasir, L. Establishment and characterization of equine fibroblast cell lines transformed in vivo and in vitro by BPV-1: Model systems for equine sarcoids. Virology 2008, 373, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, G.; Iovane, G.; Marcante, M.L.; Poggiali, F.; Roperto, F.; Roperto, S.; Venuti, A. Presence of bovine papillomavirus type 2 DNA and expression of the viral oncoprotein E5 in naturally occurring urinary bladder tumours in cows. J. Gen. Virol. 2003, 84, 2921–2926. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Foster, S.A.; McDougall, J.K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996, 380, 79–82. [Google Scholar] [CrossRef]
- Petti, L.; DiMaio, D. Specific interaction between the bovine papillomavirus E5 transforming protein and the beta receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J. Virol. 1994, 68, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Suprynowicz, F.A.; Campo, M.S.; Schlegel, R. Biologic activities of papillomavirus E5 proteins. In Papillomavirus Research: From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; Volume 1, pp. 97–114. [Google Scholar]
- Tong, X.; Howley, P.M. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 1997, 94, 4412–4417. [Google Scholar] [CrossRef] [PubMed]
- DeMasi, J.; Huh, K.W.; Nakatani, Y.; Munger, K.; Howley, P.M. Bovine papillomavirus E7 transformation function correlates with cellular p600 protein binding. Proc. Natl. Acad. Sci. USA 2005, 102, 11486–11491. [Google Scholar] [CrossRef]
- Brandt, S. Immune response to bovine papillomavirus type 1 in equine sarcoid. Vet. J. 2016, 216, 107–108. [Google Scholar] [CrossRef]
- Hainisch, E.K.; Abel-Reichwald, H.; Shafti-Keramat, S.; Pratscher, B.; Corteggio, A.; Borzacchiello, G.; Wetzig, M.; Jindra, C.; Tichy, A.; Kirnbauer, R.; et al. Potential of a BPV1 L1 VLP vaccine to prevent BPV1- or BPV2-induced pseudo-sarcoid formation and safety and immunogenicity of EcPV2 L1 VLPs in the horse. J. Gen. Virol. 2016, 98, 230–241. [Google Scholar] [CrossRef]
- Hainisch, E.K.; Brandt, S.; Shafti-Keramat, S.; Van den Hoven, R.; Kirnbauer, R. Safety and immunogenicity of BPV-1 L1 virus-like particles in a dose-escalation vaccination trial in horses. Equine Vet. J. 2012, 44, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.; Hainisch, E.K.; Shafti-Keramat, S.; Kirnbauer, R.; Corteggio, A.; Borzacchiello, G.; Tober, R.; Kainzbauer, C.; Pratscher, B.; Brandt, S. Inoculation of young horses with bovine papillomavirus type 1 virions leads to early infection of PBMCs prior to pseudo-sarcoid formation. J. Gen. Virol. 2011, 92, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Ragland, W.L.; Spencer, G.R. Attempts to relate bovine papilloma virus to the cause of equine sarcoid: Immunity to bovine papilloma virus. Am. J. Vet. Res. 1968, 29, 1363–1366. [Google Scholar] [PubMed]
- Ragland, W.L.; Spencer, G.R. Attempts to relate bovine papilloma virus to the cause of equine sarcoid: Equidae inoculated intradermally with bovine papilloma virus. Am. J. Vet. Res. 1969, 30, 743–752. [Google Scholar]
- Voss, J.L. Transmission of equine sarcoid. Am. J. Vet. Res. 1969, 30, 183–191. [Google Scholar] [PubMed]
