Abstract
Background: Recent studies have highlighted the importance of cherry and cocoa extracts consumption to protect cells from oxidative stress, paying particular attention to cocoa by-products. This study aims to investigate the protective effect of cocoa husk extract (CHE) and cherry extracts (CE) against ROS-induced oxidative stress in Human Umbilical Vein Endothelial Cells (HUVECs). Methods: CE and CHE had antioxidant activity characterized by total polyphenols content (TPC). HUVECs were treated for 2 h and 24 h with increasing TPC concentrations of CE and CHE (5-10-25-50-100 µg Gallic Acid Equivalent (GAE)/mL) and then with H2O2 for 1 h. Cell viability and ROS production were evaluated. CE and CHE polyphenols permeability on excised rat intestine were also studied. Results: CE and CHE showed a similar antioxidant activity (2.5 ± 0.01 mmol Fe2+/100 g FW (fresh weight) and 2.19 ± 0.09 mmol Fe2+/100 g FW, respectively, p > 0.05) whereas CHE had a higher TPC (7105.0 ± 96.9 mg GAE/100 g FW) than CE (402.5 ± 8.4 mg GAE/100 g), p < 0.05. The in vitro viability assay showed that both extracts were non-cytotoxic. CHE resulted in protection against ROS at lower concentrations than CE. CHE showed a 2-fold higher apparent permeability compared to CE. Conclusions: CHE represents a high-value antioxidant source, which is interesting for the food and pharmaceutical industries.
1. Introduction
Reactive oxygen species (ROS) are involved in the pathogenesis of numerous chronic and degenerative diseases. Physiologically, oxygen metabolism generates ROS, which are contrasted and neutralized by antioxidant defenses. The unbalance toward ROS formation is recognized as a critical aspect of cell damage that characterizes many disease states, such as atherosclerosis and premature aging [1,2,3].
Vascular endothelial cell lines are particularly sensitive to ROS, and damage to them is reflected in the alteration of vascular tone and permeability, and thus involved in cardiovascular dysfunction associated with hypertension, diabetes, atherosclerosis, and ischemic heart disease [4,5,6].
Therefore, the use of nutraceuticals appears essential for the prevention and control of ROS induced damages. In nature, polyphenols are the most abundant category of antioxidants. Most of them are in fruits and vegetables and are typically associated with healthy diets. Actually, epidemiological studies and associated meta-analyses have confirmed that their long-term consumption correlates with protection against the development of serious diseases such as cancers, cardiovascular diseases (CVD), diabetes, osteoporosis, and neurodegenerative pathologies [7].
Cocoa and its derivative products are rich in polyphenols, which possess an antioxidant capacity and are associated with the prevention of diseases related to oxidative stress. Most of the phenolic compounds found in cocoa are represented by flavonols, such as catechins, or flavan-3-ols (37%), anthocyanins (4%), and proanthocyanidins (58%) [8,9]. The protective activity of cocoa seems to be due to its phytochemical constituents, especially catechins.
Cocoa by-products, mainly cocoa husk, are produced worldwide in large amounts, constituting about 75% wt of whole fruit from the cocoa harvest [10]. Being a production waste, they are generally discarded, with a negative environmental impact [11]. Moreover, the cocoa by-products (bean and husks) possess nutritional and functional properties, particularly related to the presence of proanthocyanidins identified in the husks [12]. The proanthocyanidins found in husk are tannins, which can have different molecular weights, according to the degree of polymerization [13]. In vitro cellular studies have also confirmed that proanthocyanidins possess antioxidant activity [8,14]. Additionally, flavanols protect cells from oxidative stress by reducing ROS production and inhibiting the activation of caspase-3. Procyanidin B2 also increases the performance of enzymes specifically involved in antioxidant and detoxification processes [15].
Along with cocoa, cherry fruits have valuable nutritional properties, and their beneficial effects have been demonstrated against oxidative stress damage on both neuronal and intestinal epithelial cells. Similarly to cocoa, the most representative nutraceutical actives in cherries are polyphenols, including flavonoids and anthocyanins [16]. Many studies have used natural extracts on endothelial progenitor cells [17,18] or Human Umbilical Vein Endothelial Cells (HUVECs) [19,20,21] in in vitro experiments related to vascular dysfunction.
The aim of the present research was to compare the antioxidant properties of two antioxidant-rich natural products plant extracts, namely cherry and cocoa (bean and husk). The extracts were evaluated both in vitro and ex vivo in order to compare their beneficial effects on vascular related dysfunction upon oral intake.
For this purpose, we performed phenol cocoa bean and husk extraction from two different plant varieties (Costa Rica and Madagascar) that had their antioxidant activity, total polyphenols content, and phenolic composition characterized by HPLC coupled to electrospray ionization tandem mass spectrometry (ESI-MS/MS). The characterization of cherry extract (CE) has already been reported [22]. Moreover, Costa Rica cocoa husk extract (CHE) and CE were compared for their ability to permeate across excised rat intestine.
2. Materials and Methods
2.1. Materials
Hexane, acetone, and Folin-Ciocalteau reagent, and gelatin were purchased from Sigma-Aldrich (Milan, Italy). HPLC grade formic acid and methanol were purchased from VWR (Milan, Italy). HPLC grade water (18 mΩ) was obtained by a Mill-Q purification system (Millipore Corp., Bedford, MA, USA).
H2O2 was purchased from Farmac-Zabban S.p.a. (Calderara di Reno, BO, Italy). Medium 199 (M199), fetal bovine serum (FBS), penicillin-streptomycin solution, L-glutamine, and HEPES buffer were supplied by Hospira S.r.l. (Naples, Italy).
4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolium]-1,3-benzenedisulfonate (WST-1 assay), was purchased from Roche Applied Science (Mannheim, Germany), 5-(and-6)-chloromethyl-2′, 7′-dichloro-di-hydro-fluorescein diacetate, and acetyl ester (CM − -H2DCFDA) were supplied by Thermo Fisher Scientific Inc. (Waltham, MA, USA).
2.2. Sample Preparation
2.2.1. Cocoa Bean and Husk Phenol Extraction
The cocoa bean and husk phenol extraction were carried out using a procedure reported by Hammerstone et al. [23], slightly modified. Two varieties of cocoa (Costa Rica and Madagascar) were ground for 30 s in order to obtain a homogeneous material that was defatted four times with 125 mL of hexane for 20 min at 200 rpm and subsequently centrifuged for 30 min at 4000 rpm. The defatted cocoa sample was then extracted four times with acetone 70% v/v at a ratio of 1:5, stirred for 3 min, and centrifuged for 30 min at 4000 rpm. The extraction procedure was repeated four times after which the supernatants were combined, filtered with a paper filter and the organic solvent was removed by evaporation at room temperature for 48 h. To obtain a stable product the remaining water suspension was lyophilized.
The cocoa from Madagascar was selected because Bruna et al. [24], comparing the content of polyphenols in cocoa husks from different countries (Ghana, Madagascar, Ecuador, Trinidad, and Venezuela), found that Madagascar husks were the richest. The cocoa from Costa Rica was chosen because compared to that from other countries (Ivory Coast, Venezuela, Samoa, Trinidad, Brazil, Ghana, Ecuador, Jamaica), it was found to be particularly rich in epicatechin [25,26,27,28].
2.2.2. Cherry Extract (CE) Preparation and Characterization
The cherry extracts were obtained from the Crognola Capannile variety of Prunus avium L., an ancient Tuscan variety of sweet cherry, as described by Beconcini et al. [29].
The Crognola Capannile variety was used for the present study due to their high polyphenol content, as reported by Berni et al. [22].
