Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances
Abstract
1. Introduction
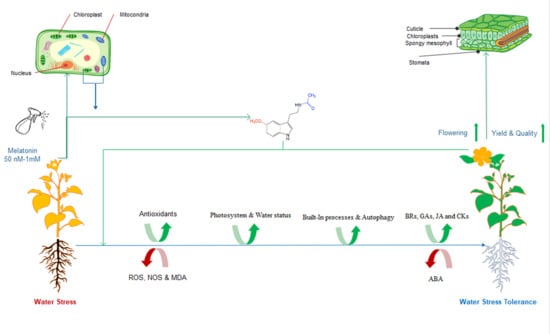
2. Melatonin-Induced Drought Stress Tolerance
2.1. An Overview
2.2. Melatonin is Involved in Drought Stress Tolerance
2.3. Mechanisms of Melatonin-Induced Drought Stress Tolerance
2.3.1. Anatomical Changes and Physiological Mechanisms
2.3.2. Molecular Mechanisms
Omics of Redox Hemostasis and Plant Built-In Processes
Omics of Energy Production, Photosynthesis, and Wax Biosynthesis
Omics of Stomatal Movement, Autophagy, and Others
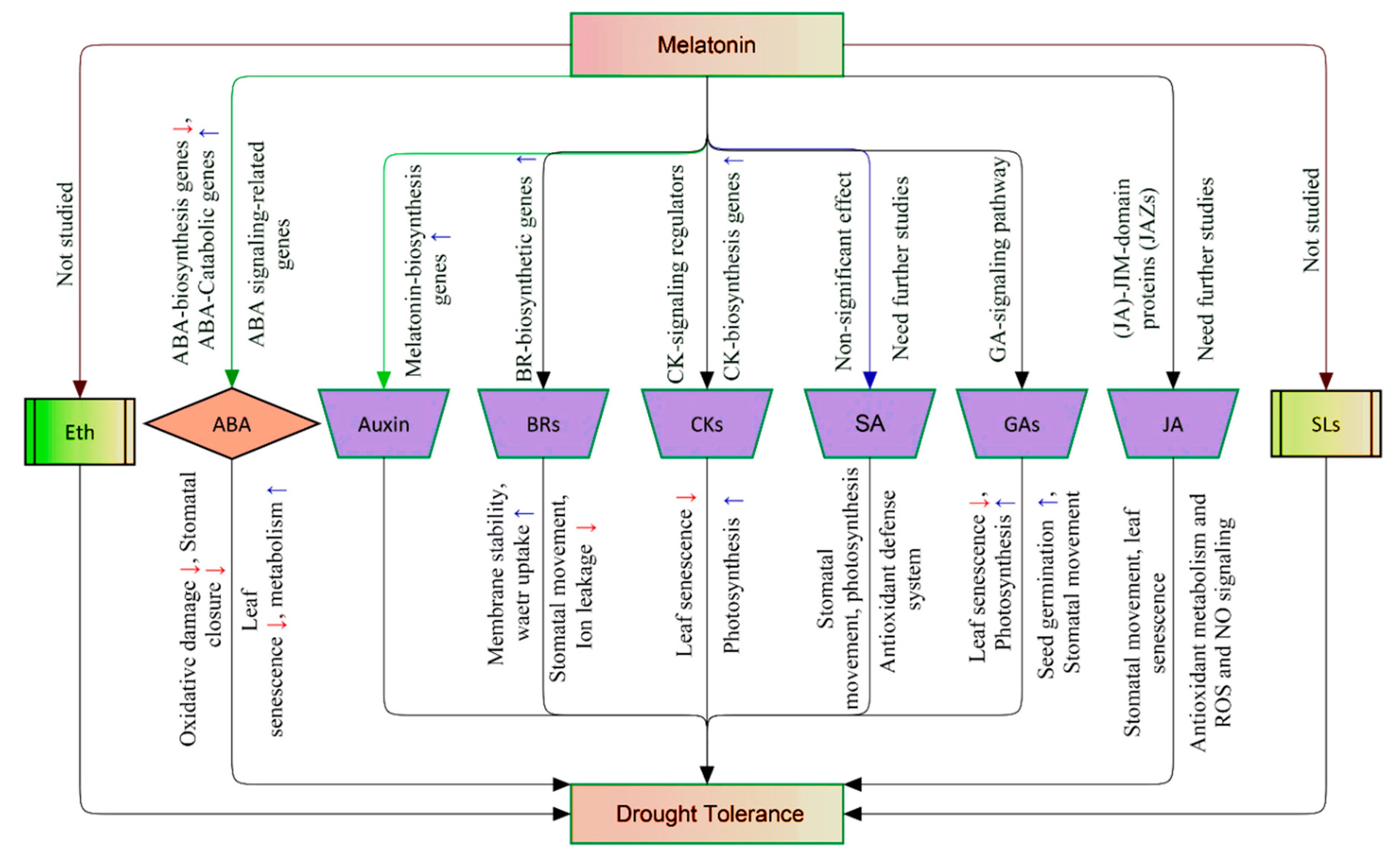
2.3.3. Melatonin Orchestrates other Phytohormones in the Regulatory–Defense Network
2.3.4. The Crosstalk of Melatonin, Nitric Oxide, and Hydrogen Sulfide in Melatonin–Water Stress Research
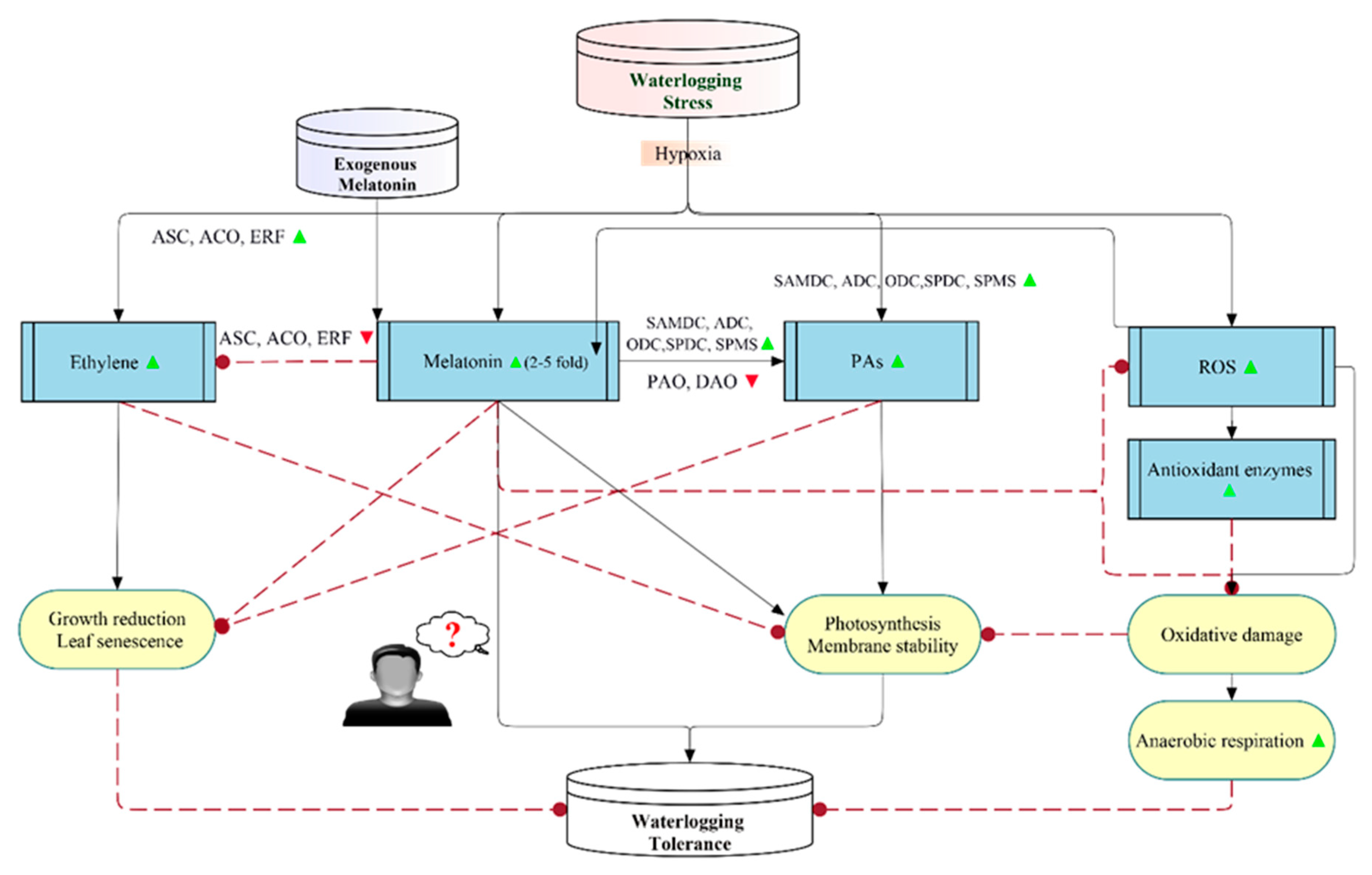
3. Melatonin-Induced Waterlogging Stress Tolerance
3.1. An Overview
3.2. Mechanisms of Melatonin-Mediated Waterlogging Stress Tolerance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xoconostle-Cázares, B.; Ramirez-Ortega, F.A.; Flores-Elenes, L.; Ruiz-Medrano, R. Drought tolerance in crop plants. Am. J. Plant Physiol. 2010, 5, 1–16. [Google Scholar]
- Stuart, M.E.; Gooddy, D.C.; Bloomfield, J.P.; Williams, A.T. A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Sci. Total Environ. 2011, 409, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Islam, M.T.; Nahar, K.; Anee, T.I. Drought stress tolerance in wheat: Omics approaches in enhancing antioxidant defense. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Zargar, S.M., Ed.; Springer: New York, NY, USA, 2016; pp. 267–307. [Google Scholar]
- Arbona, V.; Hossain, Z.; Lopez-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 2008, 132, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Lopez-Climent, M.F.; Arbona, V.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Modulation of the antioxidant system in Citrus under waterlogging and subsequent drainage. J. Plant Physiol. 2009, 166, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Boru, G.; Vantoai, T.; Alves, J.; Hua, D.; Knee, M. Responses of Soybean to Oxygen Deficiency and Elevated Root-zone Carbon Dioxide Concentration. Ann. Bot. 2003, 91, 447–453. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Sasidharan, R. Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef]
- Dennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J. Plant Res. 2019, 132, 881–901. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Mishra, A. Flooding Stress in Plants and Approaches to Overcome. In Plant Metabolites and Regulation under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 355–366. [Google Scholar]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous Application of Melatonin Induces Tolerance to Salt Stress by Improving the Photosynthetic Efficiency and Antioxidant Defense System of Maize Seedling. J. Plant Growth Regul. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and Its Effects on Plant Systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Back, K.; Lee, H.-J. 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2019, 2, 35–46. [Google Scholar]
