Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction Procedure
2.4. HPLC-DAD-ESI-Q-MS Profiling
2.5. In Vitro Antioxidant Activity
2.5.1. DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
2.5.2. ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Test
2.5.4. Carotene Bleaching Test
2.6. Cell Cultures
2.7. Inhibition of NO Production in LPS-Stimulated RAW 264.7 Cells
2.8. Immuno-Fluorescence Monitoring Nuclear Factor Kappa B (NF-κB) Translocation
2.9. Reactive Oxygen Species Assessment
2.10. Cell Viability Assay
2.11. Wound-Healing Scratch Assay
2.12. Conditioned Medium Effects Assessment
2.13. TUNEL Assay
2.14. Hemolysis Assay
2.15. Statistical Analysis
3. Results and Discussion
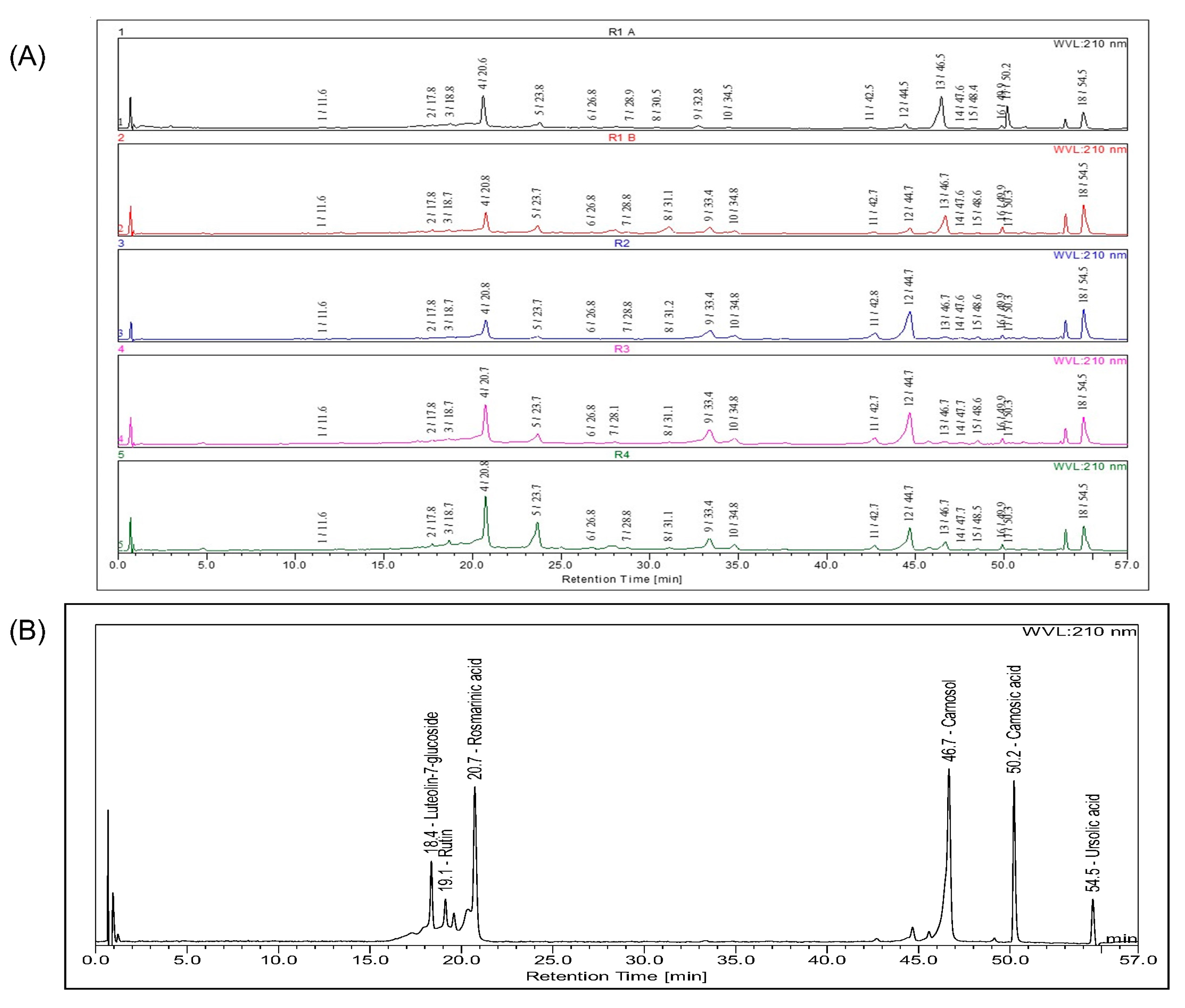
3.1. Chemical Composition
3.2. In Vitro Antioxidant Activity
3.3. Nitric Oxide Production in RAW 264.7 Cells
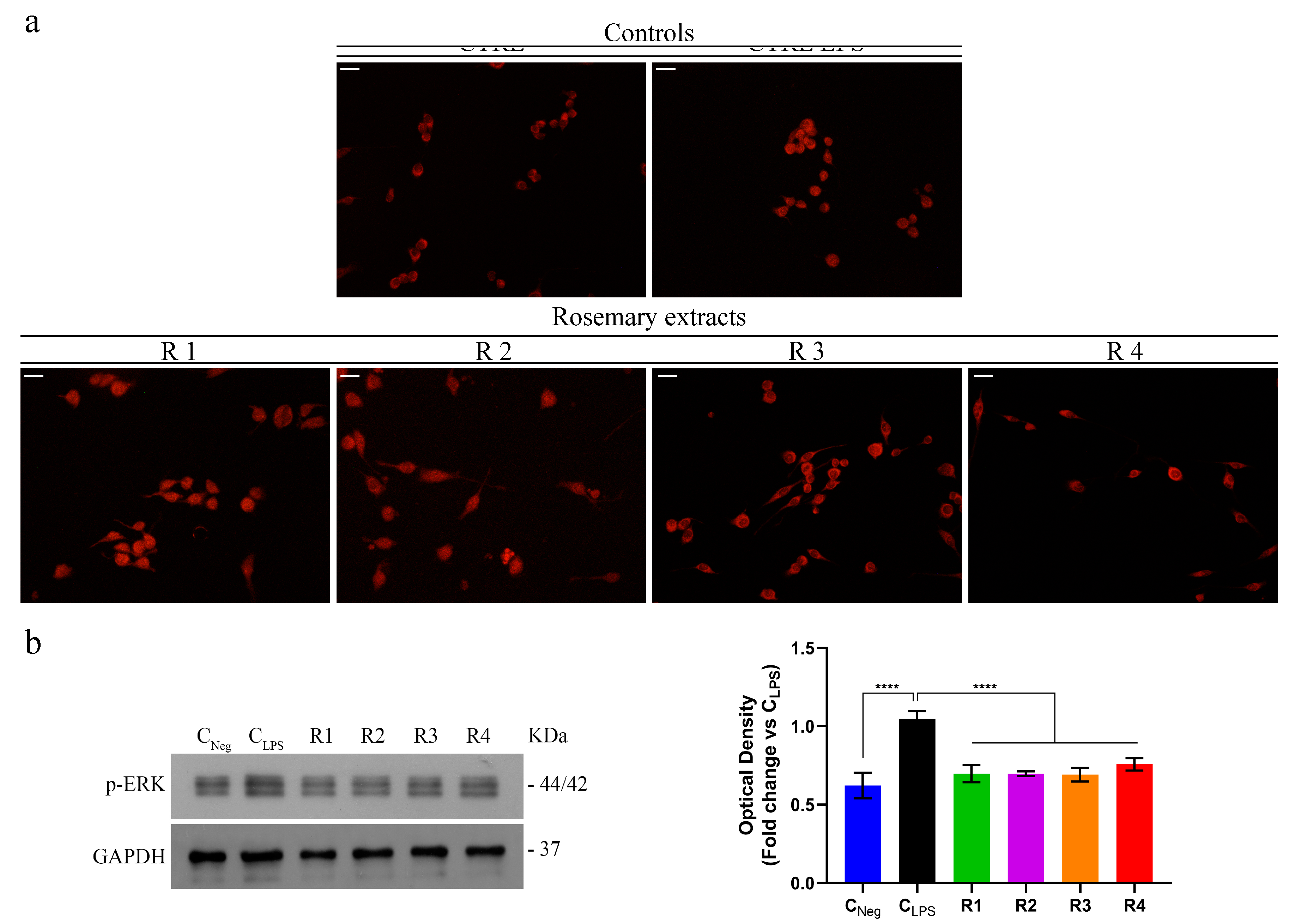
3.4. Rosemary Extracts Exert Anti-Inflammatory Effects by Reducing NF-κB Nuclear Translocation and Disrupting the MAPK/NF-κB Pathway
3.5. Rosemary Extracts Exerts Anti-Oxidant Effects by Reducing ROS Levels
3.6. Rosemary Extracts Showed a Promising Anti-Proliferative Effect on Breast Cancer Cell Lines
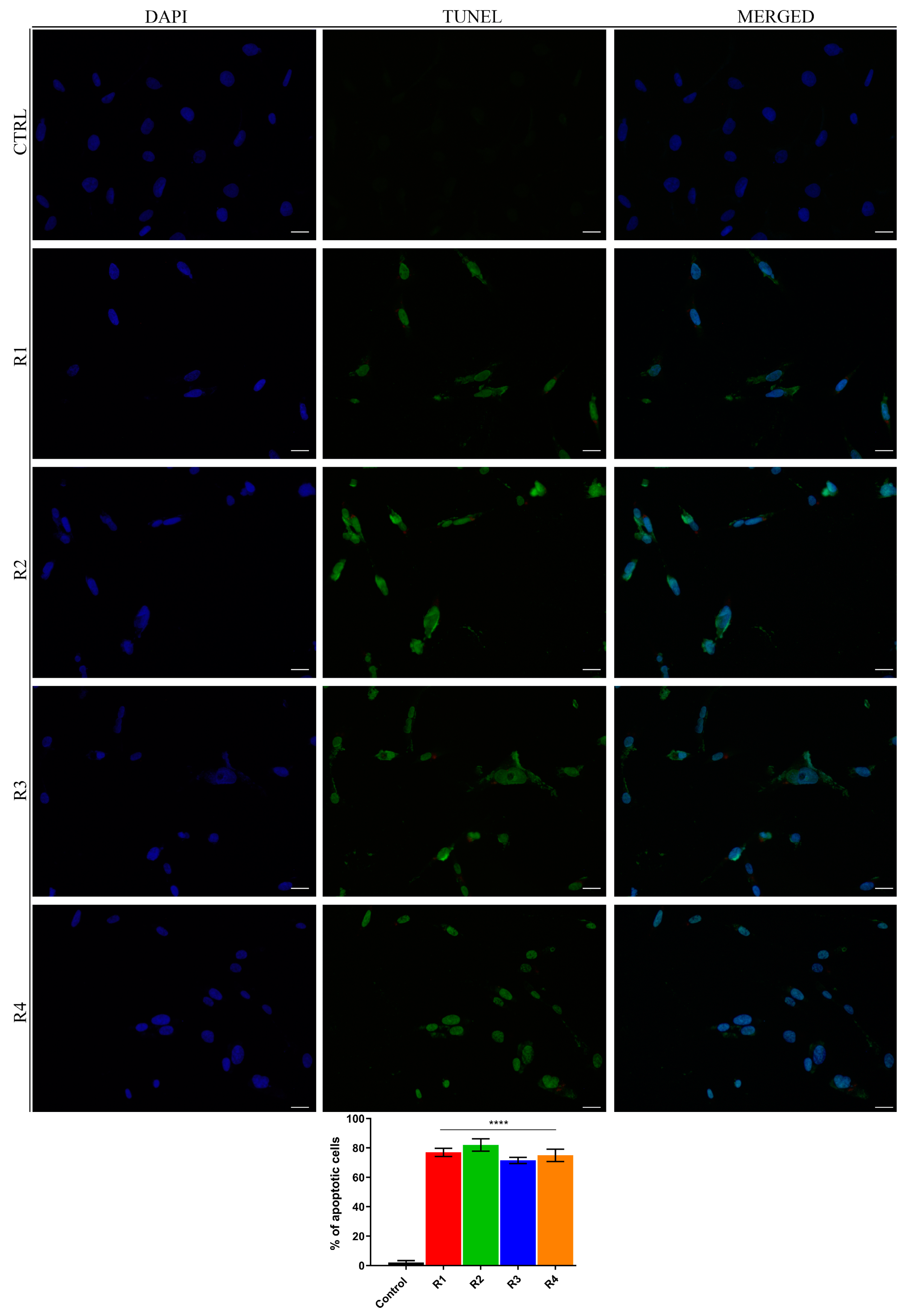
3.7. Rosemary Extracts Trigger Cell Death by Apoptosis in Breast Cancer Cells
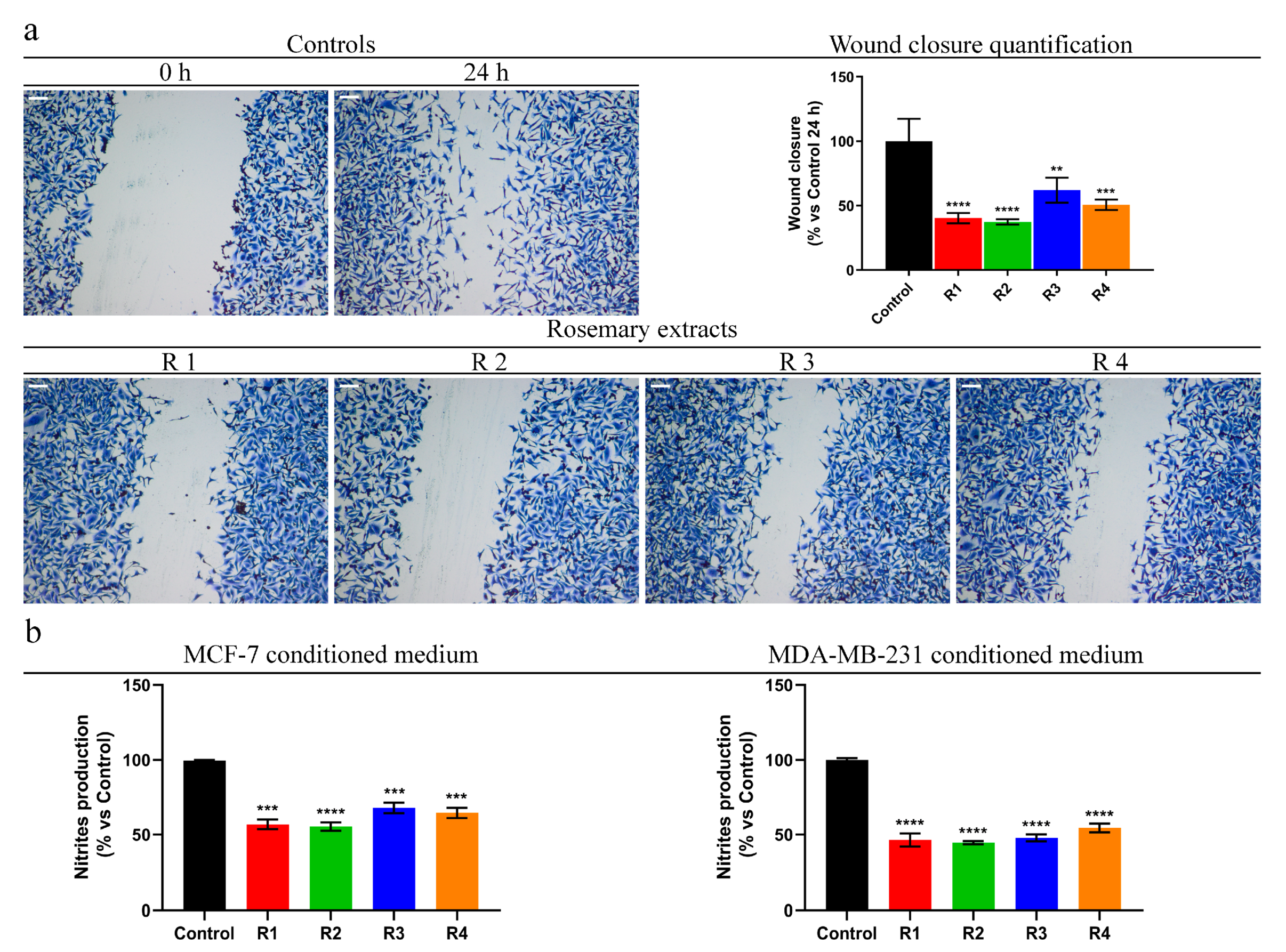
3.8. Rosemary Reduces MDA-MB-231 Cells Motility and Pro-Inflammatory Behavior of Breast Cancer Cells
3.9. Rosemary Extracts Did Not Exert Hemolytic Effects on Peripheral Blood
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Aoyagi, K.; Nakanishi, Y.; Sasaki, H.; Yoshida, T.; Honda, H. Classification of intramural metastases and lymph node metastases of esophageal cancer from gene expression based on boosting and projective adaptive resonance theory. J. Biosci. Bioeng. 2006, 102, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, W.C.; Ho, T.F.; Wu, H.S.; Wei, Y.H. Development of natural anti-tumor drugs by microorganisms. J. Biosci. Bioeng. 2011, 111, 501–511. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Comparative risk assessment collaborating, g. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Benz, C.C.; Yau, C. Ageing, oxidative stress and cancer: Paradigms in parallax. Nat. Rev. Cancer 2008, 8, 875–879. [Google Scholar] [CrossRef]
