Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Diets
2.3. Lycopene Supplementation
2.4. Euthanasia
2.5. Nutritional and Obesity Characterization
2.6. Lycopene Bioavailability Evaluation
2.7. Clinical Biochemistry
2.8. Hepatic Tissue Analysis
2.8.1. Histology
2.8.2. Total Proteins
2.8.3. Inflammatory Biomarkers
2.8.4. Redox State
Oxidative Damage to Lipids
Antioxidant Enzyme Activity
Hepatic Antioxidant Capacity
2.9. Statistical Analysis
3. Results
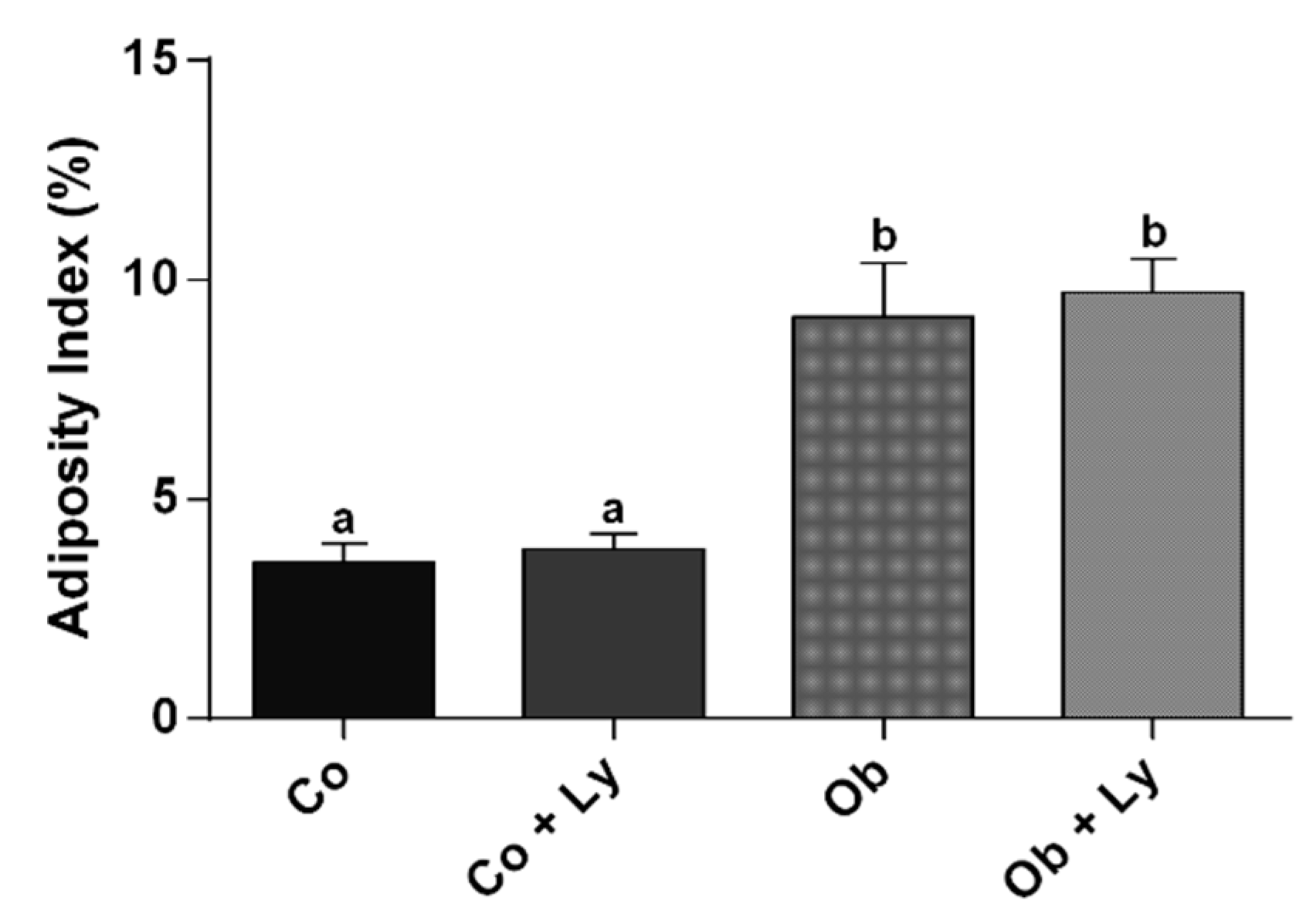
3.1. Lycopene Does Not Influence Nutritional and Adiposity Markers
3.2. Lycopene Is Available in Plasma and Liver of Supplemented Rats
3.3. Lycopene Influences Plasma Lipid Markers
