Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough?
Abstract
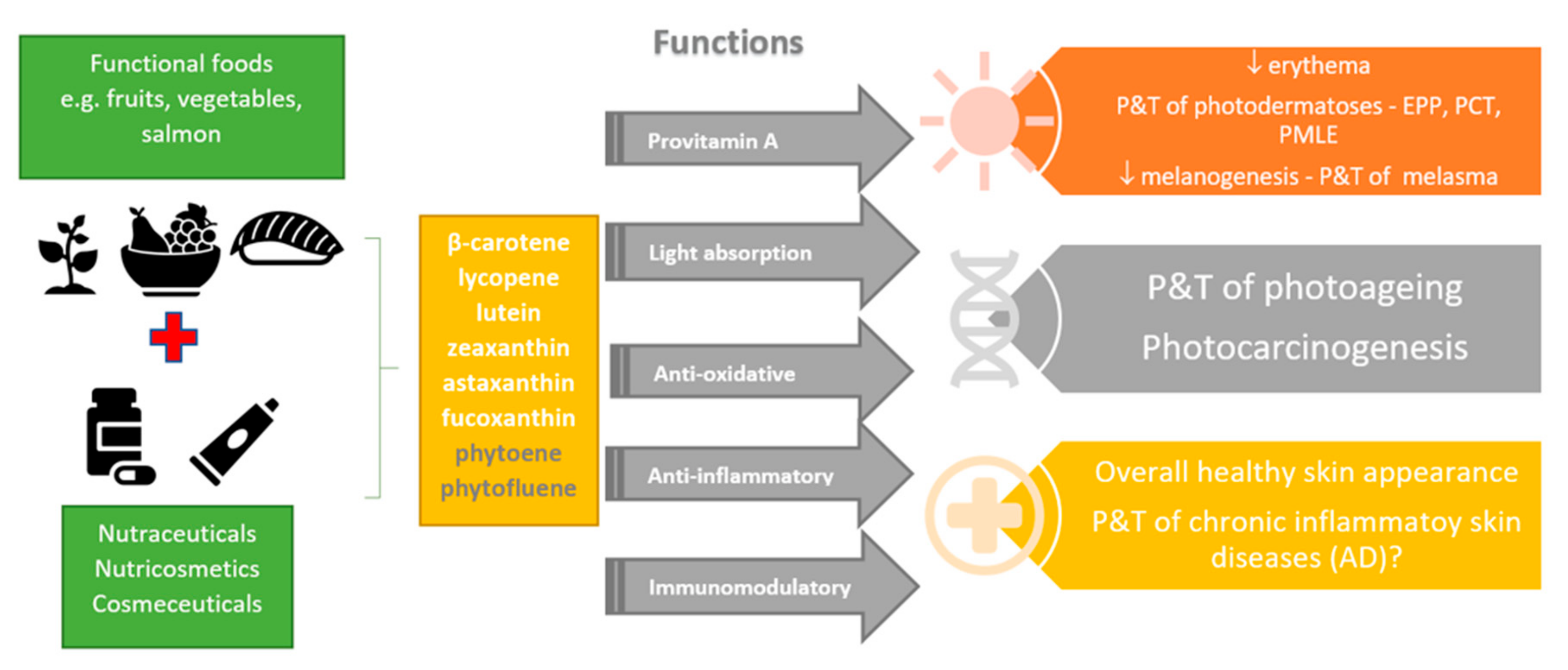
1. Introduction
2. Carotenoids
3. Carotenoids and the Skin
4. Carotenoids in Skin Photoprotection
4.1. Lycopene
4.2. Lutein
4.3. β-Carotene
4.4. Astaxanthin
4.5. Fucoxanthin
5. Carotenoids and Photocarcinogenesis
6. Carotenoids and Skin Aging
6.1. Lycopene
6.2. Lutein and Zeaxanthin
6.3. β-Carotene
6.4. Fucoxanthin
6.5. Astaxanthin
7. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Feingold, K.R.; Denda, M. Regulation of permeability barrier homeostasis. Clin. Dermatol. 2012, 30, 263–268. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Cash, K. Topical corticosteroid application and the structural and functional integrity of the epidermal barrier. J. Clin. Aesthet. Dermatol. 2013, 6, 20–27. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Schieber, A.; Weber, F. Carotenoids. Handb. Nat. Pigment. Food Beverages 2016, 101–123. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. TrAC Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Abar, L.; Vieira, A.R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D.A.; Chan, D.; Greenwood, D.C.; Norat, T. Blood concentrations of carotenoids and retinol and lung cancer risk: An update of the WCRF–AICR systematic review of published prospective studies. Cancer Med. 2016, 5, 2069–2083. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Alscher, R.G.; Hess, J.L. Antioxidants in Higher Plants; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1351369148. [Google Scholar]
- Mercadante, A.Z.; Egeland, E.S.; Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2004. [Google Scholar]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- Deming, D.M.; Erdman, J.W. Mammalian carotenoid absorption and metabolism. Pure Appl. Chem. 2007, 71, 2213–2223. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Caspers, P.; van der Pool, A.; Richter, H.; Patzelt, A.; Sterry, W.; Lademann, J. In vivo distribution of carotenoids in different anatomical locations of human skin: Comparative assessment with two different raman spectroscopy methods. Exp. Dermatol. 2009, 18, 1060–1063. [Google Scholar] [CrossRef]
- Lowe, G.M.; Bilton, R.F.; Davies, I.G.; Ford, T.C.; Billington, D.; Young, A.J. Carotenoid composition and antioxidant potential in subfractions of human low-density lipoprotein. Ann. Clin. Biochem. 1999, 36, 323–332. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef]
- Nagao, A. Bioavailability of dietary carotenoids: Intestinal absorption and metabolism. Japan Agric. Res. Q. 2014, 48, 385–392. [Google Scholar] [CrossRef]
- Kong, K.-W.; Khoo, H.-E.; Prasad, K.N.; Ismail, A.; Tan, C.-P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef]
- Stahl, W.; Sundquist, A.R.; Hanusch, M.; Schwarz, W.; Sies, H. Separation of β-carotene and lycopene geometrical isomers in biological samples. Clin. Chem. 1993, 39, 810–814. [Google Scholar]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in human skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Eroglu, A.; Hruszkewycz, D.P.; Dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Kopec, R.E.; Schwartz, S.J.; Curley, R.W.; Harrison, E.H. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 2012, 287, 15886–15895. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res 2011, 5, 7119–7131. [Google Scholar]