- Clendening, J.W.; Pandyra, A.; Boutros, P.C.; El Ghamrasni, S.; Khosravi, F.; Trentin, G.A.; Martirosyan, A.; Hakem, A.; Hakem, R.; Jurisica, I.; et al. Dysregulation of the mevalonate pathway promotes transformation. Proc. Natl. Acad. Sci. USA 2010, 107, 15051–15056. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Farrow, B.; Sugiyama, Y.; Chen, A.; Uffort, E.; Nealon, W.; Mark Evers, B. Inflammatory mechanisms contributing to pancreatic cancer development. Ann. Surg. 2004, 239, 763–771. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–196. [Google Scholar] [CrossRef]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Bonesi, M.; Brindisi, M.; Armentano, B.; Curcio, R.; Sicari, V.; Loizzo, M.R.; Cappello, M.S.; Bedini, G.; Peruzzi, L.; Tundis, R. Exploring the anti-proliferative, pro-apoptotic, and antioxidant properties of Santolina corsica Jord. & Fourr. (Asteraceae). Biomed. Pharmacother. 2018, 107, 967–978. [Google Scholar] [CrossRef]
- da Rocha, A.B.; Lopes, R.M.; Schwartsmann, G. Natural products in anticancer therapy. Curr. Opin. Pharmacol. 2001, 1, 364–369. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; Di Marzio, L.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Peiris-Pages, M.; Sanchez-Alvarez, R.; Bartella, L.; Di Donna, L.; Dolce, V.; Sindona, G.; Sotgia, F.; Cappello, A.R.; Lisanti, M.P. Bergamot natural products eradicate cancer stem cells (CSCs) by targeting mevalonate, Rho-GDI-signalling and mitochondrial metabolism. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Saija, A.; Cristani, M.; Cimino, F.; D’Arrigo, M.; Trombetta, D.; Rao, F.; Ruberto, G. Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves-chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia 2011, 82, 546–556. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Dabulici, C.M.; Sarbu, I.; Vamanu, E. The bioactive potential of functional products and bioavailability of phenolic compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Vulic, J.; Seregelj, V.; Kalusevic, A.; Levic, S.; Nedovic, V.; Tumbas Saponjac, V.; Canadanovic-Brunet, J.; Cetkovic, G. Bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walker, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary extract as a potential anti-hyperglycemic agent: Current evidence and future perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef]
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fons, L.; Garzon, M.T.; Micol, V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J. Agric. Food Chem. 2010, 58, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Yamada, K.; Naemura, A.; Yamashita, T.; Arai, R. Testing various herbs for antithrombotic effect. Nutrition 2005, 21, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yuan, J.; Liu, F.; Ye, J. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2005, 39, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Chandrika, U.G.; Abeysekera, A.M.; Menichini, F.; Frega, N.G. Antioxidant and antibacterial activities on foodborne pathogens of Artocarpus heterophyllus Lam. (Moraceae) leaves extracts. J. Food Sci. 2010, 75, M291–M295. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT-Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Brindisi, M.; Fiorillo, M.; Frattaruolo, L.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Cholesterol and mevalonate: Two metabolites involved in breast cancer progression and drug resistance through the ERRα Pathway. Cells 2020, 9, 1819. [Google Scholar] [CrossRef]
- Mazzotta, S.; Frattaruolo, L.; Brindisi, M.; Ulivieri, C.; Vanni, F.; Brizzi, A.; Carullo, G.; Cappello, A.R.; Aiello, F. 3-Amino-alkylated indoles: Unexplored green products acting as anti-inflammatory agents. Future Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Fiorillo, M.; Brindisi, M.; Curcio, R.; Dolce, V.; Lacret, R.; Truman, A.W.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Thioalbamide, A thioamidated peptide from amycolatopsis alba, affects tumor growth and stemness by inducing metabolic dysfunction and oxidative stress. Cells 2019, 8, 1408. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Toth, F.; Brindisi, M.; Sotgia, F.; Lisanti, M.P. Deferiprone (DFP) Targets Cancer Stem Cell (CSC) Propagation by Inhibiting Mitochondrial Metabolism and Inducing ROS Production. Cells 2020, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.V.; Deep, G.; Gangar, S.C.; Jain, A.K.; Agarwal, C.; Agarwal, R. Silibinin inhibits prostate cancer cells- and RANKL-induced osteoclastogenesis by targeting NFATc1, NF-kappaB, and AP-1 activation in RAW264.7 cells. Mol. Carcinog. 2014, 53, 169–180. [Google Scholar] [CrossRef]
- Armentano, B.; Curcio, R.; Brindisi, M.; Mancuso, R.; Rago, V.; Ziccarelli, I.; Frattaruolo, L.; Fiorillo, M.; Dolce, V.; Gabriele, B.; et al. 5-(Carbamoylmethylene)-oxazolidin-2-ones as a promising class of heterocycles inducing apoptosis triggered by increased ROS Levels and mitochondrial dysfunction in breast and cervical cancer. Biomedicines 2020, 8, 35. [Google Scholar] [CrossRef]
- Burci, L.M.; da Silva, C.B.; Rondon, J.N.; da Silva, L.M.; de Andrade, S.F.; Miguel, O.G.; de Fatima Gaspari Dias, J.; Miguel, M.D. Acute and subacute (28 days) toxicity, hemolytic and cytotoxic effect of Artocarpus heterophyllus seed extracts. Toxicol. Rep. 2019, 6, 1304–1308. [Google Scholar] [CrossRef]
- Crasci, L.; Lauro, M.R.; Puglisi, G.; Panico, A. natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Nowsheen, S.; Aziz, K.; Kryston, T.B.; Ferguson, N.F.; Georgakilas, A. The interplay between inflammation and oxidative stress in carcinogenesis. Curr. Mol. Med. 2012, 12, 672–680. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Dai, C.; Li, B.; Zhou, Y.; Li, D.; Zhang, S.; Li, H.; Xiao, X.; Tang, S. Curcumin attenuates quinocetone induced apoptosis and inflammation via the opposite modulation of Nrf2/HO-1 and NF-kB pathway in human hepatocyte L02 cells. Food Chem. Toxicol. 2016, 95, 52–63. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, C.H.; Lee, S.K.; Ha, S.H.; Park, J.; Chung, T.W.; Ha, K.T.; Suh, S.J.; Chang, Y.C.; Chang, H.W.; et al. Anti-Inflammatory effect of ascochlorin in LPS-Stimulated RAW 264.7 macrophage cells is accompanied with the down-regulation of iNOS, COX-2 and proinflammatory cytokines through NF-kappaB, ERK1/2, and p38 Signaling Pathway. J. Cell. Biochem. 2016, 117, 978–987. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Medicherla, K.; Ketkar, A.; Sahu, B.D.; Sudhakar, G.; Sistla, R. Rosmarinus officinalis L. extract ameliorates intestinal inflammation through MAPKs/NF-kappaB signaling in a murine model of acute experimental colitis. Food Funct. 2016, 7, 3233–3243. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Itharat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Reglero, G.; Ramirez de Molina, A. Rosemary (Rosmarinus officinalis L.) extract as a potential complementary agent in anticancer therapy. Nutr. Cancer 2015, 67, 1221–1229. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Puthenveetil, A.G.; Johnson, J.J. Polyphenols from the Mediterranean herb rosemary (Rosmarinus officinalis) for prostate cancer. Front. Pharmacol. 2013, 4, 29. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martin-Hernandez, R.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez de Molina, A. Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS ONE 2014, 9, e98556. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Sanchez-Martinez, R.; Vargas, T.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez de Molina, A. Modulation of estrogen and epidermal growth factor receptors by rosemary extract in breast cancer cells. Electrophoresis 2014, 35, 1719–1727. [Google Scholar] [CrossRef]
- Bortner, C.D.; Oldenburg, N.B.; Cidlowski, J.A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995, 5, 21–26. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Obeid, E.; Nanda, R.; Fu, Y.X.; Olopade, O.I. The role of tumor-associated macrophages in breast cancer progression. Int. J. Oncol. 2013, 43, 5–12. [Google Scholar] [CrossRef]