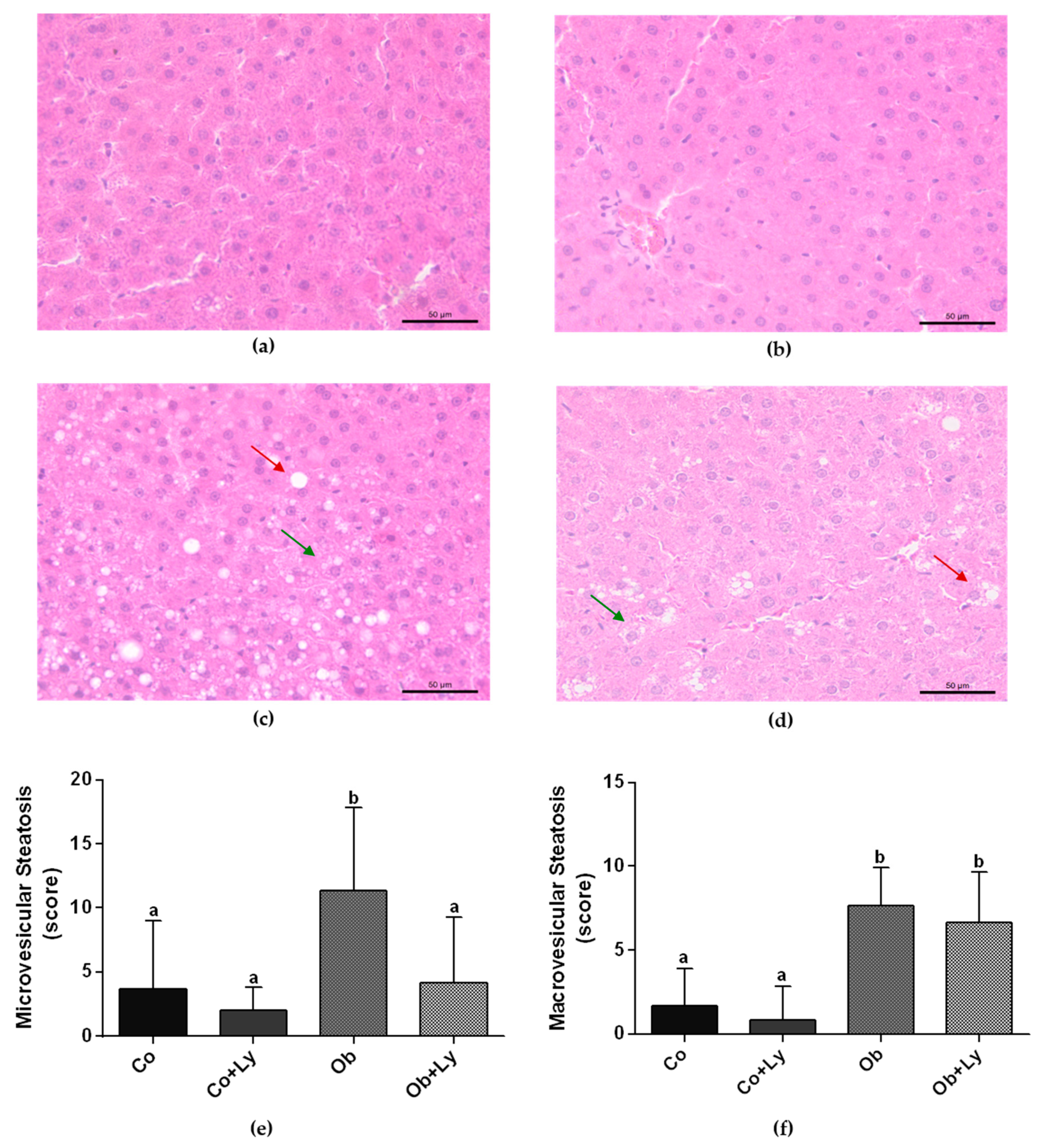
3.4. Lycopene Ameliorates Obesity-Related Hepatic Steatosis
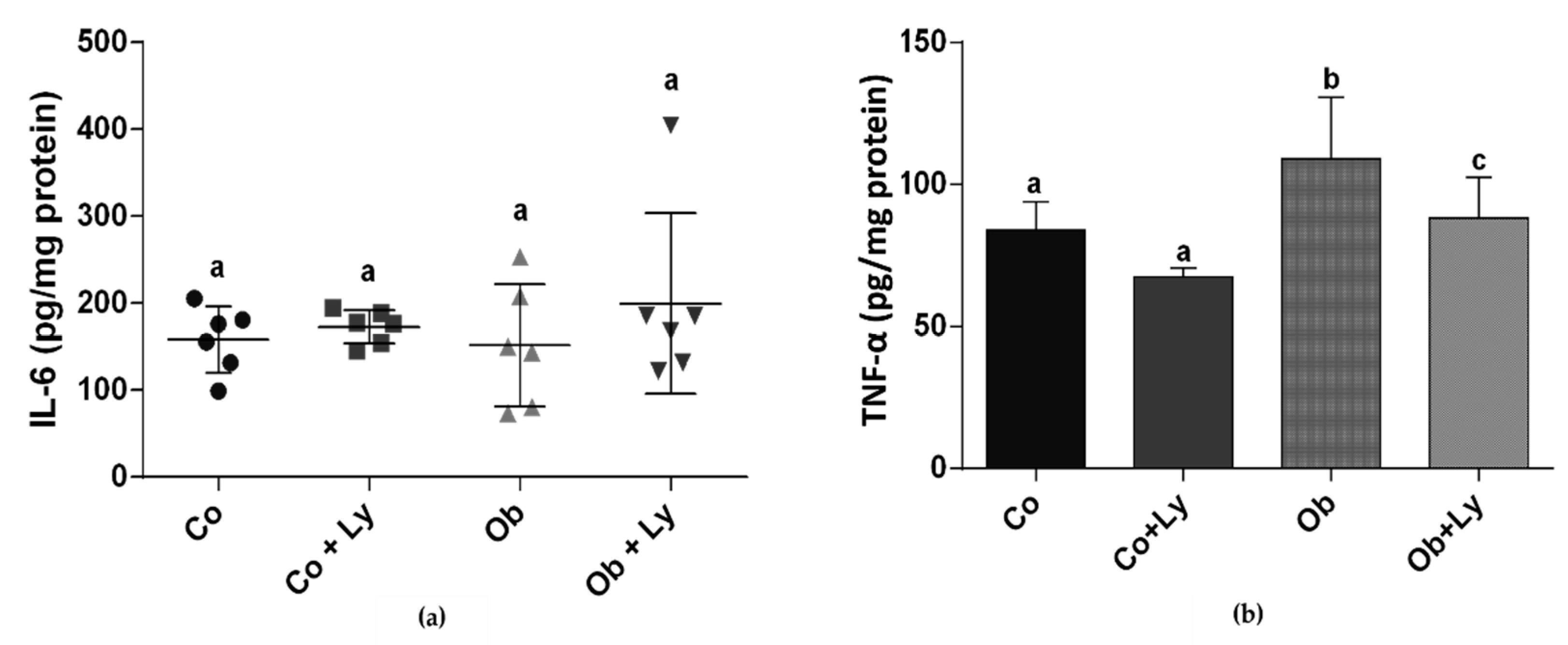
3.5. Lycopene Ameliorates TNF-α Levels in Hepatic Tissue