- Stanley, M. Host defence and persistent human papillomavirus infection. Curr. Opin. Virol. 2021, 51, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Bennett, L.; Campo, M.S.; Nasir, L. Bovine papillomavirus type 1 E2 and E7 proteins down-regulate Toll Like Receptor 4 (TLR4) expression in equine fibroblasts. Virus Res. 2010, 149, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Nicolson, L.; Marchetti, B.; Gault, E.A.; Campo, M.S.; Nasir, L. Transcriptional changes induced by bovine papillomavirus type 1 in equine fibroblasts. J. Virol. 2008, 82, 6481–6491. [Google Scholar] [CrossRef]
- Nasir, L.; Brandt, S. Papillomavirus associated diseases of the horse. Vet. Microbiol. 2013, 167, 159–167. [Google Scholar] [CrossRef]
- Marchetti, B.; Gault, E.A.; Cortese, M.S.; Yuan, Z.; Ellis, S.A.; Nasir, L.; Campo, M.S. Bovine papillomavirus type 1 oncoprotein E5 inhibits equine MHC class I and interacts with equine MHC I heavy chain. J. Gen. Virol. 2009, 90, 2865–2870. [Google Scholar] [CrossRef]
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. Int. J. Cancer 2018, 142, 224–229. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Cutarelli, A.; Catoi, A.F.; Uberti, B.D.; Cuccaro, B.; Roperto, S. Bovine delta papillomavirus E5 oncoprotein negatively regulates the cGAS-STING signaling pathway in cattle in a spontaneous model of viral disease. Front. Immunol. 2022, 13, 937736. [Google Scholar] [CrossRef]
- De Falco, F.; Cutarelli, A.; Gentile, I.; Cerino, P.; Uleri, V.; Catoi, A.F.; Roperto, S. Bovine Delta Papillomavirus E5 Oncoprotein Interacts with TRIM25 and Hampers Antiviral Innate Immune Response Mediated by RIG-I-Like Receptors. Front. Immunol. 2021, 12, 658762. [Google Scholar] [CrossRef]
- Brostrom, H. Equine sarcoids. A clinical and epidemiological study in relation to equine leucocyte antigens (ELA). Acta Vet. Scand. 1995, 36, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Broström, H.; Fahlbrink, E.; Dubath, M.L.; Lazary, S. Association between equine leucocyte antigens (ELA) and equine sarcoid tumors in the population of Swedish halfbreds and some of their families. Vet. Immunol. Immunopathol. 1988, 19, 215–223. [Google Scholar] [CrossRef]
- Meredith, D.; Elser, A.H.; Wolf, B.; Soma, L.R.; Donawick, W.J.; Lazary, S. Equine leukocyte antigens: Relationships with sarcoid tumors and laminitis in two pure breeds. Immunogenetics 1986, 23, 221–225. [Google Scholar] [CrossRef]
- Triulzi, T.; Tagliabue, E.; Balsari, A.; Casalini, P. FOXP3 expression in tumor cells and implications for cancer progression. J. Cell Physiol. 2013, 228, 30–35. [Google Scholar] [CrossRef]
- Gajewski, T.F. The expanding universe of regulatory T cell subsets in cancer. Immunity 2007, 27, 185–187. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Fang, Y.; Wu, S.; Liu, L.; Fu, D.; Shen, X. Regulatory T cell: A protection for tumour cells. J. Cell. Mol. Med. 2012, 16, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Hicks, C. Both tumour cells and infiltrating T-cells in equine sarcoids express FOXP3 associated with an immune-supressed cytokine microenvironment. Vet. Res. 2016, 47, 55. [Google Scholar] [CrossRef] [PubMed]
- Geisshusler, H.; Marti, E.; Stoffel, M.H.; Kuhni, K.; Stojiljkovic, A.; von Tscharner, C.; Vidondo, B.; Gerber, V.; Koch, C. Quantitative analysis of infiltrating immune cells and bovine papillomavirus type 1 E2-positive cells in equine sarcoids. Vet. J. 2016, 216, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ebert, L.M.; Tan, B.S.; Browning, J.; Svobodova, S.; Russell, S.E.; Kirkpatrick, N.; Gedye, C.; Moss, D.; Ng, S.P.; MacGregor, D.; et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008, 68, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, S.; Wei, H.; Pang, X.; Zhang, H. Roles of Foxp3 in the occurrence and development of cervical cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8717–8730. [Google Scholar] [PubMed]