- Yang, W.J.; Du, Y.T.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, Y.Z.; Chen, M.; Min, D.H. Overexpression of TaCOMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jin, X.J.; Zhang, Y.X. Melatonin confers drought stress tolerance in soybean (Glycine max L.) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism. Photosynthetica 2019, 57, 812–819. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Yang, Q.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol. Biochem. 2019, 142, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ni, Z.; Hu, R.; Lin, L.; Deng, H.; Wang, J.; Tang, Y.; Sun, G.; Wang, X.; Li, H.; et al. Melatonin Alleviates Drought Stress by a Non-Enzymatic and Enzymatic Antioxidative System in Kiwifruit Seedlings. Int. J. Mol. Sci. 2020, 21, 852. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Xia, H.; Liang, D. Effects of exogenous melatonin and abscisic acid on the antioxidant enzyme activities and photosynthetic pigment in ‘Summer Black’ grape under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 012013. [Google Scholar] [CrossRef]
- Karaca, P.; Cekic, F.Ö. Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Melatonin improves the multiple stress tolerance in pepper (Capsicum annuum). Sci. Hortic. 2019, 256, 108509. [Google Scholar] [CrossRef]
- Campos, C.N.; Avila, R.G.; Dazio de Souza, K.R.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop. Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wu, C.; Tie, W.; Yan, Y.; He, G.; Hu, W. Strand-specific RNA-seq based identification and functional prediction of lncRNAs in response to melatonin and simulated drought stresses in cassava. Plant Physiol. Biochem. 2019, 140, 96–104. [Google Scholar] [CrossRef]
- Zou, J.N.; Jin, X.J.; Zhang, Y.X.; Ren, C.Y.; Zhang, M.C.; Wang, M.X. Effects of melatonin on photosynthesis and soybean seed growth during grain filling under drought stress. Photosynthetica 2019, 57, 512–520. [Google Scholar] [CrossRef]
- Li, H.; Mo, Y.; Cui, Q.; Yang, X.; Guo, Y.; Wei, C.; Yang, J.; Zhang, Y.; Ma, J.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef]
- Shi, H.; Qian, Y.; Tan, D.X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef]
- Lee, H.-J.; Back, K. 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J. Pineal Res. 2016, 61, 303–316. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Deng, X.P.; Wang, S.W.; Yin, L.N.; Chen, D.Q.; Xiong, B.L.; Wang, X.Y. Effects of melatonin on growth, photosynthetic characteristics and antioxidant system in seedling of wheat under drought stress. J. Triticeae Crop. 2015, 35, 1275–1283. [Google Scholar]
- Li, D.; Zhang, D.; Wang, H.; Li, H.; Song, S.; Li, H.; Li, R. Effects of melatonin on germination and amino acid content in different wheat varieties seeds under polyethylene glycol stress. BioRxiv 2019, 2019, 710954. [Google Scholar]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Li, X.; Tan, D.X.; Jiang, D.; Liu, F. Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef]