- Knowles, H.; Leek, R.; Harris, A.L. Macrophage infiltration and angiogenesis in human malignancy. In Novartis Foundation Symposium; Discussion 200-184, 259-169; John Wiley: Chichester, UK; New York, NY, USA, 2004; Volume 256, pp. 189–200. [Google Scholar]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R.; et al. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ochoa, Á.; Borrás-Linares, I.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; González-Álvarez, I.; Arráez-Román, D.; Micol, V.; Segura-Carretero, A. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Foods 2017, 33, 202–210. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Borras-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; Gonzalez-Alvarez, I.; Ibanez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the intestinal permeability of rosemary (Rosmarinus officinalis L.) extract polyphenols and terpenoids in Caco-2 cell monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-inflammatory and antioxidant activities from the basolateral fraction of Caco-2 cells exposed to a rosmarinic acid enriched extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef]
- Doolaege, E.H.; Raes, K.; De Vos, F.; Verhé, R.; De Smet, S. Absorption, distribution and elimination of carnosic acid, a natural antioxidant from Rosmarinus officinalis, in rats. Plant Foods Hum. Nutr. 2011, 66, 196–202. [Google Scholar] [CrossRef]
- Yan, H.; Wang, L.; Li, X.; Yu, C.; Zhang, K.; Jiang, Y.; Wu, L.; Lu, W.; Tu, P.J. High-performance liquid chromatography method for determination of carnosic acid in rat plasma and its application to pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 776–781. [Google Scholar] [CrossRef]
- Achour, M.; Saguem, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems. J. Sci. Food Agric. 2018, 98, 3741–3751. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F. Correlations between microbiota bioactivity and bioavailability of functional compounds: A mini-review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef]

| S. rosmarinus | Site of Collection (Southern Italy) | Extraction Procedure | Yield (%) |
|---|---|---|---|
| R1 | Cirò Superiore (Ionian coast) | Maceration | 11.63 ± 1.34 |