2.3. HPLC-PDA/UVvis-ESI-MS/MS Analysis of Cocoa Extracts
Cocoa extracts were dissolved in methanol at a final concentration of 2.5 mg/mL, then centrifuged for 5 min at 1145× g. The supernatants (20 μL injection volume) were subjected to HPLC coupled with a photodiode array (PDA)/UVvis and an ion trap ESI-MS. The LC system was composed of a Surveyor autosampler, a Surveyor LC pump, a Surveyor PDA/UVvis detector, and a LCQ Advantage ion trap ESI mass spectrometer (ThermoFinnigan, San Jose, CA, USA) equipped with Xcalibur 3.1 software. Qualitative analysis was performed on a C-18 column (Luna, 4.6 × 150 mm, 5 µm, Phenomenex, Bologna, Italy) using a mixture of methanol (solvent A) and formic acid in water 0.1% v/v (solvent B) as eluent. A linear gradient of increasing 5 to 55% A was developed within 50 min at a flow rate of 0.8 mL/min, with a splitting system of 2:8 to MS detector (160 μL/min) and PDA detector (640 μL/min), respectively. PDA/UV spectra were recorded in a range of 200–600 nm, using 254, 280, and 325 nm as preferential channels, while ESI-MS experiments were achieved in negative ion mode (scan range of m/z 150-2000). Ionization parameters were used as previously reported [30].
2.4. Antioxidants Determination
The total antioxidant potential of cocoa by-product extracts and CE freeze-dried samples was determined by a ferric reducing antioxidant power (FRAP) assay, as previously reported [31]. The FRAP value of the samples, expressed as mmol of Fe2+ per 100 g FW, was determined from a standards curve built up using ferrous sulfate.
2.5. Total Polyphenolic Content
The total polyphenols content (TPC) of cocoa by-product extract and CE was determined by the Folin–Ciocalteau method [32]. The results were expressed as gallic acid equivalent (GAE) on a dry weight basis following a previously reported procedure [33].
2.6. HUVEC Isolation and Culture
HUVECs were collected from umbilical cords obtained from healthy donors women post-labor, treated anonymously. The age of the donors ranged from 24 to 43 years. Ethical approval (authorization number 2943), was granted by the local Ethics Committee (Full name: Comitato Etico per la Sperimentazione Clinica Area Vasta Nord Ovest c/o Azienda Ospedaliero-Universitaria Pisana (AOUP), Pisa”). HUVECs were collected following the procedure described by Jeffe et al. [34]. Briefly, HUVECs after isolation and centrifugation were plated on gelatin pre-coated flasks and incubated for 24 h at 37 °C, using 5% CO2 in a complete growth medium made with 10% FBS. After 24 h, the growth medium was replaced to remove the excess of red blood cells.
2.7. Cell Treatment
HUVECs between passage P2–P4 were treated for 2 h and 24 h with increasing polyphenol concentrations of cocoa husk Costa Rica extract (CHE) and Crognola Capannile cherry extract (CE) (5, 10, 25 or 50 μg GAE/mL), in growth medium with 5% FBS. Cells in medium only were used as a positive control. Then, cells were washed with PBS and treated with 50 µM of commercial H2O2 for 1 h to induce oxidative stress [33]. At the end of each treatment, cells were analyzed for viability and ROS production.
2.8. Cell Viability
At the end of each treatment, HUVECs were incubated with tetrazolium salt (10 µL/well) for 3 h at 37 °C, in 5% CO2 and the formazan dye formed was quantified at 450 nm with a multiplate reader (Thermo Scientific Multiskan FC Microplate Photometer), correlating the with the number of active cells. The viability was expressed as the percentage of viable cells.
2.9. ROS Production
ROS production was evaluated using CM − -H2DCFDA, a fluorescent probe. HUVECs during the last 15 min of treatment with CHE, CE or H2O2, were incubated in the dark at room temperature with CM − -H2DCFDA (10 µL/well) dissolved in PBS. ROS production was detected measuring the increase in fluorescence over time at excitation of 488 nm and emission of 510 nm using a microplate reader (Thermo Scientific Fluoroskan Ascent Microplate Fluorometer). ROS production was expressed as a percentage of ROS accumulation.
2.10. Permeation Study of CHE and CE
For the ex-vivo permeation studies, we used the intestinal mucosa excised from non-fasting male Wistar rats (weight 250–300 g), using the procedure reported by Fabiano et al. [35]. The ex vivo experiments on excised rat intestine were carried out with the aim of assessing the permeability of the intestinal epithelium to the antioxidants present in the different extracts. The isolated rat intestine was chosen from among the known intestinal models because its tight junctions are similar in number and tightness to those of the human intestine [36]. The formulation tested were 1 mL of CE solution (15.5 µg/mL GAE concentration [33], or CHE (280 µg/mL GAE concentration). At 30 min intervals of a total of 240 min, the apical to basolateral transport of CHE or CE was investigated, analyzing the receiving phase (50 µL) by the Folin–Ciocalteau reagent for TPC.
2.11. Cocoa Extract and CE Stability Studies
The stability of CHE and CE was evaluated according to the procedure reported by Beconcini et al. [33]. At 30 min intervals of a total of 240 min, 50 µL of CHE or CE volume was withdrawn and analyzed by the Folin–Ciocalteau reagent for TPC.
2.12. Statistical Analysis
The GraphPad Prism Software vs. 7.0 (GraphPad Software Inc., La Jolla, CA, USA) was used for the statistical analysis of data. All results were presented as means ± standard deviation (SD) of at least three independent experiments. The significant difference (p-value < 0.05) between groups of values was evaluated by a one-way ANOVA or Turkey’s or Bonferroni’s multiple comparisons.