- Zhang, M.; He, S.; Zhan, Y.; Qin, B.; Jin, X.; Wang, M.; Zhang, Y.; Hu, G.; Teng, Z.; Wu, Y. Exogenous melatonin reduces the inhibitory effect of osmotic stress on photosynthesis in soybean. PLoS ONE 2019, 14, e0226542. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Deqing, C.; Liang, D. Effects of exogenous melatonin and abscisic acid on osmotic adjustment substances of ‘Summer Black’ grape under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 012012. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.-C.; Ren, S.; Guo, Y.-D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Yan, W.; Hongyan, L.; Xuejiao, M.; Xuejuan, W.; Yuanbing, Z. Effect of Foliar Spraying Exogenous Melatonin on Physiological and Biochemical Characteristics of Dendranthema morifolium. Acta Bot. Boreali-Occident. Sin. 2016, 36, 2241–2246. [Google Scholar]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Alam, M.N.; Wang, Y.; Chan, Z. Physiological and biochemical analyses reveal drought tolerance in cool-season tall fescue (Festuca arundinacea) turf grass with the application of melatonin. Crop Past. Sci. 2018, 69, 1041–1049. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Amiri, H.; Ismaili, A. Improving drought stress tolerance in fenugreek (Trigonella foenum-graecum) by exogenous melatonin. Plant Biosyst. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 2013, 55, 149–155. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013, 138, 1212–1214. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, H.; Hu, W.; Chen, L.; He, C.; Shi, H. Comparative Transcriptional Profiling of Melatonin Synthesis and Catabolic Genes Indicates the Possible Role of Melatonin in Developmental and Stress Responses in Rice. Front. Plant Sci 2016, 7, 676. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Role of Melatonin to Enhance Phytoremediation Capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous Melatonin Improves Tolerance to Water Deficit by Promoting Cuticle Formation in Tomato Plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Sun, F.; Gao, X.; Xie, K.; Zhang, C.; Liu, S.; Xi, Y. Proteomic analysis of melatonin-mediated osmotic tolerance by improving energy metabolism and autophagy in wheat (Triticum aestivum L.). Planta 2018, 248, 69–87. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Xiong, Y.; Liu, C.; Wang, J.; Wang, G.; Cai, Y. Overexpression of a maize plasma membrane intrinsic protein ZmPIP1;1 confers drought and salt tolerance in Arabidopsis. PLoS ONE 2018, 13, e0198639. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Burgess, P.; Huang, B. Mechanisms of Hormone Regulation for Drought Tolerance in Plants. In Drought Stress Tolerance in Plants, Vol 1: Physiology and Biochemistry; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Swizerland, 2016; pp. 45–75. [Google Scholar]
- Behnam, B.; Iuchi, S.; Fujita, M.; Fujita, Y.; Takasaki, H.; Osakabe, Y.; Yamaguchi-Shinozaki, K.; Kobayashi, M.; Shinozaki, K. Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013, 20, 315–324. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, C. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 2007, 58, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hwang, I. Cytokinin: Perception, signal transduction, and role in plant growth and development. J. Plant Biol. 2007, 50, 98–108. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burgess, P.; Huang, B. Transcriptional regulation of hormone-synthesis and signaling pathways by overexpressing cytokinin-synthesis contributes to improved drought tolerance in creeping bentgrass. Physiol. Plant. 2017, 161, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Melatonin is involved in skotomorphogenesis by regulating brassinosteroid biosynthesis in rice plants. J. Pineal Res. 2018, 65, e12495. [Google Scholar] [CrossRef]
