Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Study Outcomes
2.3. Healthy Individuals for Comparison
2.4. SARS-CoV-2 Serological Assessment
2.5. Surrogate Neutralization for Omicron
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
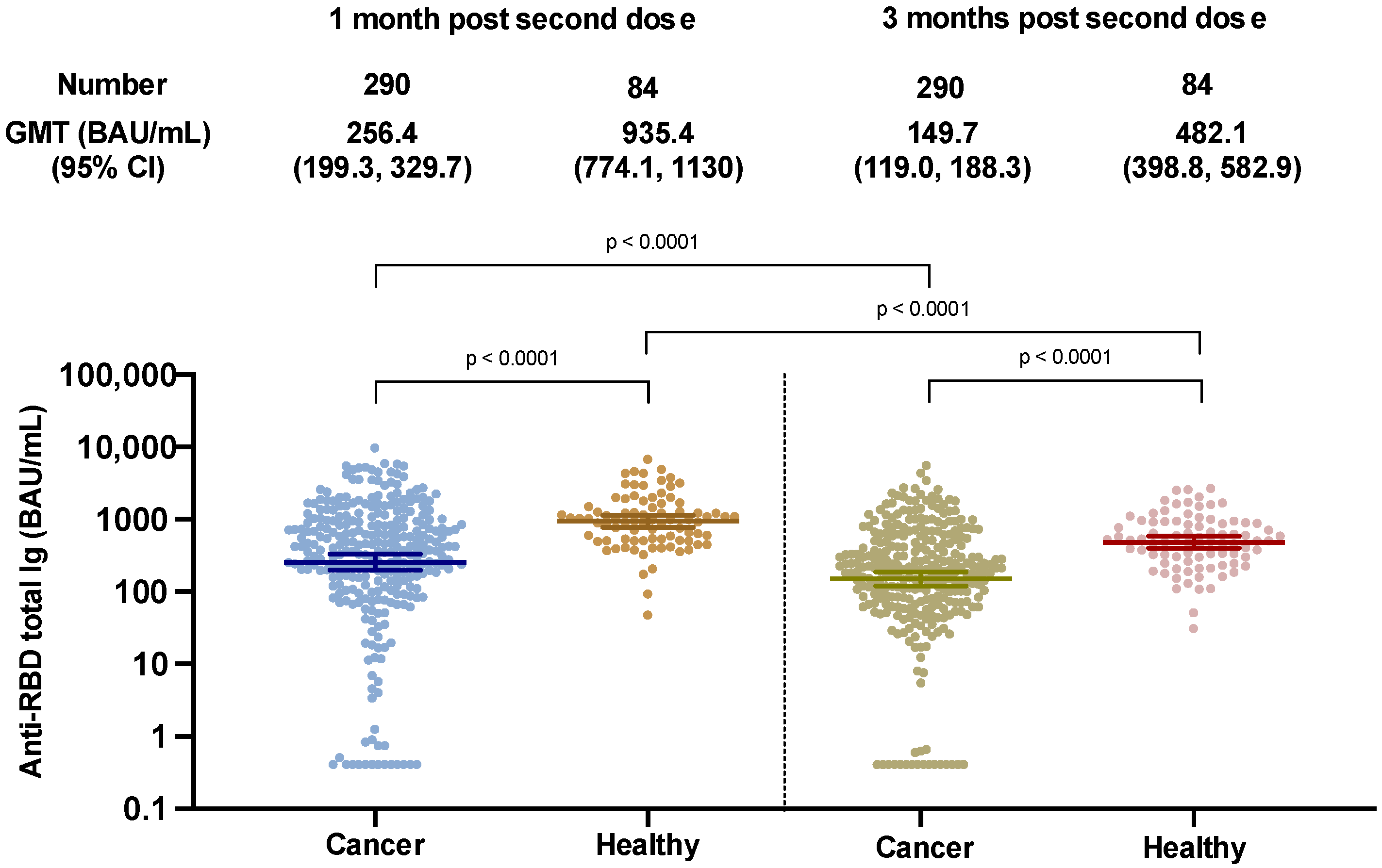
3.2. Anti-RBD Total Ig Response at 12 Weeks Post Second ChAdOx1 nCoV-19 Vaccine
3.3. Decay Rate of Anti-SARS-CoV-2 Ig
3.4. Late Titer Elevation in SARS-CoV-2 Binding Antibody in Solid Cancer Patients
3.5. Clinical Factors Associated with Adequate and Non-Adequate Immunologic Response
3.6. Effect of Anticancer Treatment on Antibody Response
3.7. Effect of Treatment Cessation on Antibody Response
3.8. Surrogate Neutralization against Omicron Variant of Concern
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez-MacGregor, M.; Lei, X.; Zhao, H.; Scheet, P.; Giordano, S.H. Evaluation of COVID-19 Mortality and Adverse Outcomes in US Patients With or Without Cancer. JAMA Oncol. 2022, 8, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Seth, G.; Sethi, S.; Bhattarai, S.; Saini, G.; Singh, C.B.; Aneja, R. SARS-CoV-2 Infection in Cancer Patients: Effects on Disease Outcomes and Patient Prognosis. Cancers 2020, 12, 3266. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, A.; Desilets, A.; Papneja, N.; Cvetkovic, L.; Groleau, C.; Lakehal, Y.A.; Shbat, L.; Richard, C.; Malo, J.; Belkaid, W.; et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: A multicentre observational cohort study. Eur. J. Cancer 2020, 139, 181–187. [Google Scholar] [CrossRef]
- de Joode, K.; Dumoulin, D.W.; Tol, J.; Westgeest, H.M.; Beerepoot, L.V.; van den Berkmortel, F.; Mutsaers, P.; van Diemen, N.G.J.; Visser, O.J.; Oomen-de Hoop, E.; et al. Dutch Oncology COVID-19 consortium: Outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur. J. Cancer 2020, 141, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.; Favier, B.; Assaad, S.; Zrounba, P.; Blay, J.Y. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann. Oncol. 2021, 32, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Berger, N.A.; Xu, R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Bivona, C.R.; Martin, G.A.; Zhang, J.; Liu, B.; He, J.; Li, K.H.; Nelson, M.; Williamson, S.; Doolittle, G.C.; et al. Evaluation of the Durability of the Immune Humoral Response to COVID-19 Vaccines in Patients with Cancer Undergoing Treatment or Who Received a Stem Cell Transplant. JAMA Oncol. 2022, 8, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Barberi, V.; Pellini, R.; Morrone, A.; Giannarelli, D.; Cognetti, F. Clinical characteristics limiting the durability of humoral response to BNT162b2 in patients with solid cancer. Ann. Oncol. 