Anti-SARS-CoV-2S Antibody Levels in Healthcare Workers 10 Months after the Administration of Two BNT162b2 Vaccine Doses in View of Demographic Characteristic and Previous COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Determining Antibody Levels
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
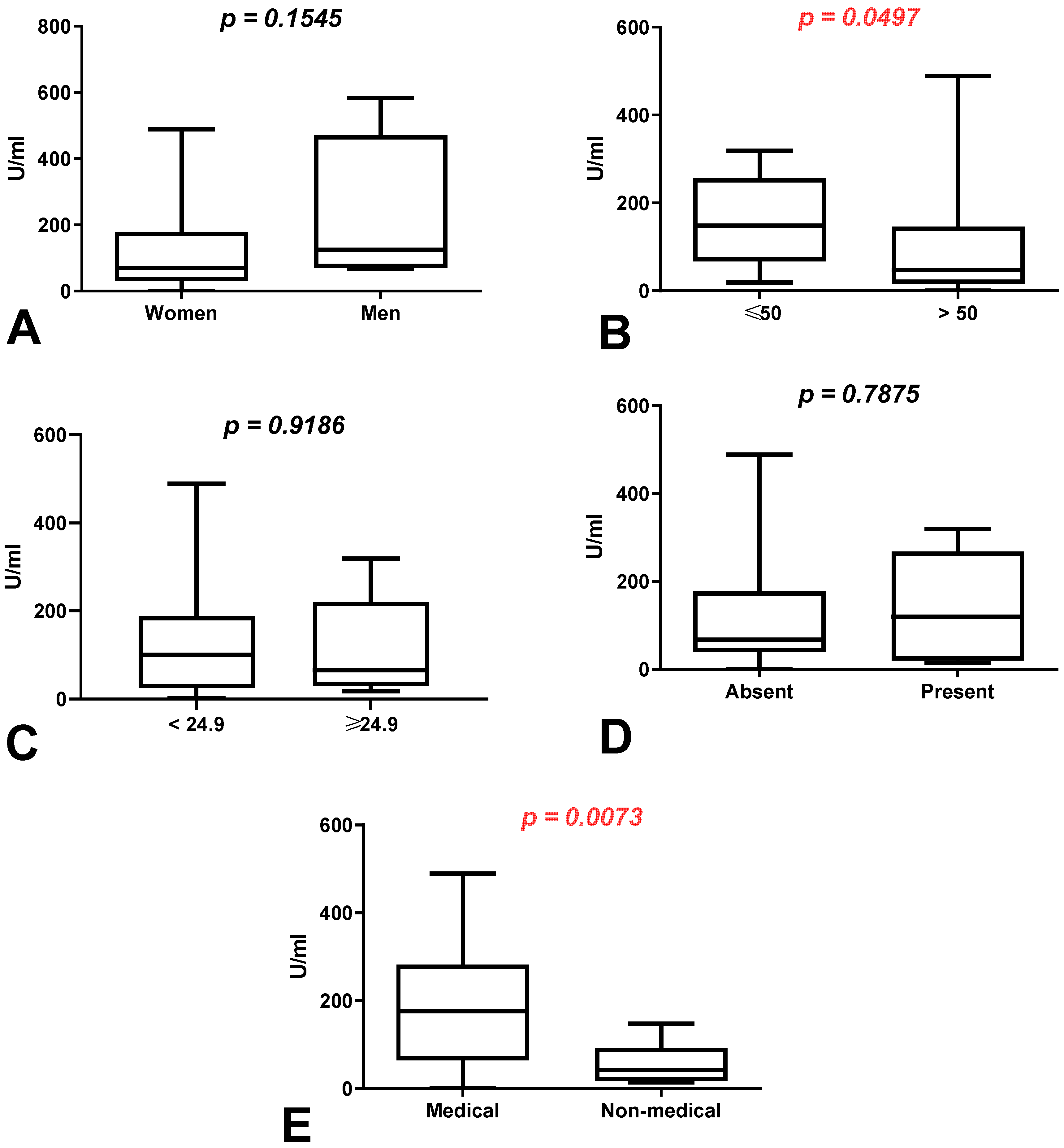
3.2. Comparison of Total Anti-SARS-CoV-2 Antibody Levels According to Sex, Age, BMI, Coexisting Diseases and Work Type in Group of Workers with History of COVID-19
3.3. Comparison of Total Anti-SARS-CoV-2 Antibody Levels According to Sex, Age, BMI, Coexisting Diseases and Work Type in Group of Workers without History of COVID-19
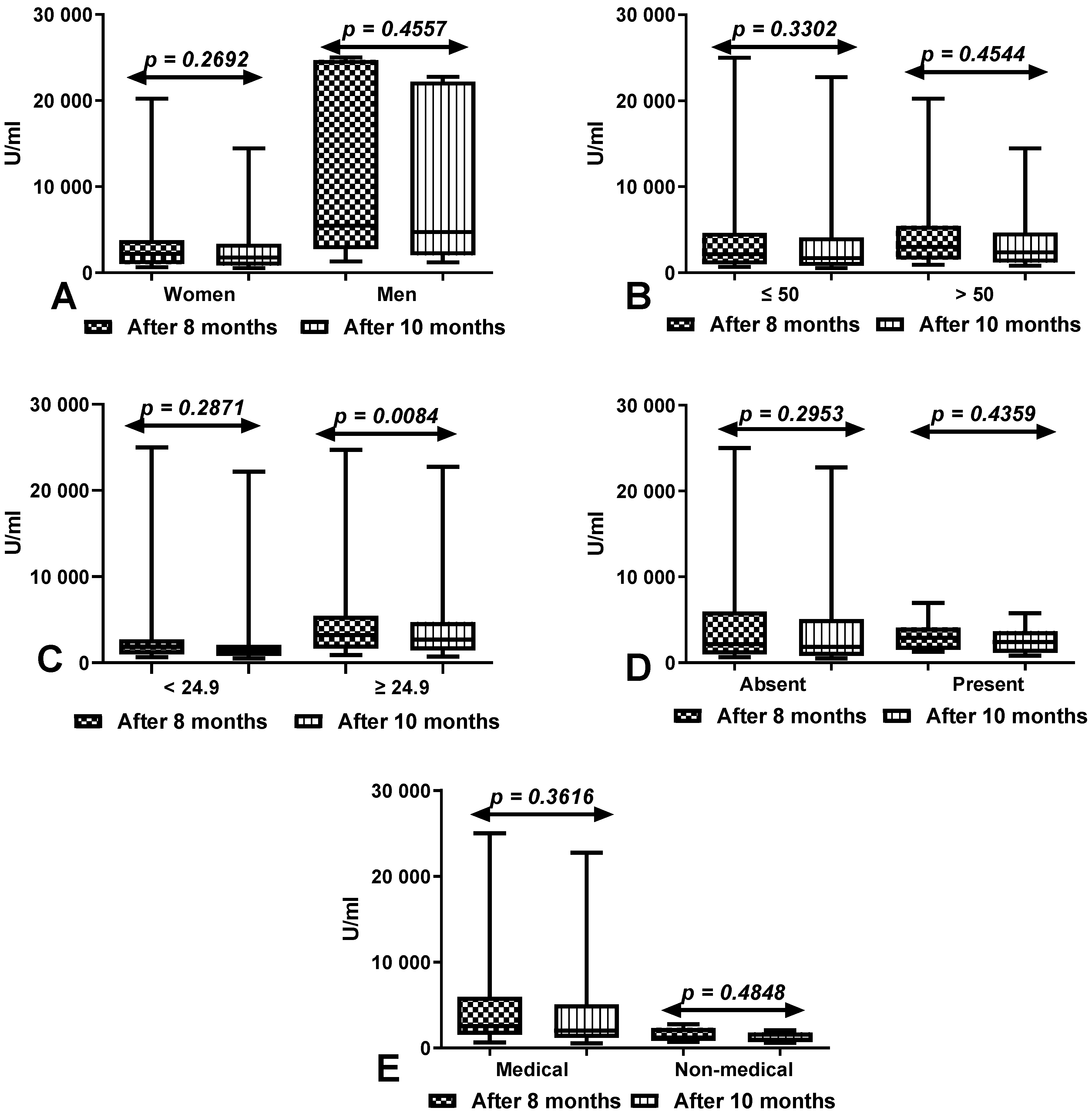
3.4. Comparison of Total Anti-SARS-CoV-2 Antibody Levels between First (after 8 Months) and Second Determination (after 10 Months) According to Sex, Age, BMI, Coexisting Diseases and Work Type in Group of Workers with History of COVID-19
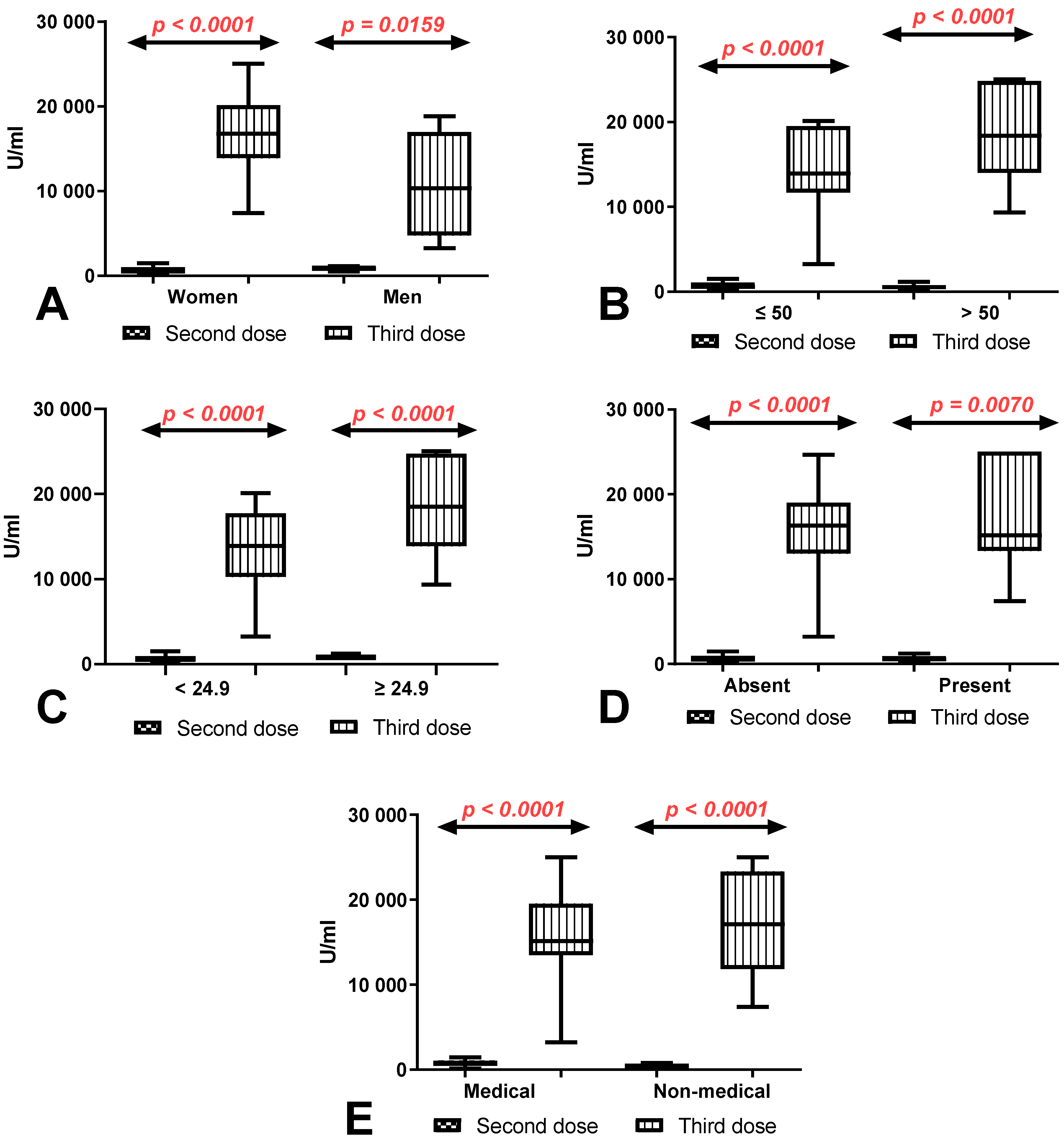
3.5. Comparison of Total Anti-SARS-CoV-2 Antibody Levels between First (after 8 Months) and Second Determination (after 10 Months) According to Sex, Age, BMI, Coexisting Diseases and Work Type in Group of Workers with and without History of COVID-19
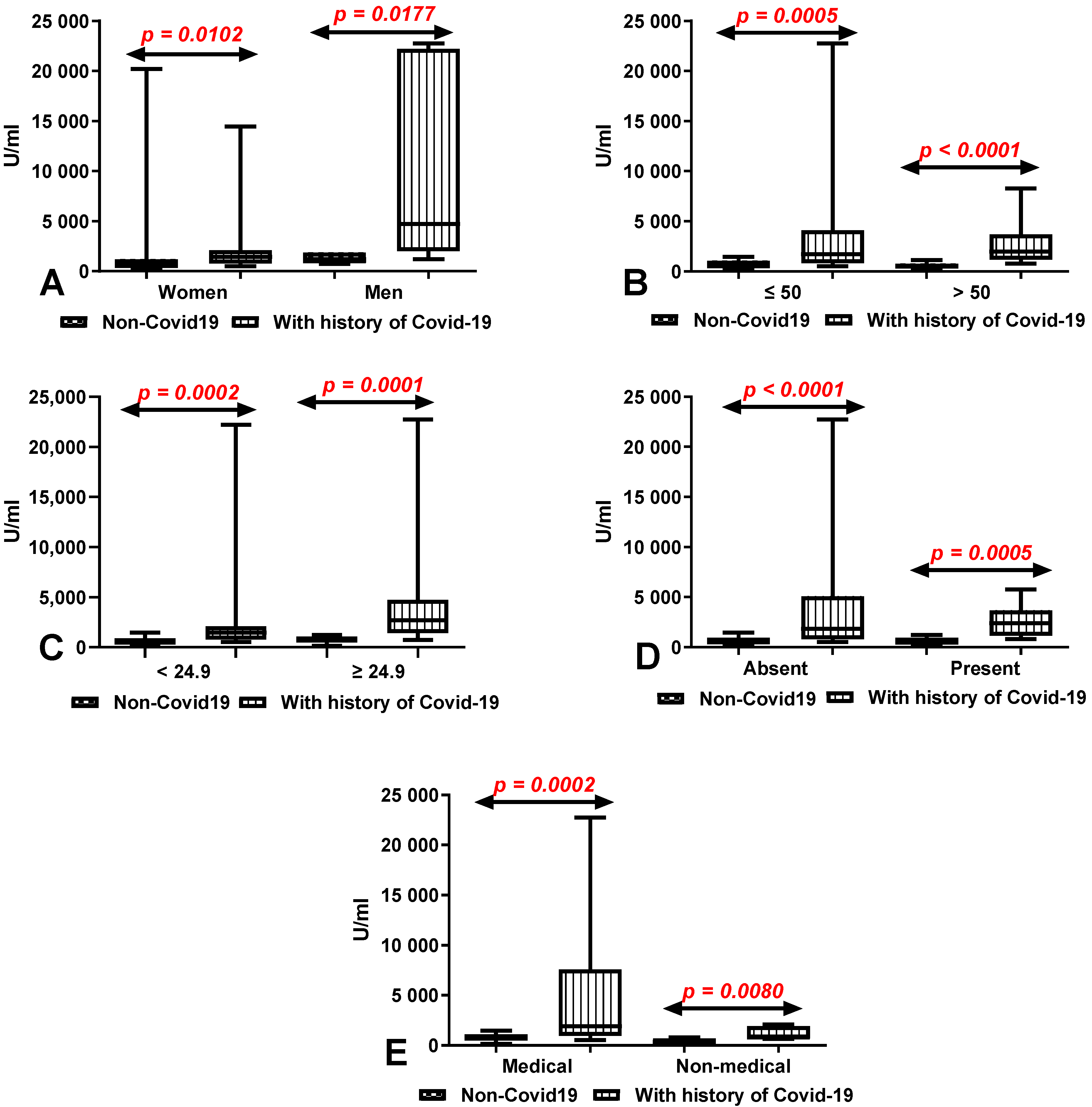
3.6. Comparison of Total Anti-SARS-CoV-2 Antibody Levels after 10 Months on Second Determinations According to Sex, Age, BMI, Coexisting Diseases and Work Type in Group of Workers with and without History of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and MRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zuiani, A.; Fischinger, S.; Mullur, J.; Atyeo, C.; Travers, M.; Lelis, F.J.N.; Pullen, K.M.; Martin, H.; Tong, P.; et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell 2020, 183, 1496. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2020, 184, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.-V.; Lai, L.; Sahoo, M.K.; Floyd, K.; Sibai, M.; Solis, D.; Flowers, M.W.; Hussaini, L.; Ciric, C.R.; Bechnack, S.; et al. Infection and Vaccine-Induced Neutralizing Antibody Responses to the SARS-CoV-2 B.1.617.1 Variant. bioRxiv 2021, 1, 234–240. [Google Scholar] [CrossRef]
- Faustini, S.E.; Shields, A.M.; Banham, G.; Wall, N.; Al-Taei, S.; Tanner, C.; Ahmed, Z.; Efstathiou, E.; Townsend, N.; Plant, T.; et al. Cross Reactivity of Spike Glycoprotein Induced Antibody against Delta and Omicron Variants before and after Third SARS-CoV-2 Vaccine Dose. medRxiv 2022, 2, 143–148. [Google Scholar] [CrossRef]
- Zhang, Z.; Kolls, J.K.; Oliver, P.; Good, D.; Schwarzenberger, P.O.; Joshi, M.S.; Ponthier, J.L.; Lancaster, J.R. Activation of Tumor Necrosis Factor-Alpha-Converting Enzyme-Mediated Ectodomain Shedding by Nitric Oxide. J. Biol. Chem. 2000, 275, 15839–15844. [Google Scholar] [CrossRef] [Green Version]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized Vaccinology: A Review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Higgins, V.; Fabros, A.; Kulasingam, V. Quantitative Measurement of Anti-SARS-CoV-2 Antibodies: Analytical and Clinical Evaluation. J. Clin. Microbiol. 2021, 59, e03149-20. [Google Scholar] [CrossRef]