- Xia, X.-J.; Gao, C.-J.; Song, L.-X.; Zhou, Y.-H.; Shi, K.A.I.; Yu, J.-Q. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant Cell Environ. 2014, 37, 2036–2050. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K.; Tuberosa, R. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl Jasmonate-Induced Alteration in Lipid Peroxidation, Antioxidative Defence System and Yield in Soybean Under Drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Tung, S.A.; Samad, R.A.; Wang, L.; Khan, I.; Rehman, N.u.; Shah, A.N.; Shahzad, B. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant 2015, 38, 25. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C. Stomatal regulation of plant water status. In Plant Abiotic Stress; Jenks, M.A., Hasegawa, P.M., Eds.; Wiley: New York, NY, USA, 2014; pp. 47–67. [Google Scholar]
- Balbi, V.; Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2007, 177, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.-H.; Lee, D.J.; Bae, H.-J. Gibberellic acid of Arabidopsis regulates the abscisic acid-induced inhibition of stomatal opening in response to light. Plant Sci. 2009, 176, 136–142. [Google Scholar] [CrossRef]
- Teszlák, P.; Kocsis, M.; Gaál, K.; Nikfardjam, M.P. Regulatory effects of exogenous gibberellic acid (GA3) on water relations and CO2 assimilation among grapevine (Vitis vinifera L.) cultivars. Sci. Hortic. 2013, 159, 41–51. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Li, Z.; Lu, G.Y.; Zhang, X.K.; Zou, C.S.; Cheng, Y.; Zheng, P.Y. Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed (Brassica napus L.). Seed Sci. Technol. 2010, 38, 432–440. [Google Scholar] [CrossRef]
- Krugman, T.; Peleg, Z.; Quansah, L.; Chague, V.; Korol, A.B.; Nevo, E.; Saranga, Y.; Fait, A.; Chalhoub, B.; Fahima, T. Alteration in expression of hormone-related genes in wild emmer wheat roots associated with drought adaptation mechanisms. Funct. Integr. Genom. 2011, 11, 565–583. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Reiter, R.J.; He, C.; Liu, G.; Lei, Q.; Zuo, B.; Zheng, X.D.; Li, Q.; Kong, J. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 2014, 56, 134–142. [Google Scholar] [CrossRef]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Bhuyan, M.H.M.B.; Hasanuzzaman, M.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Lu, M.; Hao, C.; Pu, Z.; Guo, M.; Hou, D.; Chen, L.Y.; Huang, X. Melatonin-Nitric Oxide Crosstalk and Their Roles in the Redox Network in Plants. Int. J. Mol. Sci. 2019, 20, 6200. [Google Scholar] [CrossRef]
- Jin, Z.; Shen, J.; Qiao, Z.; Yang, G.; Wang, R.; Pei, Y. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011, 414, 481–486. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, L.; Jiao, C.; Su, M.; Yang, T.; Zhou, L.; Peng, R.; Wang, R.; Wang, C. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol. Biochem. 2013, 70, 278–286. [Google Scholar] [CrossRef]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2019, 82, 25–34. [Google Scholar] [CrossRef]