3. Results
3.1. Phenolic Profile of Cocoa Extracts
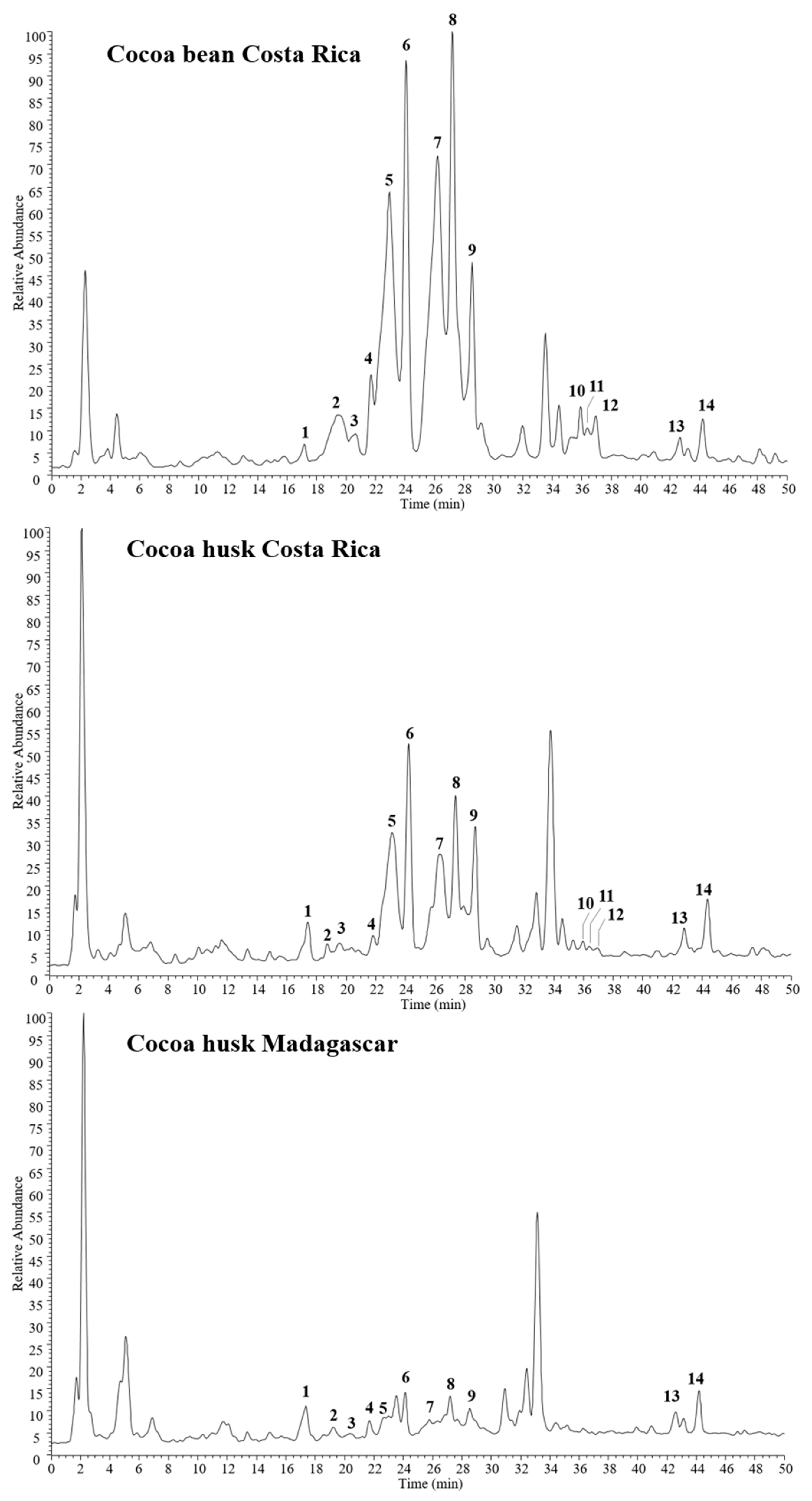
The phenolic composition of cocoa bean and husk extracts was characterized by HPLC-PDA/UVvis-ESI-MS/MS experiments. Cocoa Costa Rica extracts (bean and husk) showed similar chemical profiles (Figure 1), with 14 major phenol compounds identified: one caffeoyl derivative, caffeoyl aspartate (1); one flavan-3-ol, catechin/epicatechin (9); three procyanidin B isomers (5, 6, and 10) together with seven procianydin C isomers (2, 3, 4, 7, 8, 11, and 12); two flavonol glycosides, quercetin 3-O-glucoside (13), and quercetin 3-O-arabinoside (14).
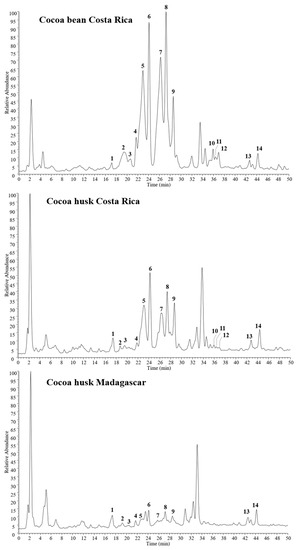
Figure 1.
HPLC-electrospray ionization (ESI)-MS/MS profiles of phenols detected in cocoa husk and bean extracts in negative ion mode. Peak data are listed in Table 1.
In contrast, the Madagascar cocoa husk extract showed a less rich phenolic content, mainly with regard to procyanidins. Indeed, compounds 5-9, which were the most representative constituents in cocoa husk Costa Rica extract, were significantly reduced in Madagascar cocoa husk, while compounds 10, 11, and 12 were not detected at all. These findings are in agreement with previous studies, reporting a high different phenol content in cocoa products with different geographic origins, i.e., Costa Rica and Madagascar [24].
All compounds were tentatively identified by comparison of their elution order, UV data, and both full and fragmentation mass spectra (Table 1) with data reported in the literature [13,37,38]. Compounds 2–4, 7, 8, 11, and 12 showed the same molecular deprotonated ion [M − -H]− at m/z 865 and two strong UV absorptions at 242–258 and 277–280 nm, suggesting a trimeric B-type procyanidin structure for all isomers. This finding was confirmed by fragmentation mass spectra, all showing the same diagnostic product ions at m/z 739, 713, 695, 451, 407, and 287, together with a base peak ion at m/z 577, represented by the dimeric form [30].

Table 1.
ESI-MS/MS, UV, and chromatographic data (retention time, tR) of compounds 1-14 detected in the cocoa bean and husk extracts Costa Rica and cocoa husk Madagascar.
Since all the spectra are superimposable, it is not possible to distinguish between the isomeric forms [39], but all compounds can be assigned as procyanidin C isomers, previously found in cocoa extract [37]. Likewise, full MS of peaks 5, 6, and 10 were characterized by type-B procyanidin dimers, as deduced by the deprotonated molecule [M − -H]− at m/z 577 and product ions at m/z 451, 425, 407, and 289. Also, in this case, the exact structure cannot be assigned only on the basis of spectral data. However, it can be assumed all three molecules to be isomers of procyanidin B, previously reported in cocoa extract [37]. In addition to these oligomers, also their monomer was detected (compound 9, [M − -H]− at m/z 289), corresponding to catechin or epicatechin, as deduced by diagnostic product ions at m/z 245 and 205 [30].
Along with flavan-3-ols, also two flavonol glycosides were identified in all three analyzed cocoa extracts, as evidenced by UV absorptions at 267–268 and 354–355 nm. Compounds 13 ([M − -H]− at m/z 463) and 14 ([M − -H]− at m/z 433) were quercetin derivatives showing the loss of a hexose ([M − -H-162]− at m/z 301) and a pentose units ([M − -H-132]− at m/z 301), respectively. According to data reported in previous work [40], 13 and 14 were identified as quercetin 3-O-glucoside and quercetin 3-O-arabinoside, respectively.
Finally, a caffeoyl derivative (compound 1, [M − -H]− at m/z 293) was detected in all cocoa samples, showing the loss of an aspartate residue ([M − -H-115]− at m/z 301) in the ESI-MS/MS experiments. Thus, compound 1 was identified as N-caffeoyl aspartate, previously reported in cocoa source [37]. Some other peaks remained unidentified; however, based on UV data, they were not attributed to phenol derivatives.
3.2. Cherry and Cocoa By-Product Extracts Characterization
FRAP and Folin–Ciocalteu methods on CE reported that Crognola had the highest antioxidant content and TPC (402.5 ± 8.4 mg GAE/100 g FW) among the six varieties of Prunus avium L. studied. Flavonoid molecules of quercetin and catechins were 59.32 ± 3.2 µg/g FW and 292.76 ± 1.9 µg/g FW, respectively. HPLC analysis also showed the presence of anthocyanins, represented mainly by cyanidin-3-glucoside (227.37 ± 1.2 µg/g FW).
In Table 2, the antioxidant content and the TPC of the two extracts under study are reported. CE and CHE antioxidant content was not significantly different (p > 0.05), whereas CHE had a higher TPC than CE (p < 0.05).

Table 2.
Cherry Extract (CE) and Cocoa Husk Extract (CHE) characterization. a Determined by FRAP. b Determined by Folin–Ciocalteau. * Significantly different from each other (p < 0.05). TPC = total polyphenols content.
