Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Study Medication
2.4. Blood Sampling and Blood Analyses
2.5. Echocardiography
2.6. Pharmacokinetic Assessment
2.7. Q10 Concentration by High-Performance Liquid Chromatography-Electrochemical Detection
2.8. Statistical Analyses
3. Results
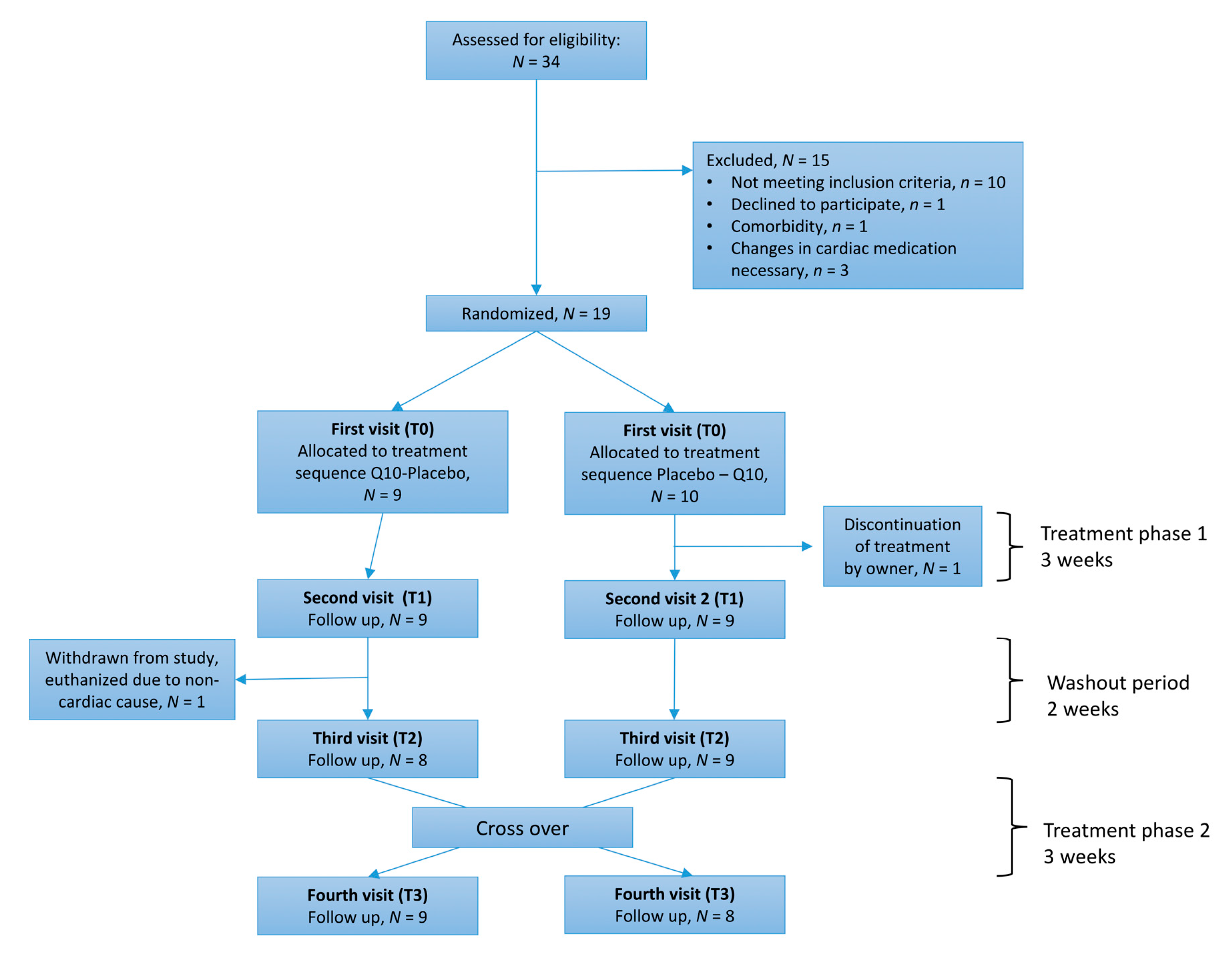
3.1. Study Population
3.2. Adherence
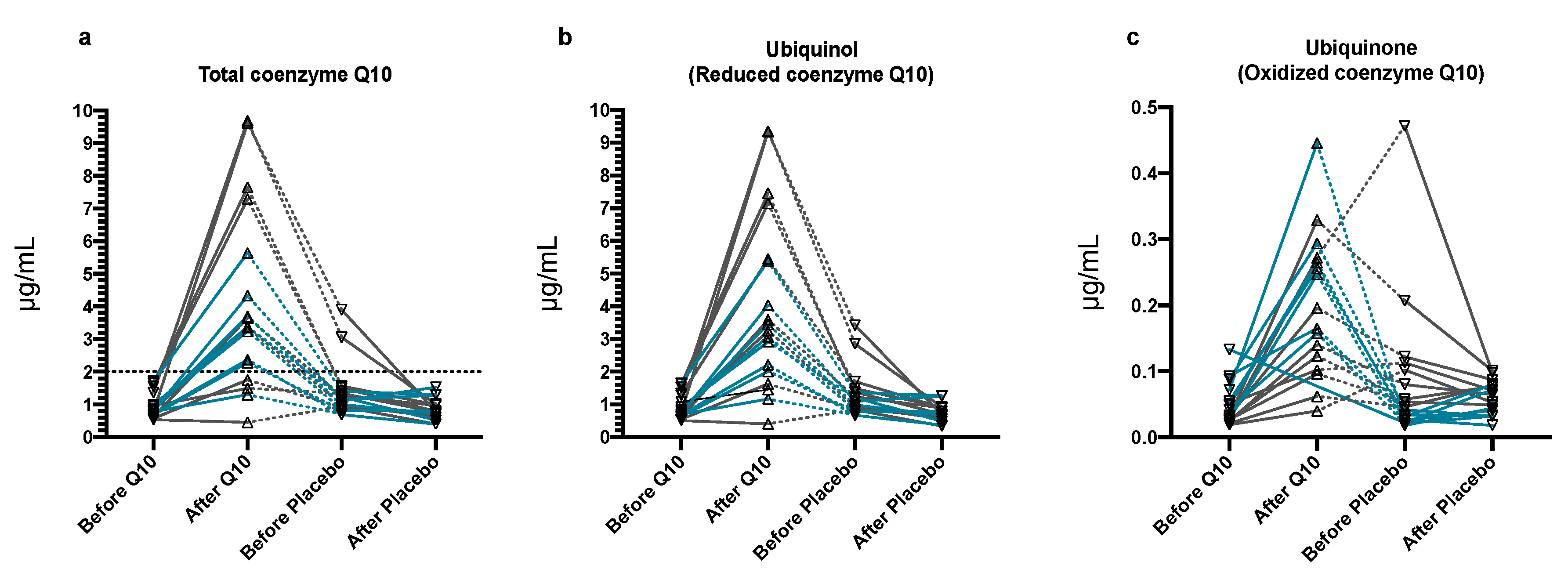
3.3. Plasma Q10 Concentrations
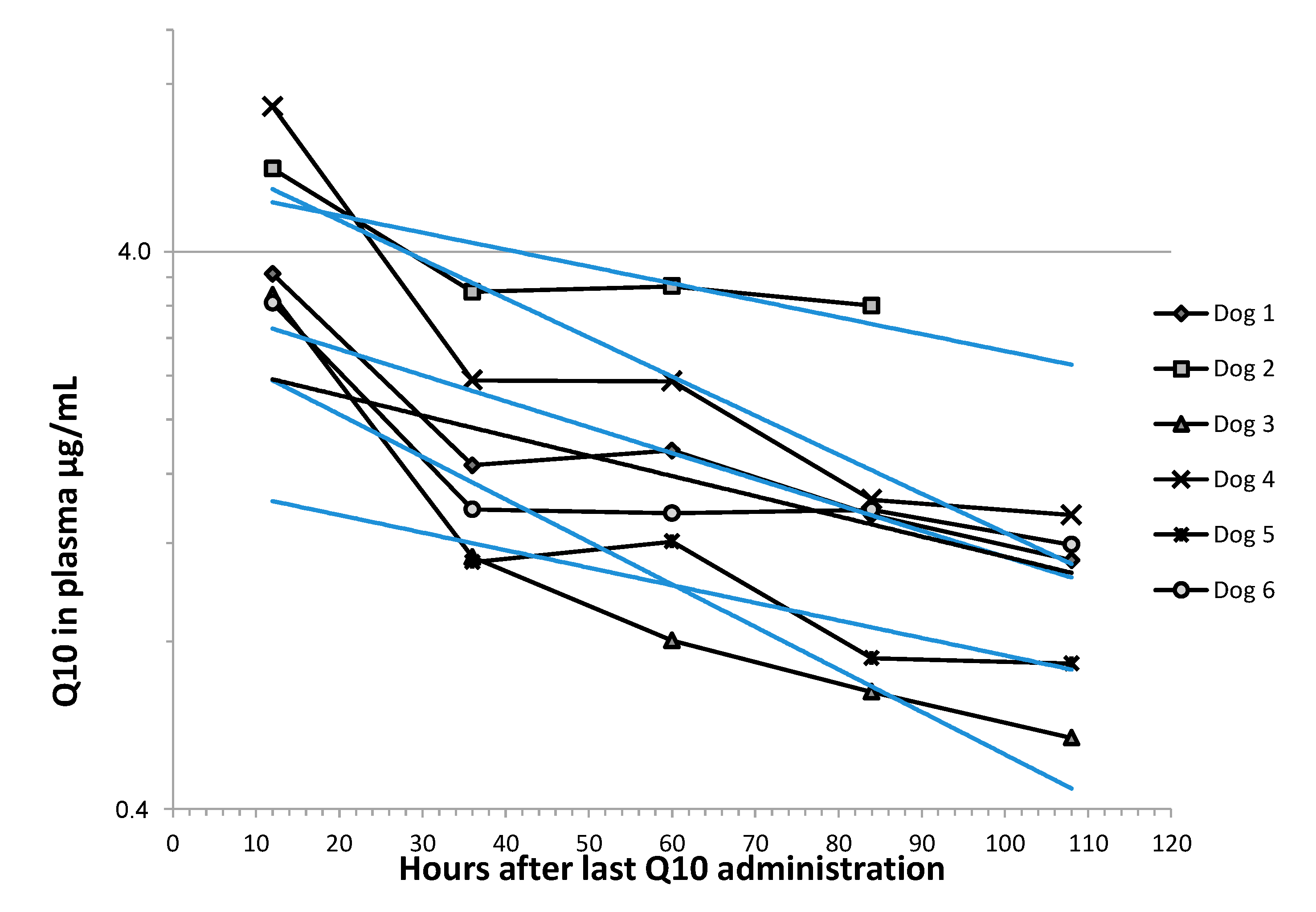
3.4. Pharmacokinetic Analyses
3.5. Secondary Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egenvall, A.; Bonnett, B.N.; Häggström, J. Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J. Vet. Intern. Med. 2006, 20, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Darke, P. Valvular incompetence in cavalier King Charles spaniels. Vet. Rec. 1987, 120, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Häggström, J.; Hansson, K.; Kvart, C.; Swenson, L. Chronic valvular disease in the cavalier King Charles spaniel in Sweden. Vet. Rec. 1992, 131, 549–553. [Google Scholar] [PubMed]
- Häggström, J.; Boswood, A.; O’Grady, M.; Jons, O.; Smith, S.; Swift, S.; Borgarelli, M.; Gavaghan, B.; Kresken, J.-G.; Patteson, M.W.; et al. Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study. J. Vet. Intern. Med. 2008, 22, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Formiggini, G.; Genova, M.L. The role of Coenzyme Q in mitochondrial electron transport. Mitochondrion 2007, 7, S8–S33. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.S.; Morrison, J.A.; Tang, P.H.; Degrauw, T.; Pesce, A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta 2003, 332, 123–132. [Google Scholar] [CrossRef]
- Stride, N.; Larsen, S.; Hey-Mogensen, M.; Sander, K.; Lund, J.T.; Gustafsson, F.; Køber, L.; Dela, F. Decreased mitochondrial oxidative phosphorylation capacity in the human heart with left ventricular systolic dysfunction. Eur. J. Heart Fail. 2013, 15, 150–157. [Google Scholar] [CrossRef]