- Shi, H.; Chen, Y.; Tan, D.-X.; Reiter, R.J.; Chan, Z.; He, C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015, 59, 102–108. [Google Scholar] [CrossRef]
- Mukherjee, S.; David, A.; Yadav, S.; Baluška, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 2014, 152, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Feng, C.; Kong, J.; Wang, L.; Wang, N.; Zheng, X.; Zhou, Y.; Chan, D. Use of Product Containing Melatonin as Effective Component for Improving Waterlogging Stress Resistance in Plants. CN105076136-A, CN105076136-B, CN105076136-A, 25 November 2015. A01N-043/38 201612. [Google Scholar]
- Zheng, X.; Zhou, J.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin Improves Waterlogging Tolerance of Malus baccata (Linn.) Borkh. Seedlings by maintaining aerobic respiration, photosynthesis and ROS Migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Zhang, Z.; Liu, N.; Li, D.; Hu, L. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a phytomelatonin-rich extract from cultured plants with excellent biochemical and functional properties as an alternative to synthetic melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef]

| Common Name | Scientific Name | Drought Treatment | Melatonin Treatment | Effects | Reference | |
|---|---|---|---|---|---|---|
| Concentration* | Application Form | |||||
| Model Plants | ||||||
| Arabidopsis | Arabidopsis thaliana | Water withholding (21 d) | 50 µM | Supplemented with nutrient solution | Stress-responsive genes ▲, soluble sugars ▲ | [40] |
| Field Crops | ||||||
| Rice | Oryza sativa | Water drainage from vessels (5 d) | 100 μM | Pretreatment in growing distilled water | Plant growth ▲, osmoprotectants proline ▲, stress-responsive genes ▲, mitochondrial structure ▲, ROS ▼, electroleakage ▼ | [41] |
| Maize | Zea mays | Water withholding (8 d), melatonin application during recovery, followed by withholding (8 d). | 1 mM | Supplemented with irrigation | Photoprotection (PSII efficiency) ▲ | [34] |
| Maize | Z. mays | 30–60% SWC (8d) | 100 µM | Foliar application | Recovering after rehydration ▲, photosynthesis ▲, stomatal conductance ▲, transpiration rates ▲, cell turgor and water holding capacity ▲, enzymatic and nonenzymatic antioxidants ▲, osmotic potential ▼, ROS ▼ | [42] |
| Maize | Z. mays | 20% PEG6000(3 d) | 10–100 μM | Foliar application pre-treatment | Photosynthesis ▲, antioxidant enzymes ▲, carbon fixation ▲, amino acids and secondary metabolites biosynthesis ▲, ROS ▼ | [26] |
| Maize | Z. mays | Water withholding (7 d) | 100 µM | Two methods (root-irrigation and foliar application) | Photosynthesis ▲, ROS ▼ | [43] |
| Maize | Z. mays | 40–45% field capacity (50 d) | 50 µM (foliar spray) and 100 µM (soil drench) | Foliar application or soil treatment | Photosynthesis ▲, antioxidant enzymes ▲, ROS ▼ | [44] |
| Wheat | Triticum aestivum | 40% and 60% field capacity (7 d) | 500 µM | Soil application | Chloroplast structure▲, photosynthesis ▲, cell turgor and water holding capacity ▲, GSH and AsA contents ▲, antioxidant enzymes▲, GSH–AsA cycle-related genes ▲, ROS ▼, membrane damage ▼ | [45] |
| Wheat | T. aestivum | 30% pot holding capacity (8 d) | 100 µM | Soil application | Recovering after rehydration ▲, biomass and root/shoot ratio ▲, water holding capacity ▲, chlorophyll ▲, photosynthesis ▲, ROS ▼, MDA ▼ | [46] |