- Wolszczak-Biedrzycka, B.; Bié Nkowska, A.; Dorf, J.; Leitão, J.H. Assessment of Post-Vaccination Antibody Response Eight Months after the Administration of BNT1622b2 Vaccine to Healthcare Workers with Particular Emphasis on the Impact of Previous COVID-19 Infection. Vaccines 2021, 9, 1508. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the Bnt162b2 Mrna Covid-19 Vaccine in Subjects with Prior Sars-Cov-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of Antibody Levels after Vaccination with MRNA SARS-CoV-2 Vaccine in Individuals with or without Prior Infection. JAMA J. Am. Med. Assoc. 2021, 326, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.F.; Sulbarán, A.C.M.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; de la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to Bnt162b2 Vaccine in Healthcare Workers with and without Prior Sars-Cov-2 Infection. Vaccines 2021, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Apostolakou, F.; Gumeni, S.; Gavriatopoulou, M.; Papadopoulos, D.; Malandrakis, P.; Papanagnou, E.-D.; Korompoki, E.; et al. Sustained but Declining Humoral Immunity Against SARS-CoV-2 at 9 Months Postvaccination With BNT162b2: A Prospective Evaluation in 309 Healthy Individuals. Hemasphere 2021, 6, e677. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.N.; et al. Effect of Previous SARS-CoV-2 Infection on Humoral and T-Cell Responses to Single-Dose BNT162b2 Vaccine. Lancet 2021, 397, 1178. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Antoine-Moussiaux, T.; della Vecchia, A.; Horeanga, A.; Papleux, E.; Vekemans, M.; Beukinga, I.; Blairon, L. Immunogenicity of MRNA-1273 COVID Vaccine after 6 Months Surveillance in Health Care Workers; a Third Dose Is Necessary. J. Infect. 2021, 83, 559–564. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021, 384, 2259. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody Titers and Protection against a SARS-CoV-2 Infection. J. Infect. 2021, 2, 67–72. [Google Scholar] [CrossRef]
- Ward, H.; Cooke, G.; Whitaker, M.; Redd, R.; Eales, O.; Brown, J.C.; Collet, K.; Cooper, E.; Daunt, A.; Jones, K.; et al. REACT-2 Round 5: Increasing Prevalence of SARS-CoV-2 Antibodies Demonstrate Impact of the Second Wave and of Vaccine Roll-out in England. medRxiv 2021, 7, 89–93. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Bayart, J.L.; Morimont, L.; Closset, M.; Wieërs, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of Nt162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; de Virgilio, A.; de Marco, F.; di Domenico, E.G.; et al. Initial Observations on Age, Gender, BMI and Hypertension in Antibody Responses to SARS-CoV-2 BNT162b2 Vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef] [PubMed]

| Workers with History of COVID-19 (n = 50) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of Anti-SARS-CoV2S Level (U/mL)) after 8 months | Level of Anti-SARS-CoV2S Level (U/mL)) after 10 months | ||||||||||||||
| Parameter | Number of workers (%) | Median | Mean | SD | Q1 | Q3 | 5 percentile | 95 percentile | Median | Mean | SD | Q1 | Q3 | 5 percentile | 95 percentile |
