Estimated Infection and Vaccine Induced SARS-CoV-2 Seroprevalence in Israel among Adults, January 2020–July 2021
Abstract
:1. Introduction
2. Materials and Methods
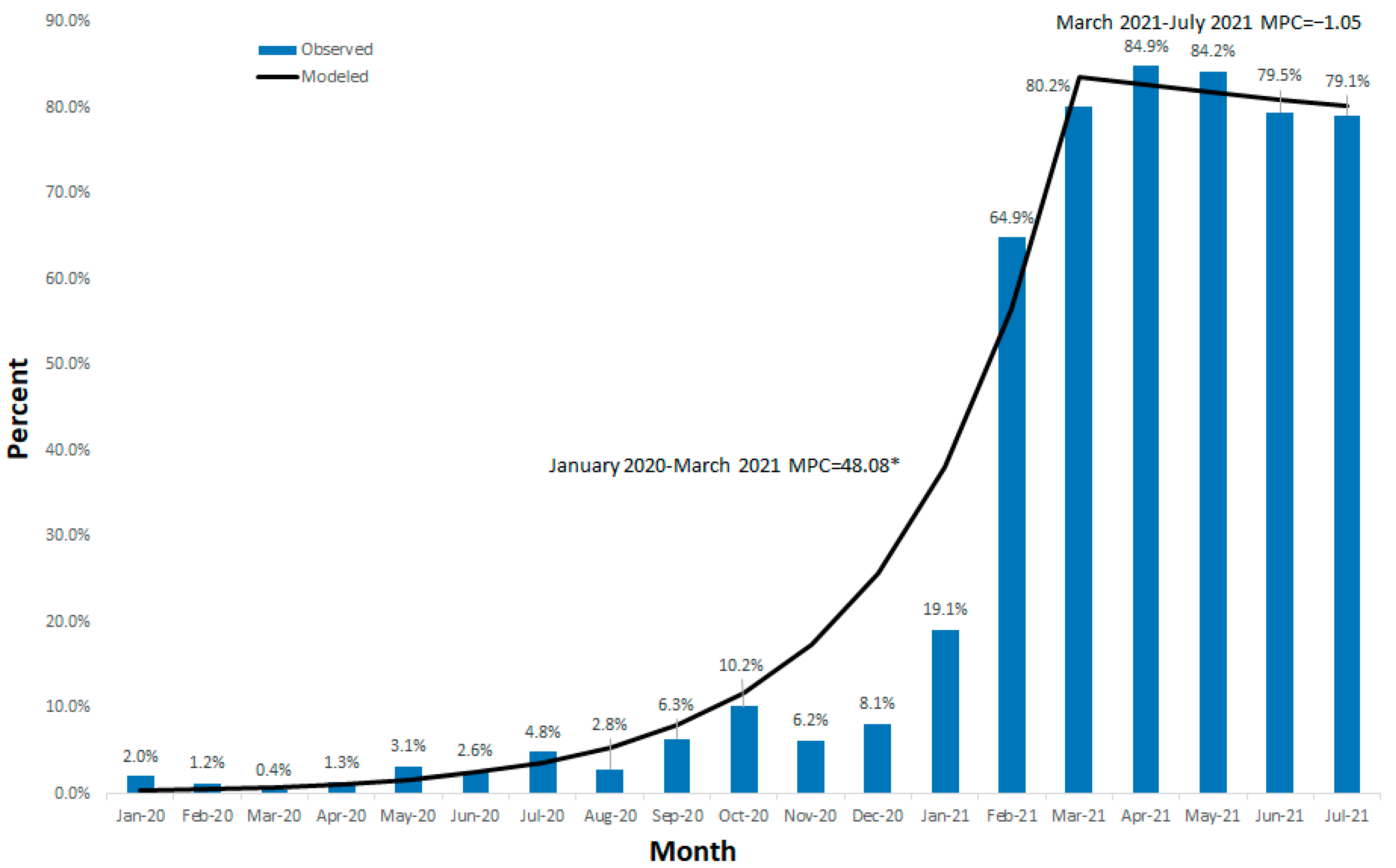
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 19 September 2022).