2022, 33, 350–352. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Massarweh, A.; Stemmer, A.; Stemmer, S.M. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021, 7, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Veyri, M.; Vozy, A.; Marot, S.; Gligorov, J.; Benderra, M.-A.; Maingon, P.; Morand-Joubert, L.; Adjoutah, Z.; Marcelin, A.G.; et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated with chemotherapy for solid cancers. Ann. Oncol. 2021, 32, 1294. [Google Scholar] [CrossRef]
- Teeyapun, N.; Luangdilok, S.; Pakvisal, N.; Sainamthip, P.; Mingmalairak, S.; Poovorawan, N.; Sitthideatphaiboon, P.; Parinyanitikul, N.; Sriuranpong, V.; Namkanisorn, T.; et al. Immunogenicity of ChAdOx1-nCoV-19 vaccine in solid malignancy patients by treatment regimen versus healthy controls: A prospective, multicenter observational study. eClinicalMedicine 2022, 52, 101608. [Google Scholar] [CrossRef]
- Pakvisal, N.; Sainamthip, P.; Teeyapun, N.; Luangdilok, S.; Wanlapakorn, N.; Yorsaeng, R.; Poovorawan, Y.; Pakvisal, P.; Susiriwatananont, T.; Zungsontiporn, N.; et al. Vaccine-Related adverse events following AZD1222 (ChAdOx1-nCoV-19) Covid-19 vaccine in solid malignancy patients receiving cancer treatment, as compared to age-matched healthy controls. Hum. Vaccines Immunother. 2022, 2094149. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Vichaiwattana, P.; Thongmee, T.; Auphimai, C.; Srimuan, D.; Thatsanatorn, T.; Assawakosri, S.; et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum. Vaccin Immunother. 2022, 18, 2029111. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. The Authorized COVID-19 Convalescent Plasma. Available online: https://www.fda.gov/media/141477/download (accessed on 30 March 2022).
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Oosting, S.F.; van der Veldt, A.A.; GeurtsvanKessel, C.H.; Fehrmann, R.S.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Chaguza, C.; Coppi, A.; Earnest, R.; Ferguson, D.; Kerantzas, N.; Warner, F.; Young, H.P.; Breban, M.I.; Billig, K.; Koch, R.T.; et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med 2022, 3, 325–334. [Google Scholar] [CrossRef] [PubMed]
- WHO. Interim Statement on the Use of Additional Booster Doses of Emergency Use Listed mRNA vaccines against COVID-19. Available online: https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19 (accessed on 21 June 2022).
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Second Booster Dose of Two COVID-19 Vaccines for Older and Immunocompromised Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and (accessed on 20 April 2022).