| Age | |||||||||||||||
| ≤50 | 31 (62%) | 1497 | 2304 | 2203 | 877.5 | 3001 | 456.9 | 8293 | 1324 | 9586 | 8965 | 615 | 4110 | 538.4 | 22,710 |
| >50 | 19 (38%) | 2564 | 3210 | 2324 | 1624 | 3751 | 901 | 10077 | 2387 | 3569 | 4235 | 1185 | 4670 | 800 | 14,466 |
| Sex | |||||||||||||||
| female | 41 (82%) | 1705 | 2914 | 3575 | 983 | 3457 | 516.5 | 10461 | 1687 | 2697 | 3159 | 829 | 3365 | 578.3 | 12,064 |
| male | 9 (18%) | 4603 | 8238 | 9597 | 2046 | 15605 | 991 | 25000 | 4531 | 8639 | 9584 | 2030 | 2105 | 1210 | 22,765 |
| Type of workers | |||||||||||||||
| medical | 43 (86%) | 2297 | 4371 | 5816 | 1304 | 4299 | 681.1 | 24051 | 2030 | 4541 | 5837 | 1214 | 5087 | 629.5 | 22,489 |
| non-medical | 7 (14%) | 941.5 | 1255 | 852.2 | 565 | 2028 | 398 | 2794 | 857 | 1187 | 588 | 721 | 1813 | 641 | 2081 |
| BMI | |||||||||||||||
| ≤24.9 | 18 (36%) | 1850 | 3926 | 6195 | 982.5 | 2719 | 661 | 25000 | 1471 | 3328 | 5520 | 809 | 2091 | 527 | 22,212 |
| >24.9 | 32 (64%) | 3244 | 5390 | 6460 | 1665 | 5455 | 901 | 24726 | 2693 | 4503 | 5490 | 1452 | 4731 | 732 | 22,765 |
| Other diseases | |||||||||||||||
| present | 23 (46%) | 2901 | 3215 | 1775 | 1543 | 4110 | 1320 | 6987 | 2497 | 2637 | 1564 | 1080 | 3843 | 561.2 | 22,599 |
| absent | 27 (54%) | 2190 | 5323 | 7318 | 996 | 5970 | 686.2 | 24,918 | 2037 | 4497 | 6341 | 1010 | 3233 | 836 | 5779 |
| Workers without History of COVID-19 (n = 50) | |||||||||||||||
| Level of Anti-SARS-CoV2S Level (U/mL)) after 8 Months | Level of Anti-SARS-CoV2S Level (U/mL)) after 10 Months | ||||||||||||||