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- The Israel Central Bureau of Statistics. Population, by Population Group, Religion, Age and Sex, District and Sub-District. Available online: https://www.cbs.gov.il/he/Pages/search/yearly.aspx (accessed on 19 September 2022).
- Coronavirius in Israel. Available online: https://datadashboard.health.gov.il/COVID-19/general (accessed on 19 September 2022).
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 19 September 2022).
- Cevik, M.; Grubaugh, N.D.; Iwasaki, A.; Openshaw, P. COVID-19 vaccines: Keeping pace with SARS-CoV-2 variants. Cell 2021, 184, 5077–5081. [Google Scholar] [CrossRef]
- Characterization and Classification of Local Authorities by the Socio-Economic Level of the Population in 2017. Local Councils and Municipalities—Rank, Cluster Membership, Population, Variable Values, Standardized Values and Ranking for the Variables Used in the Computation of the Index. Available online: https://www.cbs.gov.il/he/publications/DocLib/2021/socio_eco17_1832/h_print.pdf (accessed on 19 September 2022).
- Bassal, R.; Cohen, D.; Green, M.S.; Keinan-Boker, L. The Israel National Sera Bank: Methods, Representativeness, and Challenges. Int. J. Environ. Res. Public Health 2021, 18, 2280. [Google Scholar] [CrossRef] [PubMed]
- Indenbaum, V.; Koren, R.; Katz-Likvornik, S.; Yitzchaki, M.; Halpern, O.; Regev-Yochay, G.; Cohen, C.; Biber, A.; Feferman, T.; Cohen Saban, N.; et al. Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLoS ONE 2020, 15, e0241164. [Google Scholar] [CrossRef] [PubMed]
- Pathela, P.; Crawley, A.; Weiss, D.; Maldin, B.; Cornell, J.; Purdin, J.; Schumacher, P.K.; Marovich, S.; Li, J.; Daskalakis, D. Seroprevalence of Severe Acute Respiratory Syndrome Coronavirus 2 Following the Largest Initial Epidemic Wave in the United States: Findings From New York City, 13 May to 21 July 2020. J. Infect. Dis. 2021, 224, 196–206. [Google Scholar] [CrossRef]
- Parrott, J.C.; Maleki, A.N.; Vassor, V.E.; Osahan, S.; Hsin, Y.; Sanderson, M.; Fernandez, S.; Levanon Seligson, A.; Hughes, S.; Wu, J.; et al. Prevalence of SARS-CoV-2 Antibodies in New York City Adults, June–October 2020: A Population-Based Survey. J. Infect. Dis. 2021, 224, 188–195. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Al-Zalabani, A.H. The seroprevalence of SARS-CoV-2 IgG antibodies among asymptomatic blood donors in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 1697–1701. [Google Scholar] [CrossRef]
- O’Callaghan, M.E.; Ryan, E.; Walsh, C.; Hayes, P.; Casey, M.; O’Dwyer, P.; Culhane, A.; Duncan, J.W.; Harrold, P.; Healy, J.; et al. SARS-CoV-2 infection in general practice in Ireland: A seroprevalence study. BJGP Open 2021, 5, bjgpo.2021.0038. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; Carnalla, M.; Torres-Ibarra, L.; Romero-Martínez, M.; Martínez-Barnetche, J.; López-Martínez, I.; Aparicio-Antonio, R.; Shamah-Levy, T.; Alpuche-Aranda, C.; Rivera, J.A.; et al. Nationally representative SARS-CoV-2 antibody prevalence estimates after the first epidemic wave in Mexico. Nat. Commun. 2022, 13, 589. [Google Scholar] [CrossRef]