- Ramos, A.; Cardoso, M.J.; Ribeiro, L.; Guimarães, J.T. Assessing SARS-CoV-2 Neutralizing Antibodies after BNT162b2 Vaccination and Their Correlation with SARS-CoV-2 IgG Anti-S1, Anti-RBD and Anti-S2 Serological Titers. Diagnostics 2022, 12, 205. [Google Scholar] [CrossRef]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P.J.; et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. Npj Vaccines 2022, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.J.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar] [CrossRef] [PubMed]






| Age, Years, Median (IQR) | 61 (50, 67.75) | Cancer type | |
| BMI ± SD | 22.98 ± 4.20 | Breast | 97 (33.45%) |
| Sex | Lung | 76 (26.21%) | |
| Female | 186 (64.14%) | Colorectal | 59 (20.34%) |
| Male | 104 (35.86%) | GIST | 11 (3.79%) |
| ECOG | Head and neck | 8 (2.76%) | |
| ECOG 0–1 | 274 (94.48%) | Cholangiocarcinoma | 2 (0.69%) |
| ECOG 2 | 16 (5.51%) | Others | 37 (12.76%) |
| Initial TNM staging | Cancer treatment within 4 weeks before first vaccination | ||
| I | 15 (5.17%) | Chemotherapy | 141 (48.6%) |
| II | 47 (16.20%) | Oxaliplatin-containing regimen | 34 |
| III | 101 (34.82%) | Anthracycline | 24 |
| IV | 127 (43.79%) | Plalinum doublet | 23 |
| Comorbidities | Paclitaxel | 18 | |
| Cardiovascular disease * | 95 (45.46%) | 5-FU or Gemcitabine | 15 |
| Diabetes | 46 (15.86%) | Irinotecan-containing regimen | 15 |
| COPD | 4 (1.38%) | Docetaxel | 7 |
| Cirrhosis | 8 (2.76%) | Other | 5 |
| CKD | 9 (3.10%) | Targeted therapy/CDKi | 85 (29.3%) |
| Others | 53 (18.28%) | Immunotherapy | 35 (12%) |
| No comorbidities | 204 (70.34%) | Single agent anti-PD1/PDL1 | 33 |
| Time to blood collection from second dose | Anti-PD1 plus nati-CTLA4 | 3 | |
| 4 weeks post second dose (Days ± SD) | 28.86 ± 6.49 | Biologic agent | 16 (5.5%) |
| 12 weeks post second dose (Days ± SD) | 86.46 ± 22.78 | Anti-hormonal treatment | 13 (4.5%) |
| Neutralization against Omicron (30% Cut-Off) | |||
|---|---|---|---|
| Positive | Negative | ||
| Anti-RBD Ig > 210 U/mL | Adequate response | 2 | 35 |
| Anti-RBD Ig < 210 U/mL | Inadequate response | 0 | 3 |
| Total | 2 | 38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanchaijiraboon, P.; Teeyapun, N.; Pakvisal, N.; Sainamthip, P.; Susiriwatananont, T.; Zungsontiporn, N.; Suntronwong, N.; Vichaiwattana, P.; Klinsawat, W.; Wanlapakorn, N.; et al. Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment. Vaccines 2022, 10, 1662. https://doi.org/10.3390/vaccines10101662
Wanchaijiraboon P, Teeyapun N, Pakvisal N, Sainamthip P, Susiriwatananont T, Zungsontiporn N, Suntronwong N, Vichaiwattana P, Klinsawat W, Wanlapakorn N, et al. Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment. Vaccines. 2022; 10(10):1662. https://doi.org/10.3390/vaccines10101662
Chicago/Turabian StyleWanchaijiraboon, Passakorn, Nattaya Teeyapun, Nussara Pakvisal, Panot Sainamthip, Thiti Susiriwatananont, Nicha Zungsontiporn, Nungruthai Suntronwong, Preeyaporn Vichaiwattana, Worata Klinsawat, Nasamon Wanlapakorn, and et al. 2022. "Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment" Vaccines 10, no. 10: 1662. https://doi.org/10.3390/vaccines10101662
APA StyleWanchaijiraboon, P., Teeyapun, N., Pakvisal, N., Sainamthip, P., Susiriwatananont, T., Zungsontiporn, N., Suntronwong, N., Vichaiwattana, P., Klinsawat, W., Wanlapakorn, N., Tanasanvimon, S., Sriuranpong, V., Poovorawan, Y., & Luangdilok, S. (2022). Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment. Vaccines, 10(10), 1662. https://doi.org/10.3390/vaccines10101662