- Insel, P.M. Discovering Nutrition; Jones & Bartlett Publishers: Burlington, MA, USA, 2013; ISBN 1449632947. [Google Scholar]
- Bunea, A.; Rugină, D.; Pintea, A.; Andrei, S.; Bunea, C.; Pop, R.; Bele, C. Carotenoid and fatty acid profiles of bilberries and cultivated blueberries from Romania. Chem. Pap. 2012, 66, 935–939. [Google Scholar] [CrossRef]
- Ježek, D.; Tripalo, B.; Brnčić, M.; Karlović, D.; Rimac Brnčić, S.; Vikić-Topić, D.; Karlović, S. Dehydration of celery by infrared drying. Croat. Chem. Acta 2008, 81, 325–331. [Google Scholar]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Sprenger, J.; Hempel, J.; Kreiser, F.; Carle, R.; Schweiggert, R.M. Carotenoids from gac fruit aril (Momordica cochinchinensis [Lour.] Spreng.) are more bioaccessible than those from carrot root and tomato fruit. Food Res. Int. 2017, 99, 928–935. [Google Scholar] [CrossRef]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people—a randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications - A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Van Chuyen, H.; Eun, J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Šic Žlabur, J.; Rimac Brnčić, S.; Barba, F.J.; Grimi, N.; Koubaa, M.; Brnčić, M.; Vorobiev, E. Electrotechnologies, microwaves, and ultrasounds combined with binary mixtures of ethanol and water to extract steviol glycosides and antioxidant compounds from Stevia rebaudiana leaves. J. food Process. Preserv. 2017, 41, e13179. [Google Scholar] [CrossRef]
- Darvin, M.; Patzelt, A.; Gehse, S.; Schanzer, S.; Benderoth, C.; Sterry, W.; Lademann, J. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur. J. Pharm. Biopharm. 2008, 69, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Eichler, O.; Sies, H.; Stahl, W. Divergent Optimum Levels of Lycopene, β-Carotene and Lutein Protecting Against UVB Irradiation in Human Fibroblasts. Photochem. Photobiol. 2004, 75, 503–506. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—a review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T. Singlet oxygen and free radical reactions of retinoids and carotenoids—a review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, H.; Khovidhunkit, W.; Brown, B.E.; Fluhr, J.W.; Elias, P.M.; Feingold, K.R. Scavenger receptor class B type I is expressed in cultured keratinocytes and epidermis. Regulation in response to changes in cholesterol homeostasis and barrier requirements. J. Biol. Chem. 2002, 277, 2916–2922. [Google Scholar] [CrossRef]
- Priyadarshani, A.M.B. Insights of hypercarotenaemia: A brief review. Clin. Nutr. ESPEN 2018, 23, 19–24. [Google Scholar] [CrossRef]
- Maharshak, N.; Shapiro, J.; Trau, H. Carotenoderma--a review of the current literature. Int. J. Dermatol. 2003, 42, 178–181. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical beta-carotene protects against infra-red-light-induced free radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Dreher, F.; Maibach, H. Protective effects of topical antioxidants in humans. Curr. Probl. Dermatol. 2001, 29, 157–164. [Google Scholar] [PubMed]
- Lademann, J.; Caspers, P.J.; Van Der Pol, A.; Richter, H.; Patzelt, A.; Zastrow, L.; Darvin, M.; Sterry, W.; Fluhr, J.W. In vivo Raman spectroscopy detects increased epidermal antioxidative potential with topically applied carotenoids. Laser Phys. Lett. 2009, 6, 76–79. [Google Scholar] [CrossRef]