- Wang, X.; Lang, M.; Zhao, T.; Feng, X.; Zheng, C.; Huang, C.; Hao, J.; Dong, J.; Luo, L.; Li, X.; et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+)Treg cells in pancreatic ductal adenocarcinoma. Oncogene 2017, 36, 3048–3058. [Google Scholar] [CrossRef]
- Ashrafi, G.H.; Piuko, K.; Burden, F.; Yuan, Z.; Gault, E.A.; Muller, M.; Trawford, A.; Reid, S.W.; Nasir, L.; Campo, M.S. Vaccination of sarcoid-bearing donkeys with chimeric virus-like particles of bovine papillomavirus type 1. J. Gen. Virol. 2008, 89, 148–157. [Google Scholar] [CrossRef]
- Vacchelli, E.; Aranda, F.; Obrist, F.; Eggermont, A.; Galon, J.; Cremer, I.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Immunostimulatory cytokines in cancer therapy. Oncoimmunology 2014, 3, e29030. [Google Scholar] [CrossRef]
- Mattil-Fritz, S.; Scharner, D.; Piuko, K.; Thones, N.; Gissmann, L.; Muller, H.; Muller, M. Immunotherapy of equine sarcoid: Dose-escalation trial for the use of chimeric papillomavirus-like particles. J. Gen. Virol. 2008, 89, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Jindra, C.; Hainisch, E.K.; Rummele, A.; Wolschek, M.; Muster, T.; Brandt, S. Influenza virus vector iNS1 expressing bovine papillomavirus 1 (BPV1) antigens efficiently induces tumour regression in equine sarcoid patients. PLoS ONE 2021, 16, e0260155. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef]
- Calmette, A. Preventive Vaccination Against Tuberculosis with BCG. Proc. R. Soc. Med. 1931, 24, 1481–1490. [Google Scholar] [CrossRef]
- Cardillo, F.; Bonfim, M.; da Silva Vasconcelos Sousa, P.; Mengel, J.; Ribeiro Castello-Branco, L.R.; Pinho, R.T. Bacillus Calmette-Guerin Immunotherapy for Cancer. Vaccines 2021, 9, 439. [Google Scholar] [CrossRef]
- Murphy, J.M.; Severin, G.A.; Lavach, J.D.; Hepler, D.I.; Lueker, D.C. Immunotherapy in ocular equine sarcoid. J. Am. Vet. Med. Assoc. 1979, 174, 269–272. [Google Scholar] [PubMed]
- Wyman, M.; Rings, M.D.; Tarr, M.J.; Alden, C.L. Immunotherapy in equine sarcoid: A report of two cases. J. Am. Vet. Med. Assoc. 1977, 171, 751–779. [Google Scholar]
- Klein, W.R.; Bras, G.E.; Misdorp, W.; Steerenberg, P.A.; De Jong, W.H.; Tiesjema, R.H.; Kersjes, A.W.; Ruitenberg, E.J. Equine sarcoid: BCG immunotherapy compared to cryosurgery in a prospective randomised clinical trial. Cancer Immunol. Immunother. 1986, 21, 133–140. [Google Scholar] [CrossRef]
- Knottenbelt, D.C.; Kelly, D.F. The diagnosis and treatment of periorbital sarcoid in the horse: 445 cases from 1974 to 1999. Vet. Ophthalmol. 2000, 3, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; De Moor, A.; Vlaminck, L.; Pille, F.; Steenhaut, M. Evaluation of excision, cryosurgery and local BCG vaccination for the treatment of equine sarcoids. Vet. Rec. 2001, 149, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Komaromy, A.M.; Andrew, S.E.; Brooks, D.E.; Detrisac, C.J.; Gelatt, K.N. Periocular sarcoid in a horse. Vet. Ophthalmol. 2004, 7, 141–146. [Google Scholar] [CrossRef]
- Lavach, J.D.; Severin, G.A.; Lueker, D. Immunotherapy of periocular sarcoids in horses. Vet. Clin. N. Am. Large Anim. Pract. 1984, 6, 513–518. [Google Scholar] [CrossRef]