- Sharov, V.G.; Goussev, A.; Lesch, M.; Goldstein, S.; Sabbah, H.N. Abnormal Mitochondrial Function in Myocardium of Dogs with Chronic Heart Failure. J. Mol. Cell. Cardiol. 1998, 30, 1757–1762. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Vadhanavikit, S.; Mortensen, S.A. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc. Natl. Acad. Sci. USA 1985, 82, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef]
- Langsjoen, P.H.; Langsjoen, A.M. Coenzyme Q10 in cardiovascular disease with emphasis on heart failure and myocardial ischaemia. Asia Pac. Heart J. 1998, 7, 160–168. [Google Scholar] [CrossRef]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and heart failure—A state-of theart Review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef]
- Svete, A.N.; Verk, B.; Seliškar, A.; Tomsič, K.; Križman, P.J.; Petrič, A.D. Plasma coenzyme Q10concentration, antioxidant status, and serum N-terminal pro-brain natriuretic peptide concentration in dogs with various cardiovascular diseases and the effect of cardiac treatment on measured variables. Am. J. Vet. Res. 2017, 78, 447–457. [Google Scholar] [CrossRef]
- Schou-Pedersen, A.M.V.; Schemeth, D.; Lykkesfeldt, J. Determination of Reduced and Oxidized Coenzyme Q10 in Canine Plasma and Heart Tissue by HPLC-ECD: Comparison with LC-MS/MS Quantification. Antioxidants 2019, 8, 253. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Q-SYMBIO Study Investigators. The Effect of Coenzyme Q 10 on Morbidity and Mortality in Chronic Heart Failure. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Boswood, A.; Häggström, J.; Gordon, S.; Wess, G.; Stepien, R.; Oyama, M.; Keene, B.; Bonagura, J.; Macdonald, K.; Patteson, M.W.; et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef]
- Available online: http://www.randomization.com (accessed on 7 November 2016).
- Schulz, K.F.; Altman, D.G.; Moher, D.; Consort Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef]