- Bogdan Allemann, I.; Baumann, L. Antioxidants used in skin care formulations. Skin Therapy Lett. 2008, 13, 5–9. [Google Scholar] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Zastrow, L.; Sterry, W.; Lademann, J. Effect of supplemented and topically applied antioxidant substances on human tissue. Skin Pharmacol. Physiol. 2006, 19, 238–247. [Google Scholar] [CrossRef]
- Scarmo, S.; Cartmel, B.; Lin, H.; Leffell, D.J.; Welch, E.; Bhosale, P.; Bernstein, P.S.; Mayne, S.T. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch. Biochem. Biophys. 2010, 504, 34–39. [Google Scholar] [CrossRef]
- von Lintig, J.; Sies, H.; Mayne, S.T.; Cartmel, B.; Scarmo, S.; Jahns, L.; Ermakov, I.V.; Gellermann, W. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Arch. Biochem. Biophys. 2013, 539, 163–170. [Google Scholar]
- Sies, H.; Stahl, W. Nutritional Protection against Skin Damage from Sunlight. Annu. Rev. Nutr. 2004, 24, 173–200. [Google Scholar] [CrossRef]
- Lademann, J.; Köcher, W.; Yu, R.; Meinke, M.C.; Na Lee, B.; Jung, S.; Sterry, W.; Darvin, M.E. Cutaneous carotenoids: The mirror of lifestyle? Skin Pharmacol. Physiol. 2014, 27, 201–207. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef]
- Godar, D.E. UV Doses Worldwide - Invited Review. Photochem. Photobiol. 2005, 81, 736–749. [Google Scholar] [CrossRef]
- Sanches Silveira, J.E.P.; Myaki Pedroso, D.M. UV light and skin aging. Rev. Environ. Health 2014, 29, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Holick, M.F. Benefits and requirements of vitamin D for optimal health: A review. Altern. Med. Rev. 2005, 10, 94–111. [Google Scholar]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Timares, L.; Katiyar, S.K.; Elmets, C.A. DNA damage, apoptosis and Langerhans cells - Activators of UV-induced immune tolerance. Photochem. Photobiol. 2008, 84, 422–436. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Obermueller-Jevic, U.C. UV light, beta-carotene and human skin - Beneficial and potentially harmful effects. Arch. Biochem. Biophys. 2001, 389, 1–6. [Google Scholar] [CrossRef]
- Césarini, J.P.; Michel, L.; Maurette, J.M.; Adhoute, H.; Béjot, M. Immediate effects of UV radiation on the skin: Modification by an antioxidant complex containing carotenoids. Photodermatol. Photoimmunol. Photomed. 2003, 19, 182–189. [Google Scholar] [CrossRef]
- Randhawa, M.; Seo, I.S.; Liebel, F.; Southall, M.D.; Kollias, N.; Ruvolo, E. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS ONE 2015, 10, e0130949. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Invest. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on the skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef]
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Junqueira, H.C.; Martins, W.K.; Baptista, M.S.; Gaspar, L.R. Antioxidant role on the protection of melanocytes against visible light-induced photodamage. Free Radic. Biol. Med. 2019, 131, 399–407. [Google Scholar] [CrossRef]
- Fernández-García, E. Photoprotection of human dermal fibroblasts against ultraviolet light by antioxidant combinations present in tomato. Food Funct. 2014, 5, 285–290. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and protection against solar UV radiation. Skin Pharmacol. Physiol. 2002, 15, 291–296. [Google Scholar] [CrossRef]