| R2 | Ultrasound-assisted extraxction | 10.45 ± 1.11 | |
| R3 | Praia a Mare (Thyrrenian coast) | Maceration | 10.71 ± 1.43 |
| R4 | Ultrasound-assisted extraxction | 7.44 ± 0.87 |
| Peak | Rt | UV λ(nm) | Molecular Ion [M−H]− (m/z) | Molecular Ion [M+H]+ (m/z) | Identification | Ref. |
|---|---|---|---|---|---|---|
| 1 | 11.6 | 220/240/295/325 | 179 | Caffeic acid | [30] | |
| 2 | 17.8 | 200/225/285 | 609 | 611 | Hesperidin | [30,37] |
| 3 | 18.8 | 208/275/340 | 477 | 479 | Isorhamnetin-3-O-hexoside | [30,37] |
| 4 | 20.6 | 200/280/290/330 | 359 | 361 | Rosmarinic acid (cis,trans) 2 | [30] |
| 5 | 23.8 | 205/242/270/340 | 461 | 463 | Hispidulin-7-glucoside | [37] |
| 6 | 26.8 | 205/270/340 | 503 | 505 | Luteolin -3′-acetyl-O-glucuronide | [37] |
| 7 | 28.9 | 208/286 | 345 | 347 | Rosmanol isomer 1 | [30,37] |
| 8 | 30.4 | 205/285 | 345 | 347 | Rosmanol isomer 1 | [30,37] |
| 9 | 32.8 | 205/290 | 345 | 347 | Rosmanol isomer 1 | [30,37] |
| 10 | 34.5 | 205/290 | 315 | Dihydroxydimethoxy flavone | [37] | |
| 11 | 42.5 | 208/280/335 | 283 | 285 | Genkwanin | [30,37] |
| 12 | 44.4 | 207/291 | 359 | Rosmanol methylether isomer | [37] | |
| 13 | 46.5 | 205/286 | 329 | 331 | Carnosol 2 | [30] |
| 14 | 47.6 | 265/300/345 | 593 | Kaempferol | [30] | |
| 15 | 48.4 | 227/280 | 315 | 317 | Rosmaridiphenol | [30] |
| 16 | 49.9 | 208/233/285 | 345 | 12-O-methylcarnosic acid | [30,37] | |
| 17 | 50.1 | 204/235/287 | 331 | Carnosic acid 2 | [30,37] | |
| 18 | 54.5 | 593 3 | Oleanolic acid/Ursolic acid 2 | [30] |
| Extract | Rosmarinic Acid (Rt 20.6 min) | Triterpene Acids (Rt 54.5 min) | ||
|---|---|---|---|---|
| (*) | (% g/g) | (*) | (% g/g) | |
| R1 | 0.11 ± 0.02 | 5.39 | 0.340 ± 0.008 | 16.67 |
| R2 | 0.066 ± 0.009 | 3.84 | 0.380 ± 0.023 | 22.09 |
| R3 | 0.0384 ± 0.0067 | 2.09 | 0.393 ± 0.043 | 21.36 |
| R4 | 0.0407 ± 0.0068 | 2.37 | 0.167 ± 0.026 | 9.71 |
| S. rosmarinus | DPPH a Test IC50 (μg/mL) | ABTS b Test IC50 (μg/mL) | β-carotene Bleaching Test IC50 (μg/mL) | FRAP c Test μM Fe (II)/g 1 | |
|---|---|---|---|---|---|
| 30 min | 60 min | ||||