- Tang, P.H.; Miles, M.V.; Steele, P.; Degrauw, A.; Chuck, G.; Schroer, L.; Pesce, A. Anticoagulant effects on plasma coenzyme Q(10) estimated by HPLC with coulometric detection. Clin. Chim. Acta 2002, 318, 127–131. [Google Scholar] [CrossRef]
- Eksell, P.; Häggström, J.; Kvart, C.; Karlsson, A. Thrombocytopenia in the cavalier King Charles spaniel. J. Small Anim. Pr. 1994, 35, 153–155. [Google Scholar] [CrossRef]
- Langhorn, R.; Willesen, J.L.; Tarnow, I.; Kjelgaard-Hansen, M. Evaluation of a high-sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet. Clin. Pathol. 2013, 42, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Vet, M.; Moise, N.S.; Moses, B.L. Recommendations for Standards in Transthoracic Two-Dimensional Echocardiography in the Dog and Cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.; Moller, J.E.; Häggström, J.; Markussen, B.; Holen, A.; Falk, T.; Olsen, L. R-R interval variations influence the degree of mitral regurgitation in dogs with myxomatous mitral valve disease. Vet. J. 2014, 199, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Häggström, J.; Hansson, K.; Karlberg, B.E.; Kvart, C.; Olsson, K. Plasma concentration of atrial natriuretic peptide in relation to severity of mitral regurgitation in Cavalier King Charles Spaniels. Am. J. Vet. Res. 1994, 55, 698–703. [Google Scholar]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Lombard, C.W. Normal values of the canine M-mode echocardiogram. Am. J. Vet. Res. 1984, 45, 2015–2018. [Google Scholar]
- Wess, G.; Maurer, J.; Simak, J.; Hartmann, K. Use of Simpson’s Method of Disc to Detect Early Echocardiographic Changes in Doberman Pinschers with Dilated Cardiomyopathy. J. Vet. Intern. Med. 2010, 24, 1069–1076. [Google Scholar] [CrossRef]
- Yerramilli-Rao, P.; Beal, M.F.; Watanabe, D.; Kieburtz, K.; De Blieck, E.A.; Kitano, M.; Hosoe, K.; Funahashi, I.; Cudkowicz, M.E. Oral Repeated-Dose Toxicity Studies of Coenzyme Q10 in Beagle Dogs. Int. J. Toxicol. 2012, 31, 58–69. [Google Scholar] [CrossRef]