- Rabinovich, L.; Kazlouskaya, V. Herbal sun protection agents: Human studies. Clin. Dermatol. 2018, 36, 369–375. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Photochemoprevention by botanical antioxidants. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 297–306. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.B.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179–1184. [Google Scholar] [CrossRef]
- Stahl, W.; Krutmann, J. Systemische photoprotektion durch karotinoide. Hautarzt 2006, 57, 281–285. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef]
- Boukari, F.; Jourdan, E.; Fontas, E.; Montaudié, H.; Castela, E.; Lacour, J.-P.; Passeron, T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J. Am. Acad. Dermatol. 2015, 72, 189–190. [Google Scholar] [CrossRef]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef]
- Greul, A.K.; Grundmann, J.U.; Heinrich, F.; Pfitzner, I.; Bernhardt, J.; Ambach, A.; Biesalski, H.K.; Gollnick, H. Photoprotection of UV-irradiated human skin: An antioxidative combination of vitamins E and C, carotenoids, selenium and proanthocyanidins. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 307–315. [Google Scholar] [CrossRef]
- Wertz, K.; Seifert, N.; Hunziker, P.B.; Riss, G.; Wyss, A.; Lankin, C.; Goralczyk, R. β-carotene inhibits UVA-induced matrix metalloprotease 1 and 10 expression in keratinocytes by a singlet oxygen-dependent mechanism. Free Radic. Biol. Med. 2004, 37, 654–670. [Google Scholar] [CrossRef]
- Wertz, K.; Hunziker, P.B.; Seifert, N.; Riss, G.; Neeb, M.; Steiner, G.; Hunziker, W.; Goralczyk, R. β-carotene interferes with ultraviolet light A-induced gene expression by multiple pathways. J. Invest. Dermatol. 2005, 124, 428–434. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Köpcke, W.; Krutmann, J. Protection from sunburn with β-carotene - A meta-analysis. Photochem. Photobiol. 2008, 84, 284–288. [Google Scholar] [CrossRef]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with β-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. J. Nutr. 2018, 133, 98–101. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Pathak, M.A.; Parrish, J.; Fitzpatrick, T.B.; Kass, E.H.; Toda, K.; Clemens, W. A clinical trial of the effects of oral beta-carotene on the responses of human skin to solar radiation. J. Invest. Dermatol. 1972, 59, 349–353. [Google Scholar] [CrossRef]
- Lee, J.; Jiang, S.; Levine, N.; Watson, R.R. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc. Soc. Exp. Biol. Med. 2000, 223, 170–174. [Google Scholar] [CrossRef]
- Epstein, J.H. Effects of β-carotene on ultraviolet induced cancer formation in the hairless mouse skin. Photochem. Photobiol. 1977, 25, 211–213. [Google Scholar] [CrossRef]
- Santamaria, L.; Bianchi, A.; Arnaboldi, A.; Andreoni, L.; Bermond, P. Dietary carotenoids block photocarcinogenic enhancement by benzo (a) pyrene and inhibit its carcinogenesis in the dark. Experientia 1983, 39, 1043–1045. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Torsello, A.; Di Nicuolo, F.; Maggiano, N.; Ranelletti, F.O.; Wolf, F.I.; Calviello, G. Mechanism of activation of caspase cascade during β-carotene-induced apoptosis in human tumor cells. Nutr. Cancer 2003, 47, 76–87. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Kuttan, G. β-Carotene down-regulates inducible nitric oxide synthase gene expression and induces apoptosis by suppressing bcl-2 expression and activating caspase-3 and p53 genes in B16F-10 melanoma cells. Nutr. Res. 2007, 27, 336–342. [Google Scholar] [CrossRef]