- Shaweno, T.; Abdulhamid, I.; Bezabih, L.; Teshome, D.; Derese, B.; Tafesse, H.; Shaweno, D. Seroprevalence of SARS-CoV-2 antibody among individuals aged above 15 years and residing in congregate settings in Dire Dawa city administration, Ethiopia. Trop. Med. Health 2021, 49, 55. [Google Scholar] [CrossRef]
- Coltart, C.E.M.; Wells, D.; Sutherland, E.; Fowler, A. National cross-sectional survey of 1.14 million NHS staff SARS-CoV-2 serology tests: A comparison of NHS staff with regional community seroconversion rates. BMJ Open 2021, 11, e049703. [Google Scholar] [CrossRef] [PubMed]
- Coyer, L.; Boyd, A.; Schinkel, J.; Agyemang, C.; Galenkamp, H.; Koopman, A.D.M.; Leenstra, T.; Moll van Charante, E.P.; van den Born, B.H.; Lok, A.; et al. SARS-CoV-2 antibody prevalence and correlates of six ethnic groups living in Amsterdam, the Netherlands: A population-based cross-sectional study, June–October 2020. BMJ Open 2022, 12, e052752. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Stone, M.; Sulaeman, H.; Fink, R.V.; Dave, H.; Levy, M.E.; Di Germanio, C.; Green, V.; Notari, E.; Saa, P.; et al. Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020–May 2021. JAMA 2021, 326, 1400–1409. [Google Scholar] [CrossRef]
- Gidding, H.F.; Machalek, D.A.; Hendry, A.J.; Quinn, H.E.; Vette, K.; Beard, F.H.; Shilling, H.S.; Hirani, R.; Gosbell, I.B.; Irving, D.O.; et al. Seroprevalence of SARS-CoV-2-specific antibodies in Sydney after the first epidemic wave of 2020. Med. J. Aust. 2021, 214, 179–185. [Google Scholar] [CrossRef]
- Li, Z.; Lewis, B.; Berney, K.; Hallisey, E.; Williams, A.M.; Whiteman, A.; Rivera-González, L.O.; Clarke, K.E.N.; Clayton, H.; Tincher, T.; et al. Social vulnerability and rurality associated with higher Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection-induced seroprevalence: A nationwide blood donor study—United States, July 2020–June 2021. Clin. Infect. Dis. 2022, 75, e133–e143. [Google Scholar] [CrossRef]
- Garay, E.; Serrano-Coll, H.; Rivero, R.; Gastelbondo, B.; Faccini-Martínez, Á.; Berrocal, J.; Pérez, A.; Badillo, M.; Martínez-Bravo, C.; Botero, Y.; et al. SARS-CoV-2 in eight municipalities of the Colombian tropics: High immunity, clinical and sociodemographic outcomes. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Indenbaum, V.; Lustig, Y.; Mendelson, E.; Hershkovitz, Y.; Glatman-Freedman, A.; Keinan-Boker, L.; Bassal, R. Under-diagnosis of SARS-CoV-2 infections among children aged 0–15 years, a nationwide seroprevalence study, Israel, January 2020 to March 2021. Euro Surveill. Bull. Eur. Mal. Transm. 2021, 26, 2101040. [Google Scholar] [CrossRef]