- Belardinelli, R.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P.; Muçaj, A. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Harker-Murray, A.K.; Tajik, A.; Ishikura, F.; Meyer, D.; Burnett, J.C.; Redfield, M.M. The role of coenzyme Q10 in the pathophysiology and therapy of experimental congestive heart failure in the dog. J. Card. Fail. 2000, 6, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, C.; Xu, P.; Lin, L.; Pan, C.; Wang, L.; Xu, H. Validated HPLC method for the quantitative determination of CoQ10 in dog plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2010, 25, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, A.-A.; Gurley, B.; Khan, M.A.; Bhagavan, H.; Chopra, R.; Reddy, I.K. Bioavailability Assessment of Oral Coenzyme Q10 Formulations in Dogs. Drug Dev. Ind. Pharm. 2002, 28, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Del Pozo-Cruz, J.; Sánchez-Cuesta, A.; Cortés-Rodríguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Leong, A.; Zhou, J.; Forno, S.D.; Hall, S.; Rutolo, D. The Plasma Bioavailability of Coenzyme Q10 Absorbed from the Gut and the Oral Mucosa. J. Funct. Biomater. 2018, 9, 73. [Google Scholar] [CrossRef]
- Khatta, M.; Alexander, B.S.; Krichten, C.M.; Fisher, M.L.; Freudenberger, R.; Robinson, S.W.; Gottlieb, S.S. The effect of coenzyme Q10 in patients with congestive heart failure. Ann. Intern. Med. 2000, 132, 636–640. [Google Scholar] [CrossRef]
- Munkholm, H.; Hansen, H.H.T.; Rasmussen, K. Coenzyme Q10 treatment in serious heart failure. BioFactors 1999, 9, 285–289. [Google Scholar] [CrossRef]
- Ochiai, A.; Itagaki, S.; Kurokawa, T.; Kobayashi, M.; Hirano, T.; Iseki, K. Improvement in intestinal coenzyme q10 absorption by food intake. Yakugaku Zasshi 2007, 127, 1251–1254. [Google Scholar] [CrossRef]