- Frieling, U.M.; Schaumberg, D.A.; Kupper, T.S.; Muntwyler, J.; Hennekens, C.H. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physicians’ health study. Arch. Dermatol. 2000, 136, 179–184. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of Effect of Long-Term Supplementation with Beta Carotene on the Incidence of Malignant Neoplasms and Cardiovascular Disease. N. Engl. J. Med. 2002, 334, 1145–1149. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Stukel, T.A.; Stevens, M.M.; Mandel, J.S.; Spencer, S.K.; Elias, P.M.; Lowe, N.; Nierenberg, D.W.; Bayrd, G. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. N. Engl. J. Med. 1990, 323, 789–795. [Google Scholar] [CrossRef]
- Wertz, K.; Seifert, N.; Buchwald Hunziker, P.; Riss, G.; Wyss, A.; Hunziker, W.; Goralczyk, R. β-Carotene interference with UVA-induced gene expression by multiple pathways. Pure Appl. Chem. 2006, 78, 1539–1550. [Google Scholar] [CrossRef]
- Minami, Y.; Kawabata, K.; Kubo, Y.; Arase, S.; Hirasaka, K.; Nikawa, T.; Bando, N.; Kawai, Y.; Terao, J. Peroxidized cholesterol-induced matrix metalloproteinase-9 activation and its suppression by dietary β-carotene in photoaging of hairless mouse skin. J. Nutr. Biochem. 2009, 20, 389–398. [Google Scholar] [CrossRef]
- Marini, A.; Jaenicke, T.; Grether-Beck, S.; Le Floc’h, C.; Cheniti, A.; Piccardi, N.; Krutmann, J. Prevention of polymorphic light eruption by oral administration of a nutritional supplement containing lycopene, β-carotene, and L actobacillus johnsonii: Results from a randomized, placebo-controlled, double-blinded study. Photodermatol. Photoimmunol. Photomed. 2014, 30, 189–194. [Google Scholar] [CrossRef]
- Mathews Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.H.; Kass, E.H. Beta Carotene Therapy for Erythropoietic Protoporphyria and Other Photosensitivity Diseases. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Foley, S.; Lange, L.; Truscott, T.G. Antioxidant inhibition of porphyrin-induced cellular phototoxicity. J. Photochem. Photobiol. B 2001, 65, 177–183. [Google Scholar] [CrossRef]
- Harper, P.; Wahlin, S. Treatment options in acute porphyria, porphyria cutanea tarda, and erythropoietic protoporphyria. Curr. Treat. Options Gastroenterol. 2007, 10, 444–455. [Google Scholar] [CrossRef]
- Offord, E.A.; Gautier, J.C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes protect against development of UV-induced keratinocyte carcinoma via metabolomic alterations. Sci. Rep. 2017, 7, 5106. [Google Scholar] [CrossRef]
- Evans, J.A.; Johnson, E.J. Something New Under the Sun: Lutein—s Role in Skin Health. Am. J. Lifestyle Med. 2009, 3, 349–352. [Google Scholar] [CrossRef]
- Ascenso, A.; Ribeiro, H.; Marques, H.C.; Oliveira, H.; Santos, C.; Simões, S. Chemoprevention of photocarcinogenesis by lycopene. Exp. Dermatol. 2014, 23, 874–878. [Google Scholar] [CrossRef]
- Heinrich, U.; Tronnier, H.; Stahl, W.; Béjot, M.; Maurette, J.M. Antioxidant supplements improve parameters related to skin structure in humans. Skin Pharmacol. Physiol. 2006, 19, 224–231. [Google Scholar] [CrossRef]