3.3. Dose- and Time-Dependent Effect of CHE and CE on HUVECs Viability
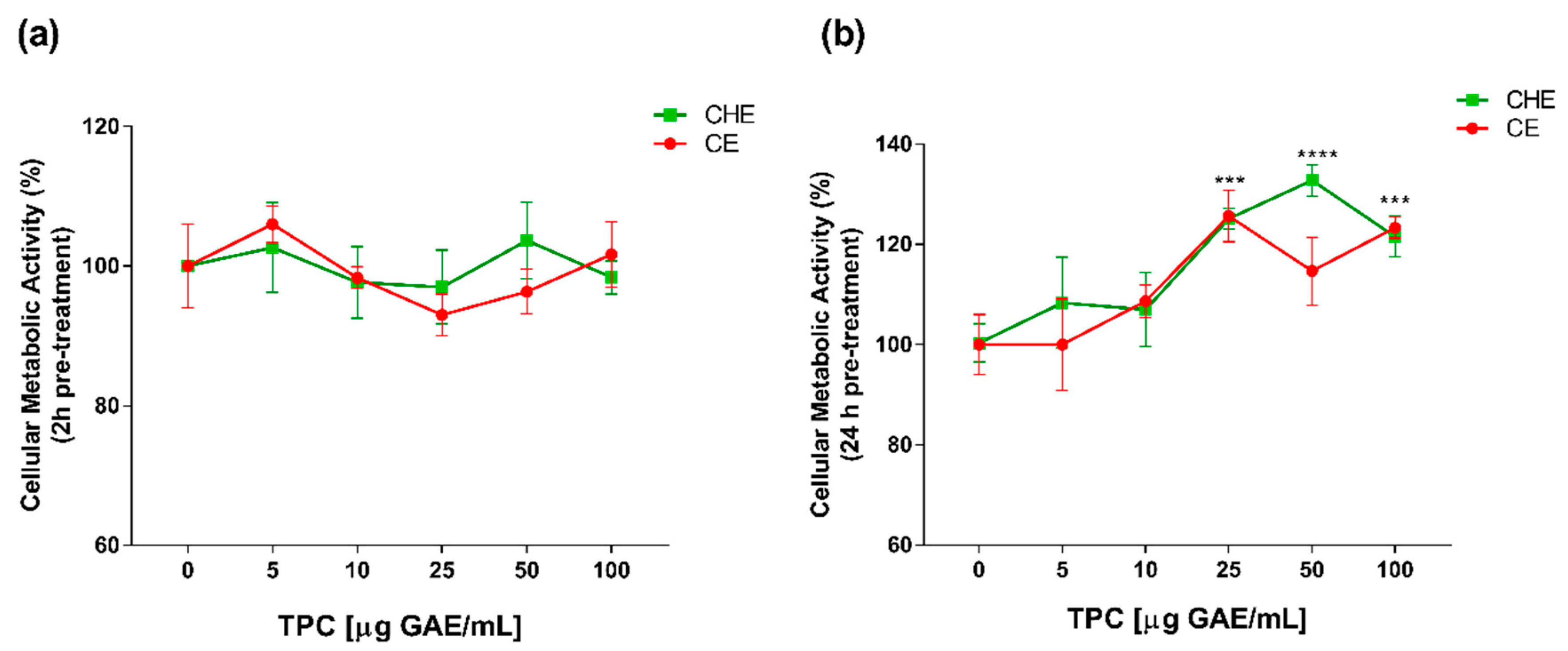
Polyphenolic concentrations of 5, 10, 25, 50 and 100 µg GAE/mL, were chosen for viability studies. Cell viability was evaluated by WST-1 colorimetric assay. Both CHE and CE polyphenols were non-cytotoxic, both after 2 h and 24 h of treatment (Figure 2a,b). After 24 h of treatment, there was an increase in cell viability at high concentrations of CHE (25 to 100 µg GAE/mL TPC) compared to control (Figure 2b). This enhancement of cell viability might be due to the incubation over time addicted to a higher concentration of polyphenols, as previously demonstrated in apple juice study on HUVECs [20].
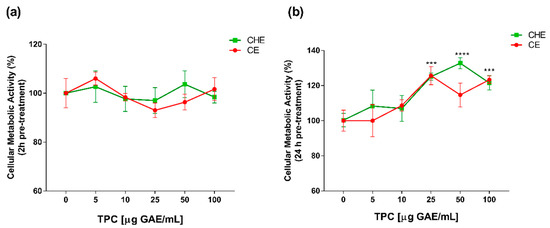
Figure 2.
Dose- and time-dependent cell metabolic activity. Human Umbilical Vein Endothelial Cells (HUVECs) were cultured for 2 h (a) and 24 h (b) in the presence of increasing concentrations of total polyphenol content (TPC) from CE or CHE (5, 10, 25, 50, and 100 µg GAE/mL). Cell metabolic activity was determined by WST-1 colorimetric assay and expressed as metabolic activity percentage compared to control (untreated cells). Graphical data are represented as mean ± SD of three separate experiments run in triplicate. (*** p < 0.005, **** p < 0.0001 vs. control).
3.4. Protective Effect from Oxidative Stress
To evaluate the antioxidant activity of CHE and CE, HUVECs were pre-treated for 2 h and 24 h with increasing polyphenols concentrations (ranging from 5–100 µg GAE/mL of TPC), and after that, an oxidative stress insult was applied by treating the cells with 50 µM H2O2 for 1 h.
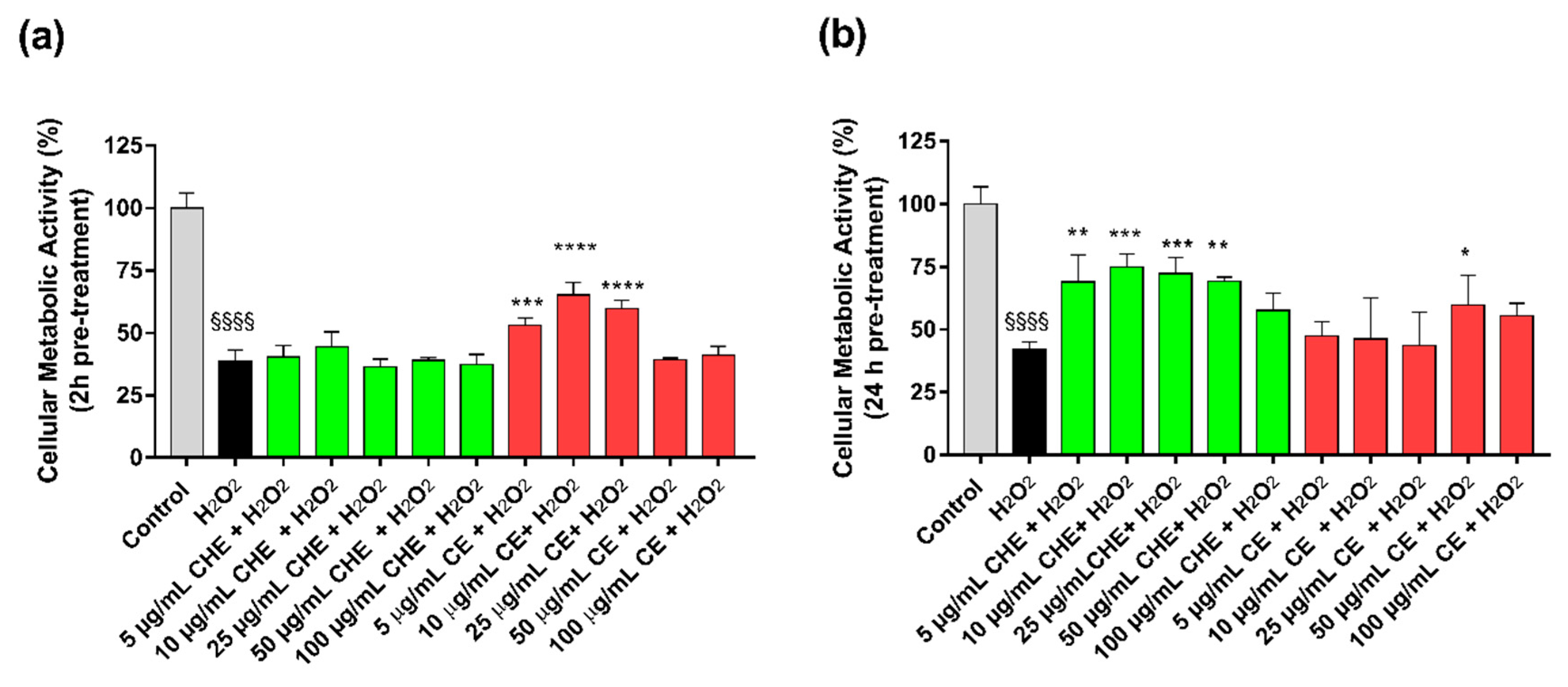
The results reported that treatment of HUVECs with H2O2 significantly reduced viable cell number compared to control (Figure 3). Viability after H2O2-induced oxidative stress was increased by a pre-treatment with CE, both after 2 h or 24 h (Figure 3b). In particular, after 24 h, only 50 µg GAE/mL TPC of CE mL had the most significant protective effect. For CHE polyphenols, only the 24 h pre-treatment significantly protected cells from oxidative stress in a concentration range of 5 µg GAE/mL TPC to 50 µg GAE/mL TPC.