- Sood, N.; Pernet, O.; Lam, C.N.; Klipp, A.; Kotha, R.; Kovacs, A.; Hu, H. Seroprevalence of Antibodies Specific to Receptor Binding Domain of SARS-CoV-2 and Vaccination Coverage Among Adults in Los Angeles County, April 2021: The LA Pandemic Surveillance Cohort Study. JAMA Netw. Open 2022, 5, e2144258. [Google Scholar] [CrossRef]
- Olariu, T.R.; Craciun, A.C.; Vlad, D.C.; Dumitrascu, V.; Marincu, I.; Lupu, M.A. SARS-CoV-2 Seroprevalence in Western Romania, March to June 2021. Medicina 2022, 58, 35. [Google Scholar] [CrossRef]
- Curtis, H.J.; Inglesby, P.; Morton, C.E.; MacKenna, B.; Green, A.; Hulme, W.; Walker, A.J.; Morley, J.; Mehrkar, A.; Bacon, S.; et al. Trends and clinical characteristics of COVID-19 vaccine recipients: A federated analysis of 57.9 million patients’ primary care records in situ using OpenSAFELY. Br. J. Gen. Pract. 2022, 72, e51–e62. [Google Scholar] [CrossRef]
- Pingali, C.; Meghani, M.; Razzaghi, H.; Lamias, M.J.; Weintraub, E.; Kenigsberg, T.A.; Klein, N.P.; Lewis, N.; Fireman, B.; Zerbo, O.; et al. COVID-19 Vaccination Coverage Among Insured Persons Aged ≥16 Years, by Race/Ethnicity and Other Selected Characteristics—Eight Integrated Health Care Organizations, United States, December 14, 2020–May 15, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Benderly, M.; Huppert, A.; Novikov, I.; Ziv, A.; Kalter-Leibovici, O. Fighting a pandemic: Sociodemographic disparities and coronavirus disease–2019 Vaccination gaps—A population study. Int. J. Epidemiol. 2022, 51, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, Y.; Anis, E.; Edelstein, M. Inequalities in initiation of COVID19 vaccination by age and population group in Israel—December 2020–July 2021. Lancet Reg. Health—Eur. 2022, 12, 100234. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Soto, A.; Pachamora Diaz, J.M.; González-Auza, L.; Merino Merino, X.J.; Schwalb, A.; Drosten, C.; Gotuzzo, E.; Talledo, M.; Arévalo Ramirez, H.; Peralta Delgado, R.; et al. High SARS-CoV-2 Seroprevalence in Rural Peru, 2021: A Cross-Sectional Population-Based Study. mSphere 2021, 6, e0068521. [Google Scholar] [CrossRef]
- Bushman, M.; Kahn, R.; Taylor, B.P.; Lipsitch, M.; Hanage, W.P. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. Cell 2021, 184, 6229–6242.e18. [Google Scholar] [CrossRef]
| Total | Period 1 | Period 2 | |||||
|---|---|---|---|---|---|---|---|
| Category | N | % | N | % | N | % | |
| Total | 9520 | 100.0 | 6042 | 100.0 | 3478 | 100.0 | |
| Age | Mean± Standard deviation; Minimum–Maximum | 45.7 ± 21.4; 16.0–103.6 | 45.8 ± 21.6; 16.0–100.6 | 45.5 ± 21.0; 16.0–103.6 | |||
| Age group (years) | 16.00–39.99 | 4397 | 46.2 | 2796 | 46.3 | 1601 | 46.0 |
| 40.00–64.99 | 2953 | 31.0 | 1828 | 30.3 | 1125 | 32.4 | |
| 65.00+ | 2167 | 22.8 | 1415 | 23.4 | 752 | 21.6 | |
| Gender | Male | 4353 | 45.7 | 2819 | 46.7 | 1534 | 44.1 |
| Female | 5167 | 54.3 | 3223 | 53.3 | 1944 | 55.9 | |
| Birth country | Israel | 7157 | 75.3 | 4471 | 74.1 | 2686 | 77.3 |