- O’Connor, I.; O’Brien, N. Modulation of UVA light-induced oxidative stress by β-carotene, lutein and astaxanthin in cultured fibroblasts. J. Dermatol. Sci. 1998, 16, 226–230. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Hix, L.M.; Lockwood, S.F.; Bertram, J.S. Upregulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives. Cancer Lett. 2004, 211, 25–37. [Google Scholar] [CrossRef]
- Scarmo, S.N. Noninvasive Measurement of Carotenoids in Human Skin as a Biomarker of Fruit and Vegetable Intake; Yale University: New Haven, CA, USA, 2009; ISBN 1109588186. [Google Scholar]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with Dietary Astaxanthin Combined with Collagen Hydrolysate Improves Facial Elasticity and Decreases Matrix Metalloproteinase-1 and -12 Expression: A Comparative Study with Placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Suganuma, K.; Shiobara, M.; Sato, Y.; Nakanuma, C.; Maekawa, T.; Ohtsuki, M.; Yazawa, K.; Imokawa, G. Anti-aging and functional improvement effects for the skin by functional foods intakes: Clinical effects on skin by oral ingestion of preparations containing Astaxanthin and Vitamins C and E. Jichi Med. Univ. J. 2012, 35, 25–33. [Google Scholar]
- Suganuma, K.; Nakajima, H.; Ohtsuki, M.; Imokawa, G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J. Dermatol. Sci. 2010, 58, 136–142. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2019, 1–6. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of astaxanthin on cutaneous wound healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Pathak, M.A.; Goukassian, D. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Invest. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef]
- Astner, S.; Wu, A.; Chen, J.; Philips, N.; Rius-Diaz, F.; Parrado, C.; Mihm, M.C.; Goukassian, D.A.; Pathak, M.A.; González, S. Dietary lutein/zeaxanthin partially reduces photoaging and photocarcinogenesis in chronically UVB-irradiated Skh-1 hairless mice. Skin Pharmacol. Physiol. 2007, 20, 283–291. [Google Scholar] [CrossRef]
- Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C.; van der Pols, J.C. Intake of antioxidant nutrients and the risk of skin cancer. Eur. J. Cancer 2007, 43, 2707–2716. [Google Scholar] [CrossRef]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef]
- Schwartz, S.; Frank, E.; Gierhart, D.; Simpson, P.; Frumento, R. Zeaxanthin-based dietary supplement and topical serum improve hydration and reduce wrinkle count in female subjects. J. Cosmet. Dermatol. 2016, 15, e13–e20. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T.G. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B Biol. 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Pirayesh Islamian, J.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386–391. [Google Scholar]
- Böhm, F.; Edge, R.; Burke, M.; Truscott, T.G. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide–ROS components from cigarette smoke. J. Photochem. Photobiol. B Biol. 2001, 64, 176–178. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Wiseman, S.; Eichler, O.; Sies, H.; Tronnier, H. Dietary Tomato Paste Protects against Ultraviolet Light–Induced Erythema in Humans. J. Nutr. 2018, 131, 1449–1451. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.A.; Close, G.L.; Jack, C.I.; Friedmann, P.S.; Jackson, M.J. Effects of oral vitamin E and β-carotene supplementation on ultraviolet radiation-induced oxidative stress in human skin. Am. J. Clin. Nutr. 2004, 80, 1270–1275. [Google Scholar] [CrossRef]