- Lagendijk, J.; Ubbink, B.; Vermaak, W.J.H. Measurement of the ratio between the reduced and oxidized forms of coenzyme Ql0 in human plasma as a possible marker of oxidative stress. J. Lipid Res. 1996, 37, 67–75. [Google Scholar]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Reimann, M.; Häggström, J.; Moller, J.E.; Lykkesfeldt, J.; Falk, T.; Olsen, L. Markers of Oxidative Stress in Dogs with Myxomatous Mitral Valve Disease are Influenced by Sex, Neuter Status, and Serum Cholesterol Concentration. J. Vet. Intern. Med. 2017, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M. Interventional nutrition for cardiac disease. Clin. Tech. Small Anim. Pr. 1998, 13, 232–237. [Google Scholar] [CrossRef]
- Fotino, A.D.; Thompson-Paul, A.M.; Bazzano, L.A. Effect of coenzyme Q₁₀ supplementation on heart failure: A meta-analysis. Am. J. Clin. Nutr. 2012, 97, 268–275. [Google Scholar] [CrossRef]
- Langsjoen, P.H. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J. Am. Coll. Cardiol. 2000, 35, 816–817. [Google Scholar] [CrossRef][Green Version]
- Weber, C.; Bysted, A.; Hølmer, G. Coenzyme Q10 in the diet-daily intake and relative bioavailability. Mol. Asp. Med. 1997, 18, 251–254. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.; Miles, L.; Tang, P.; Steele, P.; Degrauw, T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr. Res. 2002, 22, 919–929. [Google Scholar] [CrossRef]
- Li, Q.; Heaney, A.; Langenfeld-McCoy, N.; Boler, B.V.; Laflamme, D.P. Dietary intervention reduces left atrial enlargement in dogs with early preclinical myxomatous mitral valve disease: A blinded randomized controlled study in 36 dogs. BMC Vet. Res. 2019, 15, 425. [Google Scholar] [CrossRef]
| Group | Dogs Randomized to Receive Q10 in Phase 1 | Dogs Randomized to Receive Q10 in Phase 2 | p-Value |
|---|---|---|---|
| N = 9 | N = 9 | ||
| Sex male/female | 6/3 | 7/2 | >0.999 |
| Murmur intensity 3/4/5 | 0/6/3 | 1/7/1 | 0.58 |
| Age (years) | 9.2 (9.1–10.0) | 8.7 (8.3–9.0) | 0.16 |
| Bodyweight (kg) | 8.6 (8.4–9.3) | 9.0 (8.0–9.6) | 0.69 |
| ACVIM (B2/C) | 5/4 | 5/4 | 1.0 |
| Quality of Life (QoL) score | 9 (9–11) | 12 (12–14) | 0.015 * |
| Heart rate (bpm) | 124 (120–140) | 112 (100–128) | 0.17 |