| R1 | 8.83 ± 0.82 ** | 1.48 ± 0.10 | 12.53 ± 1.20 **** | 8.87 ± 0.85 **** | 95.17 ± 6.62 |
| R2 | 8.80 ± 0.81 ** | 0.94 ± 0.09 | 7.18 ± 0.76 **** | 6.53 ± 0.65 **** | 80.09 ± 5.01 |
| R3 | 11.23 ± 1.14 **** | 1.57 ± 0.14 *** | 45.57 ± 3.90 **** | 26.61 ± 2.62 **** | 97.20 ± 6.30 |
| R4 | 14.76 ± 1.42 **** | 2.01 ± 0.24 | 10.84 ± 1.05 **** | 10.75 ± 1.03 **** | 76.77 ± 5.13 |
| Positive control | |||||
| Ascorbic acid | 5.02 ± 0.80 | 1.71 ± 0.06 | |||
| Propyl gallate | 0.09 ± 0.004 | 0.09 ± 0.004 | |||
| BHT | 63.20 ± 4.32 | ||||
| IC50 ± SD (µg/mL) | |||
|---|---|---|---|
| R1 | R2 | R3 | R4 |
| 3.46 ± 0.78 | 1.71 ± 0.40 | 2.03 ± 0.20 | 5.53 ± 1.26 |
| Cell Line | R1 | R2 | R3 | R4 | |
|---|---|---|---|---|---|
| MCF-7 | IC50 (µg/mL) | 11.48 | 10.96 | 23.33 | 32.17 |
| 95% confidence interval | 8.688 to 14.90 | 8.391 to 14.06 | 17.07 to 31.77 | 27.43 to 37.72 | |
| MDA-MB-231 | IC50 (µg/mL) | 8.648 | 6.830 | 12.24 | 15.67 |
| 95% confidence interval | 5.687 to 12.50 | 4.169 to 10.31 | 7.992 to 17.94 | 12.80 to 19.09 | |
| MCF-10A | IC50 (µg/mL) | 285.6 | 265.7 | 241.9 | 212.3 |
| 95% confidence interval | 140.1 to 1456 | 137.4 to 987.8 | 140.1 to 585.9 | 93.64 to 1601 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants 2020, 9, 826. https://doi.org/10.3390/antiox9090826
Brindisi M, Bouzidi C, Frattaruolo L, Loizzo MR, Tundis R, Dugay A, Deguin B, Cappello AR, Cappello MS. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants. 2020; 9(9):826. https://doi.org/10.3390/antiox9090826
Chicago/Turabian StyleBrindisi, Matteo, Chouaha Bouzidi, Luca Frattaruolo, Monica R. Loizzo, Rosa Tundis, Annabelle Dugay, Brigitte Deguin, Anna Rita Cappello, and Maria Stella Cappello. 2020. "Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts" Antioxidants 9, no. 9: 826. https://doi.org/10.3390/antiox9090826
APA StyleBrindisi, M., Bouzidi, C., Frattaruolo, L., Loizzo, M. R., Tundis, R., Dugay, A., Deguin, B., Cappello, A. R., & Cappello, M. S. (2020). Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants, 9(9), 826. https://doi.org/10.3390/antiox9090826