- White, A.L.; Jahnke, L.S. Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of β-carotene in two Dunaliella spp. Plant Cell Physiol. 2002, 43, 877–884. [Google Scholar] [CrossRef]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012, 4, 37–41. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stahl, W.; Rock, C.L. Beta Carotene: From Biochemistry to Clinical Trials. Nutr. Rev. 2009, 58, 39–53. [Google Scholar] [CrossRef]
- Bayerl, C. Beta-carotene in dermatology: Does it help? Acta Dermatovenerol. Alp. Pannonica Adriat. 2008, 17, 160–162,164–166. [Google Scholar]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Sofiah, A.S.; Lesmana, R.; Farenia, R.; Adi, S. Astaxanthin cream alters type I procollagen and Matrix metalloproteinase-1 (MMP-1) gene expression induced by ultraviolet B irradiation in rat skin. J. Biomed. Clin. Sci. 2018, 3, 62–67. [Google Scholar]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. Biol. B 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C.; et al. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.P.; Bingham, S.; Johansson, I.; Wallström, P.; Overvad, K.; Tjønneland, A.; et al. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Ferrari, P.; Slimani, N.; Pala, V.; Johansson, I.; Nilsson, S.; Mattisson, I.; Wirfalt, E.; Galasso, R.; Palli, D.; et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Clin. Nutr. 2005, 59, 1397–1408. [Google Scholar] [CrossRef]
- Meinke, M.C.; Müller, R.; Bechtel, A.; Haag, S.F.; Darvin, M.E.; Lohan, S.B.; Ismaeel, F.; Lademann, J. Evaluation of carotenoids and reactive oxygen species in human skin after UV irradiation: A critical comparison between in vivo and ex vivo investigations. Exp. Dermatol. 2015, 24, 194–197. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef]
- Segger, D.; Schönlau, F. Supplementation with Evelle® improves skin smoothness and elasticity in a double-blind, placebo-controlled study with 62 women. J. Dermatolog. Treat. 2004, 15, 222–226. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of orally taken carotenoid-rich curly kale extract on collagen I/elastin index of the skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef]
- Cho, S.; Lee, D.H.; Won, C.-H.; Kim, S.M.; Lee, S.; Lee, M.-J.; Chung, J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 930164. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef]
- Eren, B.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Kindlund, P.J. Astaxanthin. Nutrafoods 2011, 10, 27–31. [Google Scholar] [CrossRef]
- Daubrawa, F.; Sies, H.; Stahl, W. Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. J. Nutr. 2005, 135, 2507–2511. [Google Scholar] [CrossRef]
| Carotenoid | Food Source | Photoprotective Effects | Role in Photo-Induced Carcinogenesis Prevention | Role in Photoaging Prevention | Additional Benefits |
|---|---|---|---|---|---|
| β-carotene | Pumpkin, carrots, sweet potatoes, mangos, papaya, bilberry [28,29,30,33] | Prevention of UV-induced erythema [88,100,101], ↑1 MED2 [102,103], ↓3 of the rate of mitochondrial mutation in human dermal fibroblasts after UV irradiation [96] | Delayed tumor appearance and reduced tumor growth rates [104], inhibition of photocarcinogenic enhancement by benzopyrene [105], in vitro induction of apoptosis of melanoma cells by activation of caspase-3,-8, and -9 [106] or by additional regulation of Bcl-2, p53 [107] In vivo no influence, positive or negative, on the incidence of malignant skin neoplasms, including melanoma. and NMSC4 [108,109,110] | O2 quenching,↓MMP5-1, -3, and MMP-10 [96,111],↓MMP-9 partly, by inhibiting Chol-OOHs6 formation [112] | Combination of β-carotene, lycopene and Lactobacillus johnsonii inhibits PMLE7 [113], protective role in the treatment of EPP8 and PCT9 by membrane protection against protoporphyrin IX and uroporphyrin I [114,115,116] |