| Wheat | T. aestivum | 20% PEG 6000 (7 d) | 10 and 100 μM (variety-dependent) | Seeds treatment | Germination percentage ▲, germination index ▲, germination potential ▲, radicle length and number ▲, plumule length ▲, lysine (germination-related amino acid) ▲ | [47] |
| Tartary Buckwheat | Fagopyrum tataricum | 20% field capacity (15 d) | 100 μM | Foliar application | Water status ▲, osmoprotection ▲, secondary metabolites▲, antioxidant enzymes▲, photosynthesis ▲, ROS ▼ | [48] |
| Barley | Hordeum vulgare | (Combined drought and cold) | 1 mM | Foliar or soil application | Endogenous melatonin▲, ABA ▲, water status ▲, antioxidants ▲, photosynthesis ▲, PSII efficiency ▲ | [49] |
| Soybean | Glycine max | 20% field capacity (10 d) | 50 µM | Seed coating | Seedlings growth ▲, biomass ▲, electrolyte leakage ▼ | [36] |
| Soybean | G. max | 15% PEG 6000 (7 d) | 100 µM | Supplemented with nutrient solution | Seedlings growth ▲, photosynthesis ▲ | [38] |
| Soybean | G. max | 45% RSWC (15 d) | 100 µM | Foliar application | Antioxidant enzymes ▲, osmolytes ▲, MDA ▼ | [25] |
| Soybean | G. max | 15% PEG6000 (3 d) | 100 μM | Foliar and root application | Plant growth and flowering ▲, seed yield ▲, gas exchange▲, PSII efficiency ▲, antioxidant enzymes ▲, MDA ▼ | [50] |
| Cassava | Manihot esculenta | 20% PEG 6000 (11 d) | 100 µM | Soil application | POD activity▲, ROS ▼ | [37] |
| Cotton | Gossypium hirsutum | 10% PEG 6000 (7 d) | 100 µM | Seeds pre-soaking | Number and opening of stomata in cotton testa ▲, germination parameters▲, antioxidant enzymes ▲, osmoprotection ▲, GA3 ▲, ABA ▼, ROS ▼, MDA ▼ | [51] |
| Alfalfa | Medicago sativa | Water withholding (7 d) | 10 µM | Soil application | Chlorophyll ▲, stomatal conductance ▲, osmoprotection ▲, Nitro-oxidative homeostasis ▲, cellular redox disruption ▼,MDA ▼, ROS ▼ | [52] |
| Fruits | ||||||
| Apple | Malus spp. | Water withholding (6 d) | 100 µM | Soil application | Water holding capacity ▲, chlorophyll ▲, photosynthesis ▲, antioxidants ▲, stomatal opening regulation ▲, melatonin biosynthesis genes ▲, electrolyte leakage ▼, ROS▼, ABA ▼ through ABA synthesis gene▼ and catabolic genes ▲ | [53] |
| Apple | M. domestica | 50% field capacity (3 months with sampling every month) | 100 µM | Soil application | Plant growth ▲, nutrients uptake fluxes ▲, N metabolism ▲, endogenous melatonin ▲, chlorophyll ▲, photosynthesis ▲, relative water content ▲, stomatal status ▲, electrolyte leakage ▼, ROS ▼ | [54] |
| Apple | M. domestica | 50% field capacity (3 months with sampling every month) | 100 µM | Soil application | Chlorophyll ▲, photosynthesis ▲, photoprotection ▲, antioxidant enzymes ▲, GSH and AsA contents ▲, oxidative damage ▼, leaf senescence ▼, senescence-associated gene 12 ▼, pheophorbide a oxygenase-related gene ▼, ROS▼ | [55] |
| Grape | Vitis vinifer | 10% PEG 6000 (12 d) | 50, 100 and 200 nM | Roots pretreatment | Photoprotection ▲, leaf thickness ▲, spongy tissue ▲, stoma size ▲, chloroplast structure ▲, enzymatic and nonenzymatic antioxidants ▲, osmoprotectants (free proline) ▲, ultrastructural damage ▼, oxidative injury ▼ | [33] |