- Owen, R.A.; Jagger, D.W. Clinical observations on the use of BCG cell wall fraction for treatment of periocular and other equine sarcoids. Vet. Rec. 1987, 120, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.R. Immunotherapy of squamous cell carcinoma of the bovine eye and of equine sarcoid. Tijdschr Diergeneeskd 1990, 115, 1149–1155. [Google Scholar] [PubMed]
- EMA. List of Nationally Authorised Medicinal Products—Active Substance: BCG Vaccine, PSUS_A/00000304/201803; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kollipara, R.; Ekhlassi, E.; Downing, C.; Guidry, J.; Lee, M.; Tyring, S.K. Advancements in Pharmacotherapy for Noncancerous Manifestations of HPV. J. Clin. Med. 2015, 4, 832–846. [Google Scholar] [CrossRef]
- Nogueira, S.A.; Torres, S.M.; Malone, E.D.; Diaz, S.F.; Jessen, C.; Gilbert, S. Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: A pilot study. Vet. Dermatol. 2006, 17, 259–265. [Google Scholar] [CrossRef]
- Pettersson, C.M.; Brostrom, H.; Humblot, P.; Bergvall, K.E. Topical treatment of equine sarcoids with imiquimod 5% cream or Sanguinaria canadensis and zinc chloride—An open prospective study. Vet. Dermatol. 2020, 31, 471-e126. [Google Scholar] [CrossRef] [PubMed]
- Haspeslagh, M.; Vlaminck, L.E.; Martens, A.M. Treatment of sarcoids in equids: 230 cases (2008–2013). J. Am. Vet. Med. Assoc. 2016, 249, 311–318. [Google Scholar] [CrossRef]
- Barros, M.R., Jr.; de Oliveira, T.H.A.; de Melo, C.M.L.; Venuti, A.; de Freitas, A.C. Viral Modulation of TLRs and Cytokines and the Related Immunotherapies for HPV-Associated Cancers. J. Immunol. Res. 2018, 2018, 2912671. [Google Scholar] [CrossRef]
- Jebreel, A.; Mistry, D.; Loke, D.; Dunn, G.; Hough, V.; Oliver, K.; Stafford, N.; Greenman, J. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J. Laryngol. Otol. 2007, 121, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Peghini, B.C.; Abdalla, D.R.; Barcelos, A.C.; Teodoro, L.; Murta, E.F.; Michelin, M.A. Local cytokine profiles of patients with cervical intraepithelial and invasive neoplasia. Hum. Immunol. 2012, 73, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Torres-Poveda, K.; Bahena-Roman, M.; Madrid-Gonzalez, C.; Burguete-Garcia, A.I.; Bermudez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-beta1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Smola-Hess, S.; Pfister, H. Immune evasion in genital papillomavirus infection and cervical cancer: Role of cytokines and chemokines. In Papillomavirus Research—From Natural History to Vaccines and Beyond; Caister Academic Press: Norfolk, UK, 2006; pp. 321–339. [Google Scholar]
- Klein, C.; Waldhauer, I.; Nicolini, V.G.; Freimoser-Grundschober, A.; Nayak, T.; Vugts, D.J.; Dunn, C.; Bolijn, M.; Benz, J.; Stihle, M.; et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 2017, 6, e1277306. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.H.; Joshi, J.V.; Mertia, P.N.; Agashe, S.V.; Vaidya, R.A. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac. J. Cancer Prev. 2014, 15, 3851–3864. [Google Scholar] [CrossRef] [PubMed]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Den Otter, W.; Hill, F.W.; Klein, W.R.; Koten, J.W.; Steerenberg, P.A.; De Mulder, P.H.; Rhode, C.; Stewart, R.; Faber, J.A.; Ruitenberg, E.J.; et al. Therapy of bovine ocular squamous-cell carcinoma with local doses of interleukin-2: 67% complete regressions after 20 months of follow-up. Cancer Immunol. Immunother. 1995, 41, 10–14. [Google Scholar] [CrossRef]