| Other | 2352 | 24.7 | 1563 | 25.9 | 789 | 22.7 | |
| Population group | Jews and others | 6215 | 70.8 | 3936 | 70.3 | 2279 | 71.8 |
| Arabs | 2560 | 29.2 | 1665 | 29.7 | 895 | 28.2 | |
| District | Jerusalem | 1379 | 14.5 | 867 | 14.4 | 512 | 14.8 |
| North | 2382 | 25.1 | 1461 | 24.2 | 921 | 26.6 | |
| Haifa | 892 | 9.4 | 574 | 9.5 | 318 | 9.2 | |
| Central | 855 | 9.0 | 534 | 8.9 | 321 | 9.3 | |
| Tel Aviv | 929 | 9.8 | 551 | 9.1 | 378 | 10.9 | |
| South | 2556 | 26.9 | 1711 | 28.4 | 845 | 24.4 | |
| Judea and Samaria | 501 | 5.3 | 331 | 5.5 | 170 | 4.9 | |
| Socio-demographic rank | High (6–10) | 3090 | 33.3 | 1962 | 33.3 | 1128 | 33.2 |
| Low (1–5) | 6200 | 66.7 | 3925 | 66.7 | 2275 | 66.8 | |
| Period 1 | Period 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Tested | Positive | % | 95%CI £ | Tested | Positive | % | 95%CI £ | |
| Age group (years) | 16.00–39.99 | 2796 | 116 | 4.2 | 3.4–5.0 | 1601 | 1093 | 68.3 | 65.9–70.6 |
| 40.00–64.99 | 1828 | 67 | 3.7 | 2.8–4.6 | 1125 | 835 | 74.2 | 71.6–76.8 | |
| 65.00+ | 1415 | 66 | 4.7 | 3.6–5.9 | 752 | 518 | 68.9 | 65.4–72.2 | |
| Gender | Male | 2819 | 115 | 4.1 | 3.4–4.9 | 1534 | 1095 | 71.4 | 69.0–73.6 |
| Female | 3223 | 134 | 4.2 | 3.5–4.9 | 1944 | 1351 | 69.5 | 67.4–71.5 | |
| Birth country | Israel | 4471 | 202 | 4.5 | 3.9–5.2 | 2686 | 1900 | 70.7 | 69.0–72.4 |
| Other | 1563 | 47 | 3.0 | 2.2–4.0 | 789 | 543 | 68.8 | 65.5–72.0 | |
| Population group | Jews and others | 3936 | 152 | 3.9 | 3.3–4.5 | 2279 | 1643 | 72.1 | 70.2–73.9 |
| Arabs | 1665 | 89 | 5.4 | 4.3–6.5 | 895 | 573 | 64.0 | 60.8–67.2 | |
| District | Jerusalem | 867 | 54 | 6.2 | 4.7–8.0 | 512 | 373 | 72.8 | 68.8–76.7 |
| North | 1461 | 66 | 4.5 | 3.5–5.7 | 921 | 690 | 74.9 | 72.0–77.7 | |
| Haifa | 574 | 15 | 2.6 | 1.5–4.3 | 318 | 234 | 73.6 | 68.4–78.4 | |
| Central | 534 | 14 | 2.6 | 1.4–4.4 | 321 | 241 | 75.1 | 70.0–79.7 | |
| Tel Aviv | 551 | 38 | 6.9 | 4.9–9.3 | 378 | 246 | 65.1 | 60.0–69.9 | |
| South | 1711 | 43 | 2.5 | 1.8–3.4 | 845 | 522 | 61.8 | 58.4–65.1 | |
| Judea and Samaria | 331 | 15 | 4.5 | 2.6–7.4 | 170 | 130 | 76.5 | 69.4–82.6 | |
| Socio-demographic rank | High (6–10) | 1962 | 40 | 2.0 | 1.5–2.8 | 1128 | 853 | 75.6 | 73.0–78.1 |
| Low (1–5) | 3925 | 203 | 5.2 | 4.5–5.9 | 2275 | 1569 | 69.0 | 67.0–70.9 | |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Category | OR € | 95%CI £ | p-Value | OR € | 95%CI £ | p-Value | |
| Agegroup (years) | 16.00–39.99 | Ref. | |||||
| 40.00–64.99 | 0.88 | 0.65–1.20 | 0.4099 | ||||
| 65.00+ | 1.13 | 0.83–1.54 | 0.4373 | ||||
| Gender | Male | 0.98 | 0.76–1.26 | 0.8789 | |||
| Female | Ref. | ||||||
| Birth country | Israel | 1.53 | 1.10–2.11 | 0.0102 | 1.25 | 0.87–1.80 | 0.2242 |
| Other | Ref. | Ref. | |||||
| Population group | Jews and others | Ref. | Ref. | ||||
| Arabs | 1.41 | 1.08–1.84 | 0.0127 | 1.30 | 0.89–1.91 | 0.1771 | |