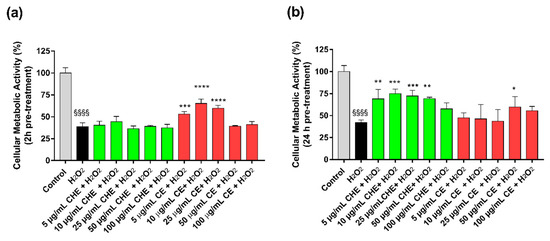
Figure 3.
Protective effects from H2O2-induced oxidative stress. HUVECs viability after 2 h (a) or 24 h (b) of pre-treatment with CE or CHE and treatment with 50 µM H2O2 for 1 h. Data are expressed as the % of viable cells compared to 100% of control (untreated cells). (*p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 vs. H2O2; §§§§ p < 0.0001 vs. control).
3.5. Antioxidant Activity
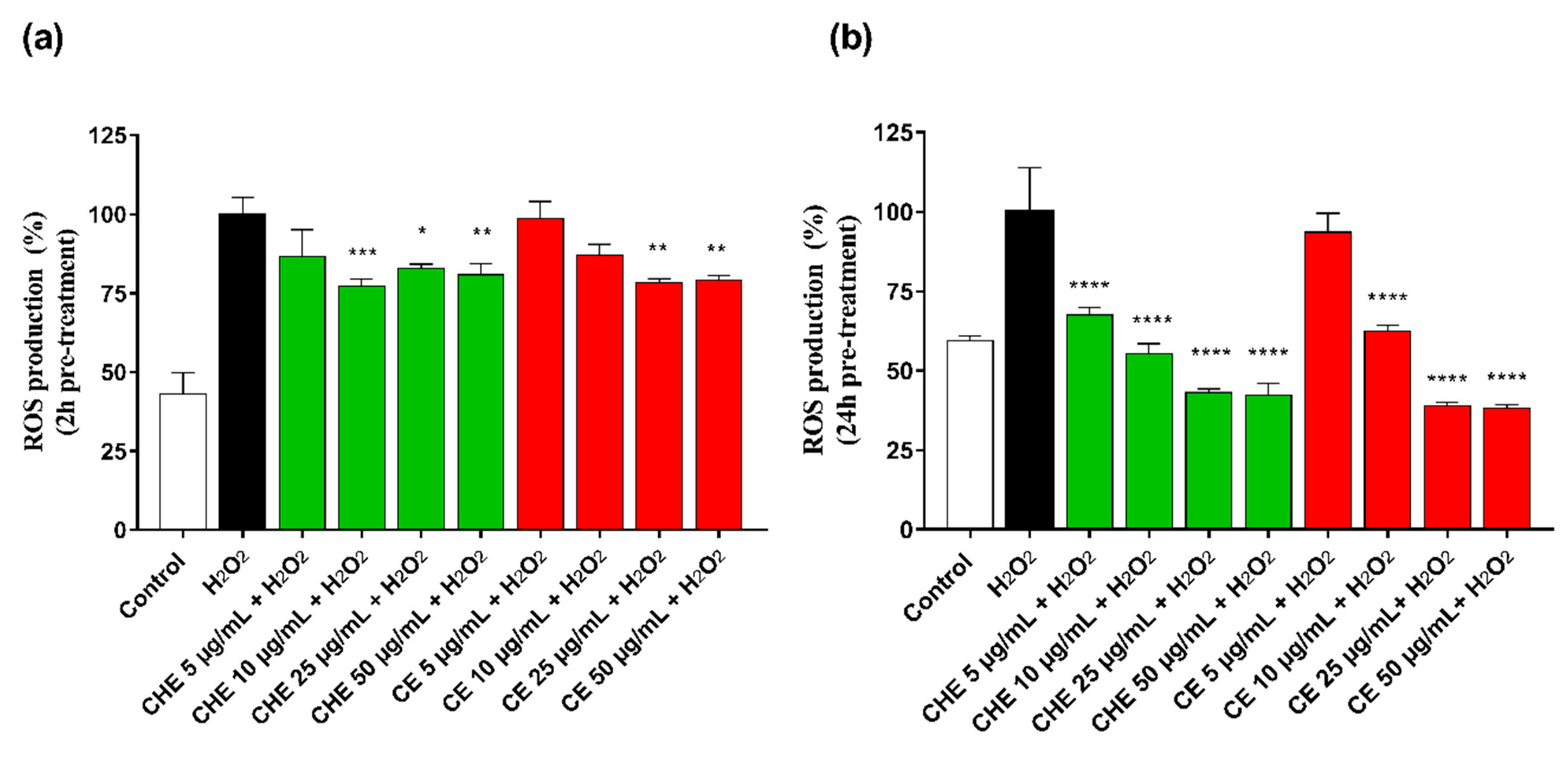
ROS accumulation in HUVECs was evaluated after 2 h and 24 h pre-treatment of CHE or CE at different concentrations (ranging 5–50 µg GAE/mL of TPC). Treatment of HUVECs with H2O2 significantly increased intracellular ROS production. As shown in Figure 4a, after 2 h pre-incubation, CHE showed to significantly reduce ROS production already at low concentration (10 µg GAE/mL TPC) compared with control, while a significantly protective effect by CE polyphenols was observed at a concentration above 25 µg GAE/mL (** p < 0.005 vs. control).
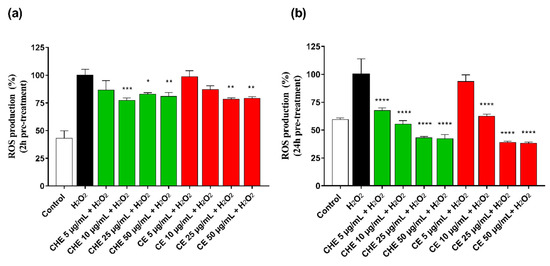
Figure 4.
Rective Oxygen Species (ROS) production by HUVECs was evaluated after 2 h (a) and 24 h (b) of incubation with different concentrations of CHE and CE (i.e., 5, 10, 25, 50, and 100 µg/mL of TPC) and 100 µM H2O2 for 1 h. Data are expressed as ROS production% by treated cells and are representative of three separate experiments run in triplicate (* p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 vs. H2O2).