| Lycopene | Tomatoes, asparagus, pink grapefruit, guava, watermelon, peaches, papaya [28,29,33] | Protection against UV-induced erythema [95,117,118,119] ↓ HO-110, ↓ ICAM-111 [79] | Inhibits mtDNA deletion [87], inhibits skin tumor formation [120], induction of apoptosis [121], chemoprevention properties in photocarcinogenesis remain contradictory [122] | ↓ MMP [79], ↓MMP-1 and ↓ reduction in fibrillin-1 [87],↓ amount of furrows and wrinkles [101,123] | PMLE prevention [113], protective role in EPP [115] |
| Astaxanthin | Microalgae, yeast, salmon, trout, krill, shrimp, crayfish and crustacea [28,29,35,39] | Protection against UV-induced erythema, ↑MED, activation of Nrf213/HO-1 AO pathway [35,37,38,124] | Inhibition of skin cancer and tyosinase in rat model [125]; apoptosis [126]; AO effect, effect on gap junctional communication important for homeostasis, growth control, and development of cells [127,128,129], may enhance immune responses and potentially exert antitumor activity [130] | ↓ wrinkle parameters [131,132], ↑ elasticity, improved skin texture, and ↓ TEWL12 [38,131,133,134], ↓ size of age spots [131], ↑ procollagen type I, ↓MMP-1, -3, -12, also MMP-13 [126,133,135], ↓ malondialdehyde; ↓ residual skin surface components [136,137], ↓ IL14-1α [132], ↓ MIF15, IL-1β, TNF-α16, preserves trans-UCA17 levels [126], ↓ mast cells [135] | Anti-inflammatory properties - ↓ iNOS18, COX-2, and inhibition of NFκB signaling [138]; ↓ TNF-α, IL-1β, IL-6— possible implication for the treatment of inflammatory diseases such as atopic dermatitis [138] and psoriasis Accelerates wound healing—↓iNOS, ↑wound healing biological markers including Col1A121 and bFGF22 [139] |
| Lutein/ Zeaxanthin | Leafy green vegetables, peas, broccoli, pumpkins, corn, red peppers, egg yolk, bilberry [28,29,30,34] | ↓ skin edema and erythema after UVR [140], ↓ masts cells number [141], ↓ melanogenesis [93], blocking of eye damage induced by blue light [90,91] | ↑ tumor-free survival time, ↓ tumor volume and multiplicity [141], ↓ PCNA23 and BrdU + epidermal cells [140], reduced incidence of SCC24 in persons who had a history of skin cancer at baseline [142] | ↓overexpression of HO-1, ICAM-1, MMP-1 Genes [79], ↓ MMP-1 and MMP-7, ↑ TIMP-2 [141,143], ↑ surface lipids, skin hydration, and skin elasticity [54,144] | Prevention of melasma, skin-lightening effects [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. https://doi.org/10.3390/antiox8080259
Balić A, Mokos M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants. 2019; 8(8):259. https://doi.org/10.3390/antiox8080259
Chicago/Turabian StyleBalić, Anamaria, and Mislav Mokos. 2019. "Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough?" Antioxidants 8, no. 8: 259. https://doi.org/10.3390/antiox8080259
APA StyleBalić, A., & Mokos, M. (2019). Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants, 8(8), 259. https://doi.org/10.3390/antiox8080259