| Parameter | Number of workers (%) | Median | Mean | SD | Q1 | Q3 | 5 percentile | 95 percentile | Median | Mean | SD | Q1 | Q3 | 5 percentile | 95 percentile |
| Age | |||||||||||||||
| ≤50 | 24 (48%) | 507.5 | 629.3 | 419.7 | 300.3 | 372.8 | 189.8 | 1707 | 476.5 | 628 | 320 | 246 | 1070 | 198 | 1476 |
| >50 | 26 (52%) | 522.5 | 673.8 | 476.1 | 372.8 | 770.8 | 372.8 | 770.8 | 514.5 | 485 | 289 | 327 | 786 | 159 | 1129 |
| Sex | |||||||||||||||
| female | 45 (90%) | 778.5 | 1675 | 3501 | 497.3 | 1547 | 222.2 | 9571 | 496 | 625 | 370 | 341 | 1005 | 166.8 | 1430 |
| male | 5 (10%) | 1222 | 1119 | 428.1 | 653 | 1512 | 512 | 1564 | 457 | 587 | 243 | 424 | 943 | 478 | 1100 |
| Type of workers | |||||||||||||||
| medical | 29 (58%) | 626.0 | 803.4 | 512.3 | 433.5 | 1222 | 210 | 1891 | 609 | 685 | 364 | 493 | 1094 | 159 | 1476 |
| non-medical | 21 (42%) | 453 | 443.9 | 202.4 | 257.5 | 552.5 | 145.7 | 791.7 | 359 | 415 | 384 | 294 | 534 | 198 | 812 |
| BMI | |||||||||||||||
| ≤24.9 | 29 (58%) | 535 | 726.5 | 467 | 382 | 1048 | 217 | 1755 | 496 | 600 | 367 | 330 | 908 | 198 | 1476 |
| >24.9 | 21 (42%) | 583 | 750.4 | 451.1 | 510 | 1142 | 225 | 1564 | 539 | 797 | 309 | 491 | 1123 | 485 | 1245 |
| Other diseases | |||||||||||||||
| present | 20 (40%) | 628.5 | 743.3 | 472.8 | 375 | 1185 | 235 | 1564 | 530 | 604 | 364 | 307 | 939 | 221 | 1245 |