- Spoormakers, T.J.; Klein, W.R.; Jacobs, J.J.; Van Den Ingh, T.S.; Koten, J.W.; Den Otter, W. Comparison of the efficacy of local treatment of equine sarcoids with IL-2 or cisplatin/IL-2. Cancer Immunol. Immunother. 2003, 52, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Loschelder-Ostrowski, J.; Winter, J.C.; Merle, R.; Klopfleisch, R.; Gehlen, H. Treatment of equine sarcoids using recombinant poxviruses expressing feline interleukin-2. Vet. Dermatol. 2021, 32, 283-e277. [Google Scholar] [CrossRef]
- Saba, C.; Eggleston, R.; Parks, A.; Peroni, J.; Sjoberg, E.; Rice, S.; Tyma, J.; Williams, J.; Grosenbaugh, D.; Leard, A.T. ALVAC-fIL2, a feline interleukin-2 immunomodulator, as a treatment for sarcoids in horses: A pilot study. J. Vet. Intern. Med. 2022, 36, 1179–1184. [Google Scholar] [CrossRef]
- Kirnbauer, R. Papillomavirus-like particles for serology and vaccine development. Intervirology 1996, 39, 54–61. [Google Scholar] [CrossRef]
- Schiller, J.T. Papillomavirus Vaccines. Papillomaviruses 2007, 1, 337–369. [Google Scholar]
- Kirnbauer, R.; Chandrachud, L.M.; O’Neil, B.W.; Wagner, E.R.; Grindlay, G.J.; Armstrong, A.; McGarvie, G.M.; Schiller, J.T.; Lowy, D.R.; Campo, M.S. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 1996, 219, 37–44. [Google Scholar] [CrossRef]
- Espy, B.M.K. How to Treat Equine Sarcoids by Autologous Implantation. AAEP Proc. 2008, 54, 68–73. [Google Scholar]
- Rothacker, C.C.; Boyle, A.G.; Levine, D.G. Autologous vaccination for the treatment of equine sarcoids: 18 cases (2009–2014). Can. Vet. J. 2015, 56, 709–714. [Google Scholar] [PubMed]
- Figge, G. Multimodale, Kombinierte Tumortherapie und deren Behandlungserfolg bei hochgradigen Equinen Sarkoiden an der Pferdeklinik der Veterinärmedizinischen Universität Wien von 2001–2020. Master Thesis, Veterinary University, Vienna, Austria, 2022. [Google Scholar]
- Garcia-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.; Brandt, S.; Sereinig, S.; Romanova, J.; Ferko, B.; Katinger, D.; Grassauer, A.; Alexandrova, G.; Katinger, H.; Muster, T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol. 1998, 72, 6437–6441. [Google Scholar] [CrossRef]
- Romanova, J.; Krenn, B.M.; Wolschek, M.; Ferko, B.; Romanovskaja-Romanko, E.; Morokutti, A.; Shurygina, A.P.; Nakowitsch, S.; Ruthsatz, T.; Kiefmann, B.; et al. Preclinical evaluation of a replication-deficient intranasal DeltaNS1 H5N1 influenza vaccine. PLoS ONE 2009, 4, e5984. [Google Scholar] [CrossRef] [PubMed]
- Wacheck, V.; Egorov, A.; Groiss, F.; Pfeiffer, A.; Fuereder, T.; Hoeflmayer, D.; Kundi, M.; Popow-Kraupp, T.; Redlberger-Fritz, M.; Mueller, C.A.; et al. A novel type of influenza vaccine: Safety and immunogenicity of replication-deficient influenza virus created by deletion of the interferon antagonist NS1. J. Infect. Dis. 2010, 201, 354–362. [Google Scholar] [CrossRef]
- Efferson, C.L.; Tsuda, N.; Kawano, K.; Nistal-Villan, E.; Sellappan, S.; Yu, D.; Murray, J.L.; Garcia-Sastre, A.; Ioannides, C.G. Prostate tumor cells infected with a recombinant influenza virus expressing a truncated NS1 protein activate cytolytic CD8+ cells to recognize noninfected tumor cells. J. Virol. 2006, 80, 383–394. [Google Scholar] [CrossRef]
- Ogbomo, H.; Michaelis, M.; Geiler, J.; van Rikxoort, M.; Muster, T.; Egorov, A.; Doerr, H.W.; Cinatl, J., Jr. Tumor cells infected with oncolytic influenza A virus prime natural killer cells for lysis of resistant tumor cells. Med. Microbiol. Immunol. 2010, 199, 93–101. [Google Scholar] [CrossRef]