CHE and CE seemed to increase their antioxidant power over incubation time, as observed in Figure 4b, and directly correlated to their polyphenolic content, indicating a time- and dose-dependent effect on cell metabolic activity.
3.6. Permeation Study of CHE and CE
For each permeation run, the value of the apparent permeability coefficient, P’app, for permeant across the excised rat intestinal mucosa was calculated from the following equation
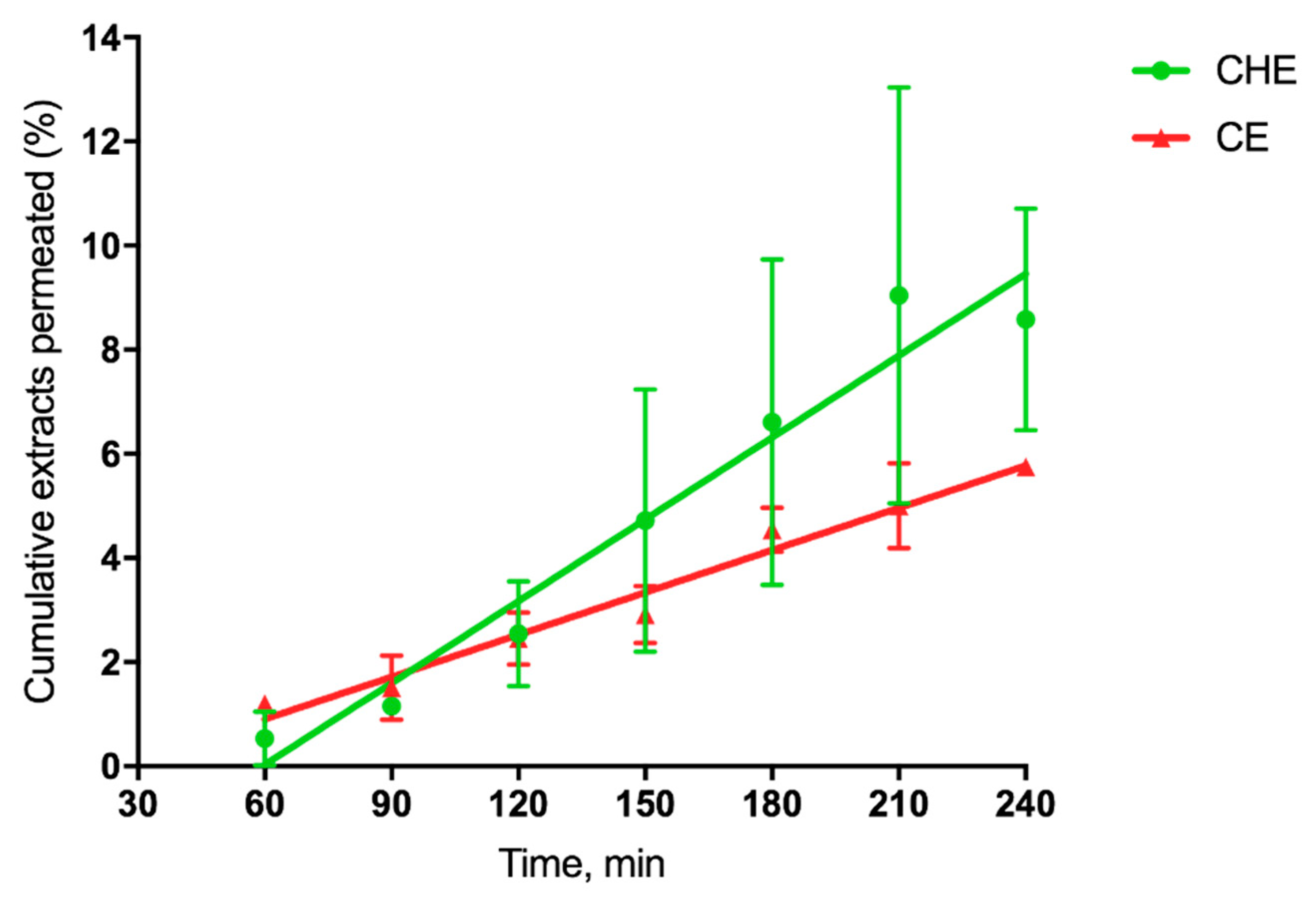
where dM/dt 1/A, the permeation flux, is the slope of the linear portion of the cumulative amount permeated per unit surface area vs. time plot, and C0 is the CHE or CE concentration (280 µg/mL or 15.5 µg/mL, respectively) introduced at the donor phase. For each plot, the quality of the linear regression, shown in Figure 5, as evidenced by r2 values being >0.9.
P’app = dM/dt × 1/(AC0)
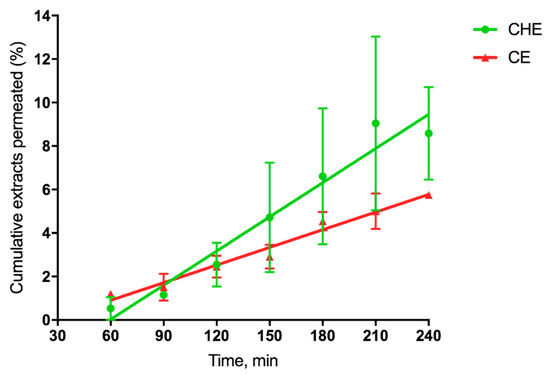
Figure 5.
Cumulative CHE and CE permeated (%) across excised rat intestine.
Such linearity allowed the application of Equation (1), as reported by [41]. The single P’app values were averaged to calculate the mean apparent permeability Papp (n = 3). Since the extract concentration was different for CHE and CE, we calculated the cumulative percentage permeated at each time, useful for comparative purposes. The relevant data reported in Table 3 shows that for cocoa extract, the apparent permeability was 2-fold higher than that corresponding to CE, as the flux was 36-fold higher than that of CE.

Table 3.
Data on CE or cocoa extract permeation across the excised rat intestine. a Apparent permeability. b Cumulative extract permeated (%) over the whole experiment time (4 h). * Significantly different from each other (p < 0.05).