| Systolic blood pressure (mmHg) | 145 (126–151) | 147 (143–167) | 0.29 |
| VHS | 12.3 (11.6–12.4) | 11.8 (11.5–12) | 0.14 |
| LVIDDN | 2.0 (1.9–2.5) | 1.9 (1.8–2.0) | 0.20 |
| FS (%) | 40.6 (40–43.3) | 40.9 (36.3–46.1) | 0.70 |
| EF (%) | 78.8 (76.4–80.2) | 77.5 (74.3–80.5) | 0.96 |
| LA/Ao | 2.1 (1.8–2.5) | 1.8 (1.6–2.0) | 0.10 |
| Hematocrit | 40.2 (38.4–41.2) | 43.7 (38.5–44.5) | 0.18 |
| Platelet count (× 106 platelets/mL) | 311 (245–372) | 253 (105–272) | 0.35 |
| Creatinine (μmol/L) | 63 (58–67) | 60 (54–78) | 0.79 |
| ALT (U/L) | 43 (35–52) | 51 (38–60) | 0.66 |
| NT-proBNP, ng/mL | 1851 (1577–3438) | 1158 (998–1282) | 0.029 * |
| cTnI (ng/mL) | 0.052 (0.039–0.074) | 0.028 (0.027–0.032) | 0.027 * |
| Total Q10 concentration in plasma (μg/mL) | 0.85 (0.63–1.36) | 1.00 (0.82–1.14) | 0.66 |
| Oxidation ratio (%) | 3.38 (2.29–3.58) | 3.07 (2.45–3.25) | 0.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christiansen, L.B.; Morsing, M.K.; Reimann, M.J.; Martinussen, T.; Birlie, Z.; Schou-Pedersen, A.M.V.; Lykkesfeldt, J.; Olsen, L.H. Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Antioxidants 2020, 9, 827. https://doi.org/10.3390/antiox9090827
Christiansen LB, Morsing MK, Reimann MJ, Martinussen T, Birlie Z, Schou-Pedersen AMV, Lykkesfeldt J, Olsen LH. Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Antioxidants. 2020; 9(9):827. https://doi.org/10.3390/antiox9090827
Chicago/Turabian StyleChristiansen, Liselotte B., Malene K. Morsing, Maria Josefine Reimann, Torben Martinussen, Zita Birlie, Anne Marie V. Schou-Pedersen, Jens Lykkesfeldt, and Lisbeth H. Olsen. 2020. "Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease" Antioxidants 9, no. 9: 827. https://doi.org/10.3390/antiox9090827
APA StyleChristiansen, L. B., Morsing, M. K., Reimann, M. J., Martinussen, T., Birlie, Z., Schou-Pedersen, A. M. V., Lykkesfeldt, J., & Olsen, L. H. (2020). Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Antioxidants, 9(9), 827. https://doi.org/10.3390/antiox9090827