- Ferko, B.; Kittel, C.; Romanova, J.; Sereinig, S.; Katinger, H.; Egorov, A. Live attenuated influenza virus expressing human interleukin-2 reveals increased immunogenic potential in young and aged hosts. J. Virol. 2006, 80, 11621–11627. [Google Scholar] [CrossRef]
- Kittel, C.; Ferko, B.; Kurz, M.; Voglauer, R.; Sereinig, S.; Romanova, J.; Stiegler, G.; Katinger, H.; Egorov, A. Generation of an influenza A virus vector expressing biologically active human interleukin-2 from the NS gene segment. J. Virol. 2005, 79, 10672–10677. [Google Scholar] [CrossRef]
- Sereinig, S.; Stukova, M.; Zabolotnyh, N.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.; Katinger, H.; Kiselev, O.; Egorov, A. Influenza virus NS vectors expressing the mycobacterium tuberculosis ESAT-6 protein induce CD4+ Th1 immune response and protect animals against tuberculosis challenge. Clin. Vaccine Immunol. 2006, 13, 898–904. [Google Scholar] [CrossRef] [PubMed]
- van Rikxoort, M.; Michaelis, M.; Wolschek, M.; Muster, T.; Egorov, A.; Seipelt, J.; Doerr, H.W.; Cinatl, J., Jr. Oncolytic effects of a novel influenza A virus expressing interleukin-15 from the NS reading frame. PLoS ONE 2012, 7, e36506. [Google Scholar] [CrossRef] [PubMed]
- Wolschek, M.; Samm, E.; Seper, H.; Sturlan, S.; Kuznetsova, I.; Schwager, C.; Khassidov, A.; Kittel, C.; Muster, T.; Egorov, A.; et al. Establishment of a chimeric, replication-deficient influenza A virus vector by modulation of splicing efficiency. J. Virol. 2011, 85, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Jindra, C.; Huber, B.; Shafti-Keramat, S.; Wolschek, M.; Ferko, B.; Muster, T.; Brandt, S.; Kirnbauer, R. Attenuated Recombinant Influenza A Virus Expressing HPV16 E6 and E7 as a Novel Therapeutic Vaccine Approach. PLoS ONE 2015, 10, e0138722. [Google Scholar] [CrossRef]
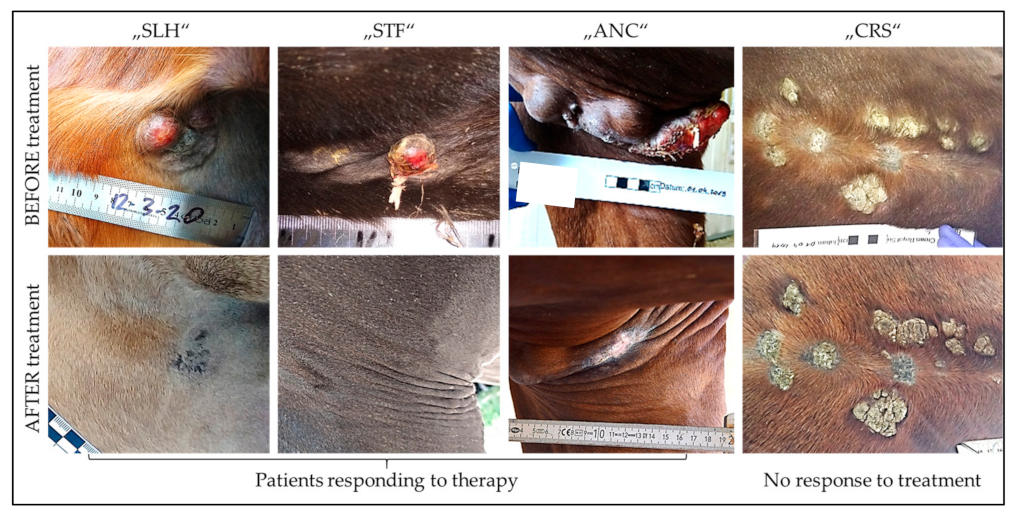
| Treatment Schedule | Used Vaccines | Number and Types of Cases | Therapeutic Outcome | |||
|---|---|---|---|---|---|---|
| COR | PAR | STD | PRD | |||
| Days 1, 3, 5/8, 10, 12 | AAA/BBB | 7 severe | 2/7 | - | 2/7 | 3/7 |
| 1 moderate | 1/1 | - | - | - | ||
| Every second month on average until COR or no further improvement | AAA | 4 severe | 1/4 | 1/4 | - | 2/4 |
| 4 moderate | 2/2 | 2/2 § | - | - | ||
| 2 mild | 2/2 | - | - | - | ||
| AA, then B * | 3 severe | 1/3 | 1/3 | - | 1/3 | |
| 1 mild | 1/1 | - | - | - | ||
| BBB | 3 severe | 1/3 | - | 2/3 | - | |
| BB, then A * | 2 severe | 1/2 | - | 1/2 | - | |
| 2 mild | 2/2 | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindra, C.; Hainisch, E.K.; Brandt, S. Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines. Vaccines 2023, 11, 769. https://doi.org/10.3390/vaccines11040769
Jindra C, Hainisch EK, Brandt S. Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines. Vaccines. 2023; 11(4):769. https://doi.org/10.3390/vaccines11040769
Chicago/Turabian StyleJindra, Christoph, Edmund K. Hainisch, and Sabine Brandt. 2023. "Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines" Vaccines 11, no. 4: 769. https://doi.org/10.3390/vaccines11040769
APA StyleJindra, C., Hainisch, E. K., & Brandt, S. (2023). Immunotherapy of Equine Sarcoids—From Early Approaches to Innovative Vaccines. Vaccines, 11(4), 769. https://doi.org/10.3390/vaccines11040769