3.7. CHE and CE Stability Studies
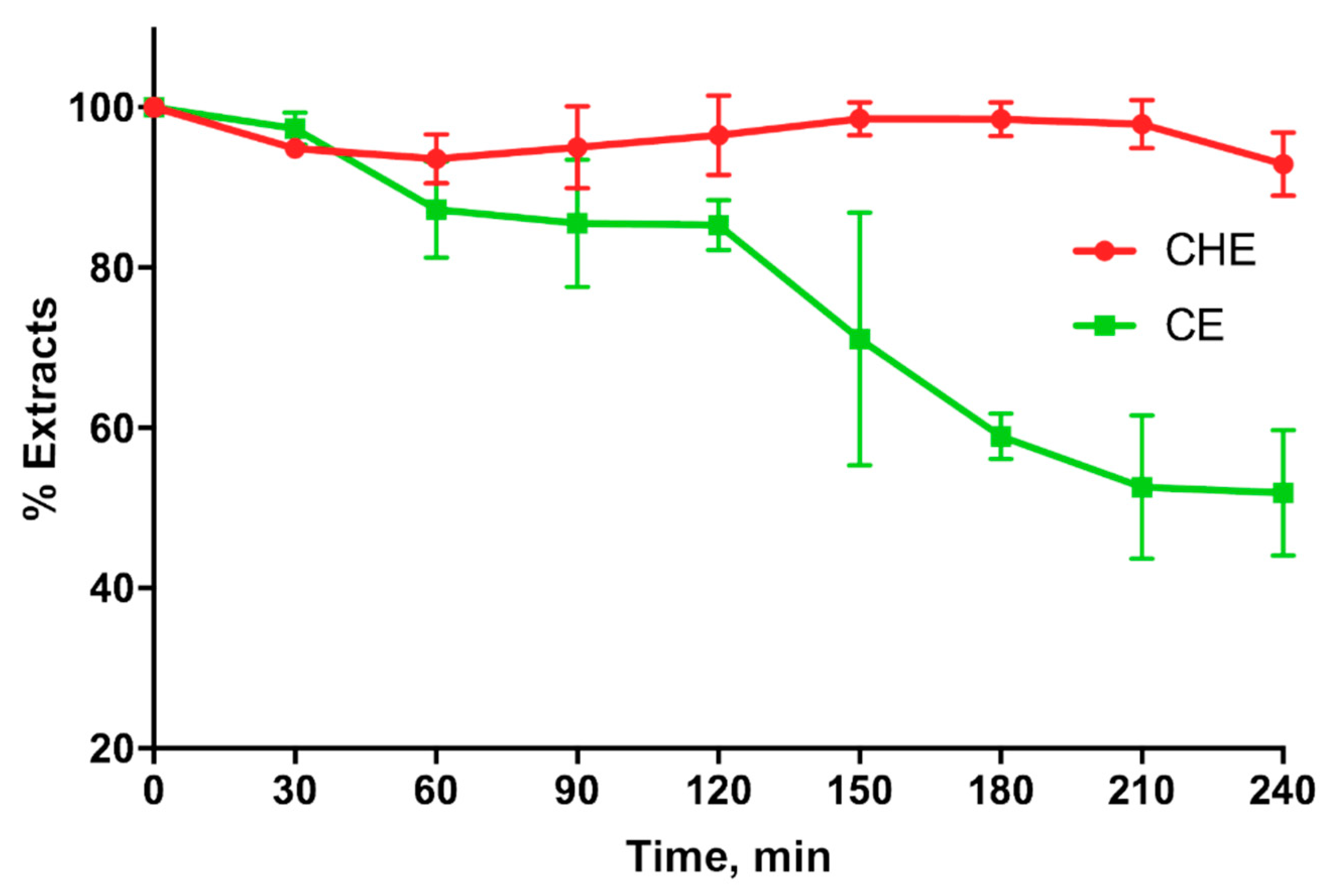
CHE and CE stability in simulated gastric fluids (SGFs) shown in Figure 6, indicates that CHE is more stable than CE in the stomach for at least 4 h. In fact, the CHE polyphenols percentage in the SGF was around 100% for the entire duration of the experiment, whereas that corresponding to CE decreased around 50% just after 3 h.
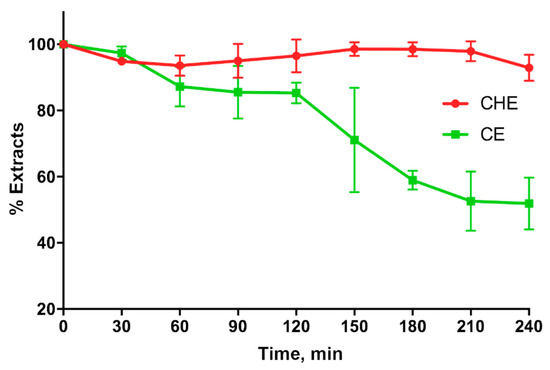
Figure 6.
Cocoa or CE stability in simulated gastric fluid.
4. Discussion
Natural products are increasingly used in scientific research for their potential effect on human health. Many studies show their beneficial effects in clinical and in vitro studies due to the presence of antioxidant molecules, mainly polyphenols. Polyphenols have shown their effects against chronic diseases, including CVD. For the treatment of CVD, prevention plays an important role. The introduction of nutraceuticals in the diet could represent the first defense mechanism of the body from oxidative stress.
In the present study, we compare the antioxidant effects of the molecules contained in an ancient variety of Tuscan cherries (Crognola Capannile) and cocoa by-product extracts (bean and husk).
Numerous studies reported that sweet cherries had high and variable concentrations of antioxidants and TPC in different varieties. A recent study by Berni et al., found the same differences in Tuscan cherries varieties [22]. The analysis confirmed the presence of a high content of antioxidant and phenolic compounds, particularly in the variety Crognola Capannile for CE and Costa Rica for cocoa by-products extract, mainly husks. Specifically, the characterization of the phenolic composition of cocoa bean and husk extracts demonstrated a high concentration, mainly with regard to procyanidins. Above all, compounds 5–9 were the most representative constituents in cocoa husk Costa Rica extract. According to previous studies, cocoa extracts were found to be rich in flavonoids, in particular, dimeric and trimeric procyanidins, among which, the B-type (characterized by a C4–C8 or C4–C6 bond between monomers catechin/epicatechin) were the most representative [37].
Several studies have tested natural products-derived polyphenols on HUVECs [19,20,21] for in vitro experiments related to vascular dysfunction. In this study, we have investigated the in vitro properties of Crognola CE, obtained from fresh fruits, and selected from the six Tuscan varieties the highest TPC and molecular content (as previously shown by Berni et al. [22]), and Costa Rica cocoa by-product extract (in particular, husk product (CHE)), for their rich phenolic content [25].
Vascular oxidative stress contributes to mechanisms of vascular dysfunction and has been implicated in playing an important role in a number of cardiovascular pathologies [42]. There are also many in vitro studies about the antioxidant properties of Prunus avium L. and cocoa by-products for the prevention of chronic diseases [12,16,43,44]. The presence of hydroxycinnamic acids, flavonoids and anthocyanins made CE interesting for in vitro experiments related to oxidative stress. Matias et al. [16] demonstrated that a 2 h pre-treatment with cherry extracts effectively alleviated oxidative stress caused by H2O2-induced injury in neuronal cells.
In a recent study, Rebollo–Hernanz et al. [44] demonstrated that cocoa by-product extracts effectively reduced inflammatory markers in macrophages and adipocytes and the production of reactive oxygen species. Moreover, extracts from cocoa by-products also modulated the phosphorylation of the insulin receptor signaling pathway and stimulated GLUT-4 translocation, increasing glucose uptake [44].
Our results show that lower concentrations of CE protected from oxidative stress in a shorter treatment time (2 h, Figure 3a), in agreement with other studies [16], whereas a higher concentration of polyphenols was required in long-term treatment (24 h, Figure 3b). These results demonstrate the indirect antioxidant potential of CHE, possibly acting through the augmentation of cellular antioxidant capacity by enhancing specific genes encoding antioxidant proteins [45].
Polyphenols showed an anti-radical activity supported by different studies [12,46,47]. In this study, the antioxidant activity of CHE and CE polyphenolic molecules has been verified through the evaluation of ROS production, both with and without H2O2-stress induction on HUVECs.
The obtained results suggest that polyphenols in sweet CE and CHE are able to inhibit ROS, protecting cells from oxidative stress. In particular, CHE showed a time- and dose-dependent effect on cell metabolic activity, probably due to an indirect antioxidant effect [45]. Specifically, CHE showed an antioxidant effect at low concentrations.
Finally, in the present study, we evaluated the ability of natural extracts to cross the excised intestinal wall. The results indicate that CHE is more able to permeate through the excised intestinal wall compared to CE, probably because the antioxidants contained in CHE are more stable than those in CE when they come into contact with the rat intestinal tissue. This hypothesis is in agreement with the results shown in Figure 6, indicating that CHE is more stable than CE in the gastric environment.