| District | Jerusalem | 2.47 | 1.36–4.49 | 0.0031 | 1.06 | 0.54–2.08 | 0.8764 |
| North | 1.76 | 0.98–3.16 | 0.0591 | 0.76 | 0.39–1.48 | 0.4154 | |
| Haifa | 1.00 | 0.48–2.08 | 0.9929 | 0.70 | 0.32–1.50 | 0.3566 | |
| Central | Ref. | Ref. | |||||
| Tel Aviv | 2.75 | 1.47–5.14 | 0.0015 | 1.71 | 0.87–3.35 | 0.1175 | |
| South | 0.96 | 0.52–1.76 | 0.8893 | 0.44 | 0.22–0.87 | 0.0190 | |
| Judea and Samaria | 1.76 | 0.84–3.70 | 0.1340 | 1.05 | 0.48–2.29 | 0.9076 | |
| Socio-demographic rank | High (6–10) | Ref. | Ref. | ||||
| Low (1–5) | 2.62 | 1.86–3.69 | <0.0001 | 2.49 | 1.64–3.80 | <0.0001 | |
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Category | OR € | 95%CI £ | p-Value | OR € | 95%CI £ | p-Value | |
| Age group (years) | 16.00–39.99 | Ref. | |||||
| 40.00–64.99 | 1.34 | 1.13–1.59 | 0.0008 | 1.36 | 1.13–1.64 | 0.0013 | |
| 65.00+ | 1.03 | 0.85–1.24 | 0.7653 | 1.02 | 0.82–1.25 | 0.8899 | |
| Gender | Male | 1.10 | 0.94–1.27 | 0.2267 | |||
| Female | Ref. | ||||||
| Birth country | Israel | 1.10 | 0.92–1.30 | 0.3005 | |||
| Other | Ref. | ||||||
| Population group | Jews and others | 1.45 | 1.23–1.71 | <0.0001 | 1.49 | 1.19–1.86 | 0.0005 |
| Arabs | Ref. | ||||||
| District | Jerusalem | 0.89 | 0.65–1.23 | 0.4777 | 1.15 | 0.79–1.68 | 0.4701 |
| North | 0.99 | 0.74–1.33 | 0.9548 | 1.55 | 1.09–2.20 | 0.0140 | |
| Haifa | 0.92 | 0.65–1.32 | 0.6659 | 1.08 | 0.74–1.59 | 0.6890 | |
| Central | Ref. | ||||||
| Tel Aviv | 0.62 | 0.44–0.86 | 0.0043 | 0.71 | 0.49–1.02 | 0.0601 | |
| South | 0.54 | 0.40–0.72 | <0.0001 | 0.85 | 0.60–1.20 | 0.3480 | |
| Judea and Samaria | 1.08 | 0.70–1.67 | 0.7329 | 1.32 | 0.82–2.11 | 0.2525 | |
| Socio-demographic rank | High (6–10) | 1.40 | 1.19–1.64 | <0.0001 | 1.23 | 0.99–1.53 | 0.0607 |
| Low (1–5) | Ref. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassal, R.; Keinan-Boker, L.; Cohen, D.; Mendelson, E.; Lustig, Y.; Indenbaum, V. Estimated Infection and Vaccine Induced SARS-CoV-2 Seroprevalence in Israel among Adults, January 2020–July 2021. Vaccines 2022, 10, 1663. https://doi.org/10.3390/vaccines10101663
Bassal R, Keinan-Boker L, Cohen D, Mendelson E, Lustig Y, Indenbaum V. Estimated Infection and Vaccine Induced SARS-CoV-2 Seroprevalence in Israel among Adults, January 2020–July 2021. Vaccines. 2022; 10(10):1663. https://doi.org/10.3390/vaccines10101663
Chicago/Turabian StyleBassal, Ravit, Lital Keinan-Boker, Dani Cohen, Ella Mendelson, Yaniv Lustig, and Victoria Indenbaum. 2022. "Estimated Infection and Vaccine Induced SARS-CoV-2 Seroprevalence in Israel among Adults, January 2020–July 2021" Vaccines 10, no. 10: 1663. https://doi.org/10.3390/vaccines10101663
APA StyleBassal, R., Keinan-Boker, L., Cohen, D., Mendelson, E., Lustig, Y., & Indenbaum, V. (2022). Estimated Infection and Vaccine Induced SARS-CoV-2 Seroprevalence in Israel among Adults, January 2020–July 2021. Vaccines, 10(10), 1663. https://doi.org/10.3390/vaccines10101663