| Grapevine | V. amurensis V. vinifera and V. labruscana | 10% PEG 6000 (4 d) | Endophyte colonization of secreted-melatonin bacteria | Bacillus amyloliquefaciens SB-9 colonization | Melatonin synthesis and its intermediates ▲, plant growth ▲, ROS ▼, MDA ▼ | [56] |
| Grape | V. vinifer | Water withholding (18 d) | 100 μM | Supplemented with irrigation | MDA ▼, relative conductivity ▼ | [57] |
| Grape | V. vinifer | Water withholding (18 d) | 100 μM | Supplemented with irrigation | Chlorophyll ▲, SOD activity ▲ | [28] |
| Kiwifruit | Actinidia. chinensis var. deliciosa | Water withholding (9 d) (RWC below 35% field capacity) | 100 μM | Supplemented with irrigation | Root vigor ▲, osmoprotectants ▲, proteins biosynthesis ▲, chlorophyll ▲, photosynthesis ▲, light energy absorption ▲, photoprotection ▲, CO2 fixation-associated genes ▲, MDA ▼, cell membranes damage ▼, stomatal closure ▼ | [58] |
| Kiwifruit | A. chinesis | water withholding (9 days) | 100 µM | Irrigation pretreatment | Water holding capacity ▲, antioxidant enzymes-related genes ▲, GSH–AsA cycle-related genes ▲, ROS ▼, MDA ▼ | [27] |
| Chinese hickory | Carya cathayensis | 30% PEG 6000 (10–40 d) | 100 µM | Foliar application pretreatment | Recovering after rehydration ▲, photosynthesis ▲, antioxidants ▲, osmoprotectants ▲, metabolic pathways-related genes ▲, antioxidant enzymes-related genes ▲, ROS ▼ | [59] |
| Vegetables | ||||||
| Tomato | Solanum lycopersicum | Water withholding for (5–20 d after moderate drought) | 0.1 mM | Supplemented with irrigation | Photosynthesis ▲, root vigor ▲, PSII efficiency ▲, antioxidants ▲, toxic substances ▼ | [60] |
| Tomato | S. lycopersicum | 10% PEG (7 d) | 200 µM | Foliar application | Chlorophyll ▲, p-coumaric acid content ▲, antioxidant enzymes ▲, MDA ▼ | [29] |
| Pepper | Capsicum annuum | 10% PEG (8 d) | 50 µM | Seed pretreatment | Water holding capacity ▲, endogenous melatonin ▲, GSH content ▲, chlorophyll ▲, carotenoids ▲, proline ▲, antioxidant enzymes ▲, MDA ▼ | [30] |
| Watermelon | Citrullus lanatus | Water withholding (4 d) | 150 µM | Root pretreatment | Wax accumulation ▲, melatonin–ABA crosstalk ▲ | [39] |
| Cucumber | Cucumis sativus | 18% PEG 6000 (days) | 100 µM | Seeds priming and nutrient solution | Seed germination ▲, root growth ▲, root/shoot ratio ▲, roots vigor ▲, chlorophyll ▲, photosynthesis ▲, chloroplasts ultrastructure ▲, antioxidant enzymes ▲, ROS ▼ | [61] |
| Rapeseed | Brassica napus | 4% PEG 6000 (7 d) | 0.05 mM | In PEG solution | Plant growth ▲, antioxidants ▲, osmoprotectants ▲, ROS ▼ | [62] |
| Rapeseed | B. napus | −0.3 and −0.4 Mpa PEG 6000 (7 d) | 500 µM | Seed priming | Chlorophyll ▲, stomatal regulation ▲, chloroplast structure ▲, cell expansion and cell wall ▲, antioxidant enzymes ▲, osmoprotectants ▲, oxidative injury ▼ | [35] |
| Ornamental and Medicinal Plants | ||||||
| Jinyu Chuju | Dendranthma morifolium | 40% field capacity (6 d) | 100 µM | Foliar application | Chlorophyll ▲, photosynthesis ▲, biomass ▲, osmoprotectants (TSS and proline) ▲, cell membrane damage ▼, relative conductivity ▼, MDA ▼ | [63] |
| Moldavian balm (Dragon head) | Dracocephalum moldavica | 40–60% field capacity | 100 µM | Foliar application | Plant growth and flowering ▲, antioxidants ▲, chlorophyll ▲, water holding capacity ▲, ROS ▼, MDA ▼ | [64] |