5. Conclusions
The two extracts studied in this work, CHE and CE, have both been shown to have antioxidant activity on HUVECs, i.e., on the cells of the endothelium of blood vessels, thus proving to be potential compounds for the prevention of CVD. Between the two extracts, CHE showed better performance on HUVECs, and greater permeability across the rat intestine than CE, perhaps due to its greater stability in the physiological environment. The results obtained in this work encourage us to continue the studies on cocoa husks extracts, which represent a waste product of cocoa processing and a cost for their disposal. Thanks to their beneficial properties, cocoa by-products might become a nutraceutical research topic for possible medical applications in CVD, as well as a potential ingredient for the food and pharmaceutical industries.
Author Contributions
Conceptualization: F.F., Y.Z. and R.D.S.; Data curation: A.F., M.D.L., and D.B.; Formal analysis: D.B.; Investigation, F.F., A.F., D.B., and M.M.C.; Methodology, F.F., A.F., M.D.L., A.M.P., D.B., and A.B.; Project administration, A.B., Y.Z., and R.D.S.; Supervision, A.B., Y.Z., and R.D.S.; Validation, F.F., A.F., M.D.L., and A.M.P.; Writing—original draft, F.F., A.F., and D.B.; Writing—review and editing, F.F., A.F., M.D.L., A.M.P., and R.D.S. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chakraborti, T.; Ghosh, S.K.; Michael, J.R.; Batabyal, S.K.; Chakraborti, S. Targets of oxidative stress in cardiovascular system. Mol. Cell. Biochem. 1998, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Pallardo, F.V.; Garcia de la Asuncion, J.; Vina, J. Mitochondria, oxidative stress and aging. Free Radic. Res. 2000, 32, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Harrison, D.G. Oxidative stress and vascular damage in hypertension. Coron. Artery Dis. 2001, 12, 455–461. [Google Scholar] [CrossRef]
- Zalba, G.; San Jose, G.; Moreno, M.U.; Fortuno, M.A.; Fortuno, A.; Beaumont, F.J.; Diez, J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension 2001, 38, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J. AOAC Int. 2010, 93, 89–96. [Google Scholar]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and its by-products: Identification and utilization. In Chocolate in Health and Nutrition; Watson, R., Preedy, V., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 7, pp. 23–37. [Google Scholar]
- Pérez, E.; Méndez, A.; León, M.; Hernández, G.; Sívoli, L. Proximal composition and the nutritional and functional properties of cocoa by-products (pods and husks) for their use in the food industry. In Chocolate Cocoa Byproducts Technology, Rheology, Styling, and Nutrition; Pérez, E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 219–234. [Google Scholar]
- Ortega, N.; Romero, M.-P.; Macia, A.; Reguant, J.; Anglès, N.; Morelló, J.; Motilva, M.-J. Comparative study of UPLC–MS/MS and HPLC–MS/MS to determine procyanidins and alkaloids in cocoa samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Counet, C.; Collin, S. Effect of the number of flavanol units on the antioxidant activity of procyanidin fractions isolated from chocolate. J. Agric. Food Chem. 2003, 51, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramiro, I.; Martin, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.A.; Rosado-Ramos, R.; Nunes, S.L.; Figueira, I.; Serra, A.T.; Bronze, M.R.; Santos, C.N.; Duarte, C.M. Protective effect of a (Poly)phenol-rich extract derived from sweet cherries culls against oxidative cell damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Di Colo, G.; D’Onofrio, C.; Fausto, C.; Balbarini, A.; Di Stefano, R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur. J. Pharm. Biopharm. 2012, 80, 176–184. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; Schiano, C.; Felice, F.; Casamassimi, A.; Balestrieri, A.; Milone, L.; Servillo, L.; Napoli, C. Effect of low doses of red wine and pure resveratrol on circulating endothelial progenitor cells. J. Biochem. 2008, 143, 179–186. [Google Scholar] [CrossRef]
- Lin, X.L.; Liu, Y.; Liu, M.; Hu, H.; Pan, Y.; Fan, X.J.; Hu, X.M.; Zou, W.W. Inhibition of hydrogen peroxide-induced human umbilical vein endothelial cells aging by allicin depends on Sirtuin1 activation. Med. Sci. Monit. 2017, 23, 563–570. [Google Scholar] [CrossRef]
- Felice, F.; Maragò, E.; Sebastiani, L.; Di Stefano, R. Apple juices from ancient Italian cultivars: A study on mature endothelial cells model. Fruits 2015, 70, 361–369. [Google Scholar] [CrossRef]
- Hafizah, A.H.; Zaiton, Z.; Zulkhairi, A.; Mohd Ilham, A.; Nor Anita, M.M.; Zaleha, A.M. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. J. Zhejiang Univ. Sci. B 2010, 11, 357–365. [Google Scholar] [CrossRef]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.-F.; Guerriero, G.; Cai, G. Functional molecules in locally-adapted crops: The case study of tomatoes, onions, and sweet cherry fruits from Tuscany in Italy. Front. Plant Sci. 2019, 9, 1983. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bruna, C.; Eichholz, I.; Rohn, S.; Kroh, L.; Huyskens-Keil, S. Bioactive compounds and antioxidant activity of cocoa hulls (Theobroma cacao L.) from different origins. J. Appl. Botany Food Qual. 2009, 83, 9–13. [Google Scholar]
- Kim, H.; Keeney, P. (-)-Epicatechin Content in Fermented and Unfermented Cocoa Beans. J. Food Sci. 2006, 49, 1090–1092. [Google Scholar] [CrossRef]
- Urbanska, B.; Kowalska, J. Comparison of the total polyphenol content and antioxidant activity of chocolate obtained from roasted and unroasted cocoa beans from different regions of the World. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Racine, K.C.; Wiersema, B.D.; Griffin, L.E.; Essenmacher, L.A.; Lee, A.H.; Hopfer, H.; Lambert, J.D.; Stewart, A.C.; Neilson, A.P. Flavanol polymerization is a superior predictor of alpha-glucosidase inhibitory activity compared to flavanol or total polyphenol concentrations in cocoas prepared by variations in controlled fermentation and roasting of the same raw cocoa beans. Antioxidants 2019, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Krysiak, W.; Oracz, J.; Sosnowska, D.; Budryn, G.; Nebesny, E. The influence of the roasting process conditions on the polyphenol content in cocoa beans, nibs and chocolates. Food Res. Int. 2016, 89, 918–929. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical profile, antioxidant and antidiabetic activities of Adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress: Mucoadhesive chitosan vs. poly(lactic-co-glycolic acid) nanoparticles. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Mattii, L.; Braca, A.; Felice, F.; Di Stefano, R.; Zambito, Y. Nanoparticles based on quaternary ammonium-chitosan conjugate: A vehicle for oral administration of antioxidants contained in red grapes. J. Drug Deliv. Technol. 2016, 32, 291–297. [Google Scholar] [CrossRef]
- Legen, I.; Salobir, J.; Kerc, J. Comparison of different intestinal epithelia as models for absorption enhancement studies. Int. J. Pharm. 2005, 291, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Lozano-Sanchez, J.; Contreras-Gámez, M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 2014, 10, 485–498. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macia, A.; Reguant, J.; Angles, N.; Morello, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef]
- Zambito, Y.; Zaino, C.; Uccello-Barretta, G.; Balzano, F.; Di Colo, G. Improved synthesis of quaternary ammonium-chitosan conjugates (N+ -Ch) for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2008, 33, 343–350. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.A.; Tripathi, A.; Dixit, P.K.; Bajaj, U.K. Role of oxidative stress in various diseases: Relevance of dietary antioxidants. J. Pharm. Exp. Ther. 2015, 4, 126–132. [Google Scholar]
- Usenik, V.; Fabčič, J.; Stampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).