| absent | 30 (60%) | 569 | 728.6 | 458.1 | 434.5 | 1055 | 217 | 1755 | 516 | 644 | 375 | 356 | 956 | 159 | 1476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolszczak-Biedrzycka, B.; Bieńkowska, A.; Zaborowska, J.E.; Smolińska-Fijołek, E.; Biedrzycki, G.; Dorf, J. Anti-SARS-CoV-2S Antibody Levels in Healthcare Workers 10 Months after the Administration of Two BNT162b2 Vaccine Doses in View of Demographic Characteristic and Previous COVID-19 Infection. Vaccines 2022, 10, 741. https://doi.org/10.3390/vaccines10050741
Wolszczak-Biedrzycka B, Bieńkowska A, Zaborowska JE, Smolińska-Fijołek E, Biedrzycki G, Dorf J. Anti-SARS-CoV-2S Antibody Levels in Healthcare Workers 10 Months after the Administration of Two BNT162b2 Vaccine Doses in View of Demographic Characteristic and Previous COVID-19 Infection. Vaccines. 2022; 10(5):741. https://doi.org/10.3390/vaccines10050741
Chicago/Turabian StyleWolszczak-Biedrzycka, Blanka, Anna Bieńkowska, Joanna Ewa Zaborowska, Elwira Smolińska-Fijołek, Grzegorz Biedrzycki, and Justyna Dorf. 2022. "Anti-SARS-CoV-2S Antibody Levels in Healthcare Workers 10 Months after the Administration of Two BNT162b2 Vaccine Doses in View of Demographic Characteristic and Previous COVID-19 Infection" Vaccines 10, no. 5: 741. https://doi.org/10.3390/vaccines10050741
APA StyleWolszczak-Biedrzycka, B., Bieńkowska, A., Zaborowska, J. E., Smolińska-Fijołek, E., Biedrzycki, G., & Dorf, J. (2022). Anti-SARS-CoV-2S Antibody Levels in Healthcare Workers 10 Months after the Administration of Two BNT162b2 Vaccine Doses in View of Demographic Characteristic and Previous COVID-19 Infection. Vaccines, 10(5), 741. https://doi.org/10.3390/vaccines10050741