| Creeping bentgrass | Agrostisstolonifera | Water withholding (14 d) | 20 μM | Foliar application | Visual quality ▲, PSII efficiency ▲, chlorophyll ▲, water holding capacity ▲, melatonin biosynthesis genes ▲, dehydration responsive genes ▲, Chlorophyll-degradation genes ▼, leaf senescence ▼, ROS ▼, MDA ▼ | [65] |
| Tall fescue | Festuca arundinacea | Water withholding (10 d) | 20 μM | Irrigation pretreatment | Plant growth ▲, chlorophyll ▲, antioxidant enzymes ▲, ROS ▼, MDA ▼ | [66] |
| Bermudagrass | Cynodon dactylon | Withholding water (21 d) | 20 and 100 μM | Irrigation pretreatment | Plant growth ▲, chlorophyll ▲, survival rate ▲, antioxidant enzymes ▲, stress-responsive genes ▲, metabolic regulation ▲, hormonal signaling-related genes regulation ▲, ROS ▼ | [67] |
| Fenugreek | Trigonella foenum-graecum | 19.5% PEG 6000(21 d) | 100 and 300 μM | Foliar application pre-treatment | Endogenous melatonin and secondary metabolites ▲, chlorophyll ▲, antioxidant enzymes ▲, ROS ▼ | [68] |
| Coffee | Coffea arabica | 40% of max moisture retention capacity (21 d) | 300 μM | Soil application | Root vigor ▲, photoprotection ▲, gas exchange ▲, carboxylation efficiency ▲, chlorophyll ▲, antioxidants ▲, MDA ▼ | [31] |
| Tea | Camellia sinensis | 20% PEG 6000 (2 d) | 100 µM | Foliar application pre-treatment | Photosynthesis ▲, GSH and AsA contents ▲, antioxidant enzymes ▲, antioxidant enzymes-related genes ▲, ROS ▼, MDA ▼ | [32] |
| Other Crops | ||||||
| Tobacco, Tomato and Cucumber | Nicotiana benthamiana, S. lycopersicum and C. sativus | Water withholding (6 d) | 10 μM | Foliar application | MDA ▼, drought tolerance ▲ | [23] |
| Species | Scientific Name | Waterlogging Treatment | Melatonin Treatment | Functions | References | |
|---|---|---|---|---|---|---|
| Concentration * | Application Form | |||||
| Apple | Malus baccata | Waterlogging stress (9 d) | 200 µM (foliar spraying) 600 µM (root irrigation) | Foliar spraying or root irrigation | Endogenous melatonin ▲, antioxidant enzymes ▲, chlorophyll ▲, photosynthesis ▲, aerobic respiration ▲, synthetic enzymes ▲ ROS ▼, MDA ▼, anaerobic respiration ▼, chlorosis and wilting ▼ | [118] |
| Alfalfa | Medicago sativa | Waterlogging stress (10 d) | 100 µM | Foliar spraying pretreatment | Endogenous melatonin ▲, gene expression regulation ▲, photosynthesis ▲, electroleakage ▼, MDA ▼, leaf senescence ▼, polyamine and ethylene metabolism reprogramming | [119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. https://doi.org/10.3390/antiox9090809
Moustafa-Farag M, Mahmoud A, Arnao MB, Sheteiwy MS, Dafea M, Soltan M, Elkelish A, Hasanuzzaman M, Ai S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants. 2020; 9(9):809. https://doi.org/10.3390/antiox9090809
Chicago/Turabian StyleMoustafa-Farag, Mohamed, Ahmed Mahmoud, Marino B. Arnao, Mohamed S. Sheteiwy, Mohamed Dafea, Mahmoud Soltan, Amr Elkelish, Mirza Hasanuzzaman, and Shaoying Ai. 2020. "Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances" Antioxidants 9, no. 9: 809. https://doi.org/10.3390/antiox9090809
APA StyleMoustafa-Farag, M., Mahmoud, A., Arnao, M. B., Sheteiwy, M. S., Dafea, M., Soltan, M., Elkelish, A., Hasanuzzaman, M., & Ai, S. (2020). Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants, 9(9), 809. https://doi.org/10.3390/antiox9090809