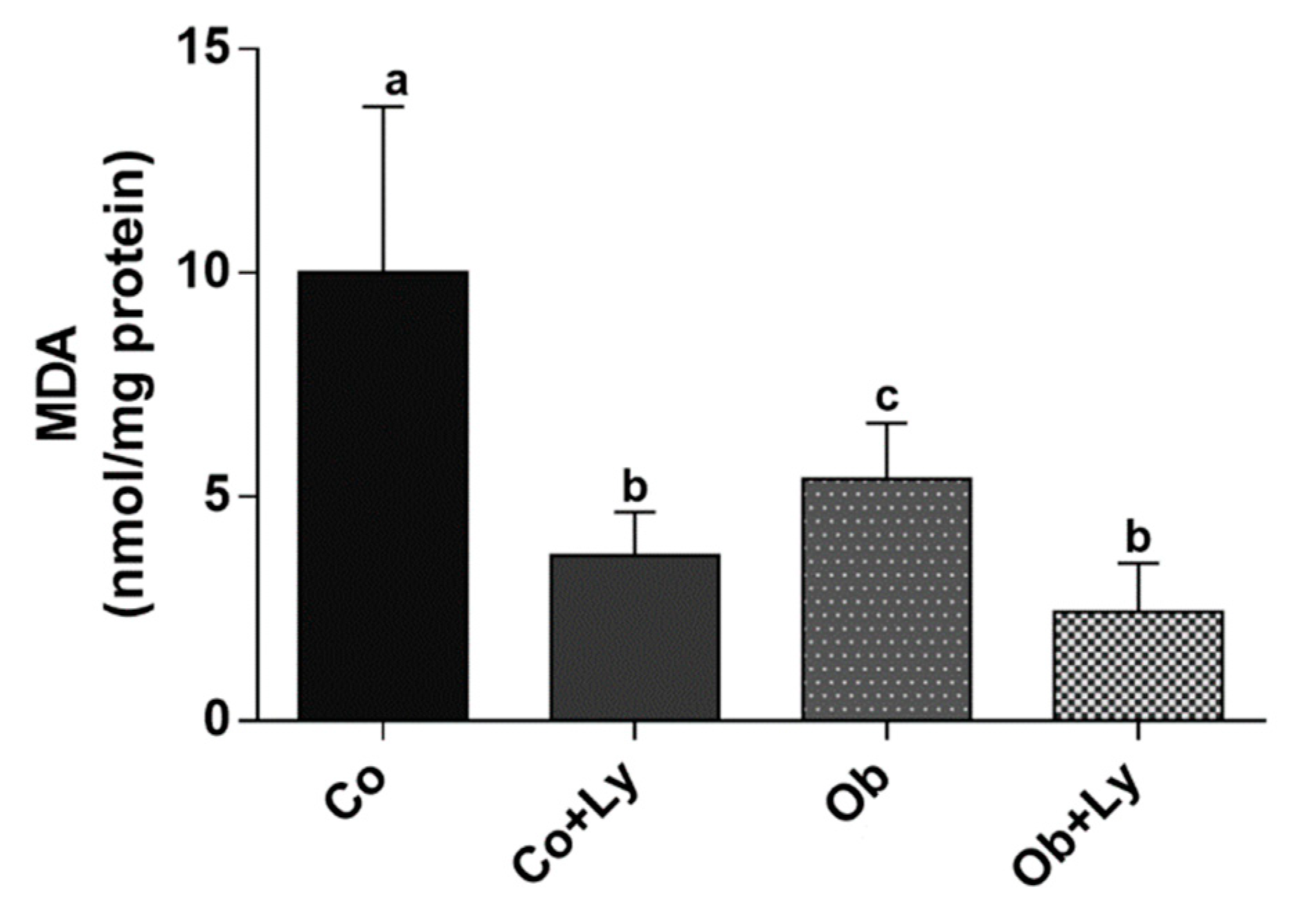
3.6. Lycopene Shows Anti-Lipid Peroxidation Activity in Hepatic Tissue
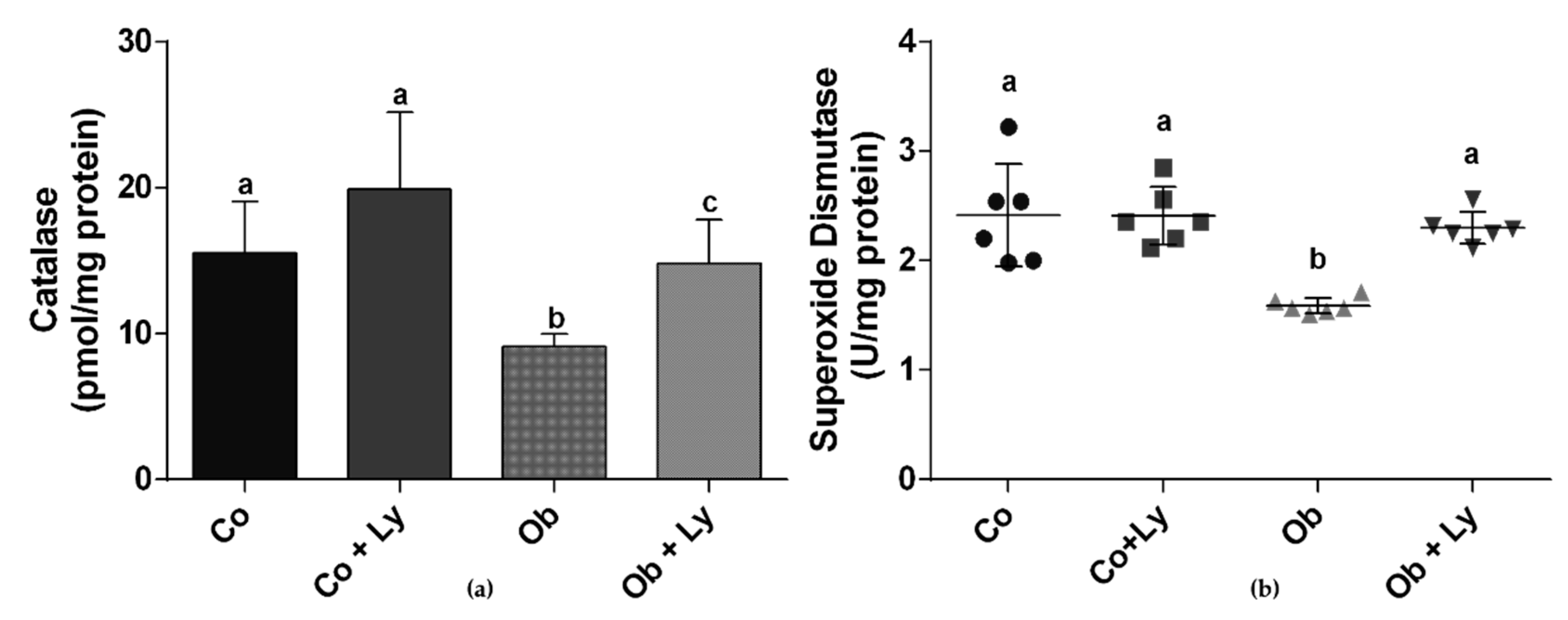
3.7. Lycopene Improves Antioxidant Enzyme Activity in Hepatic Tissue
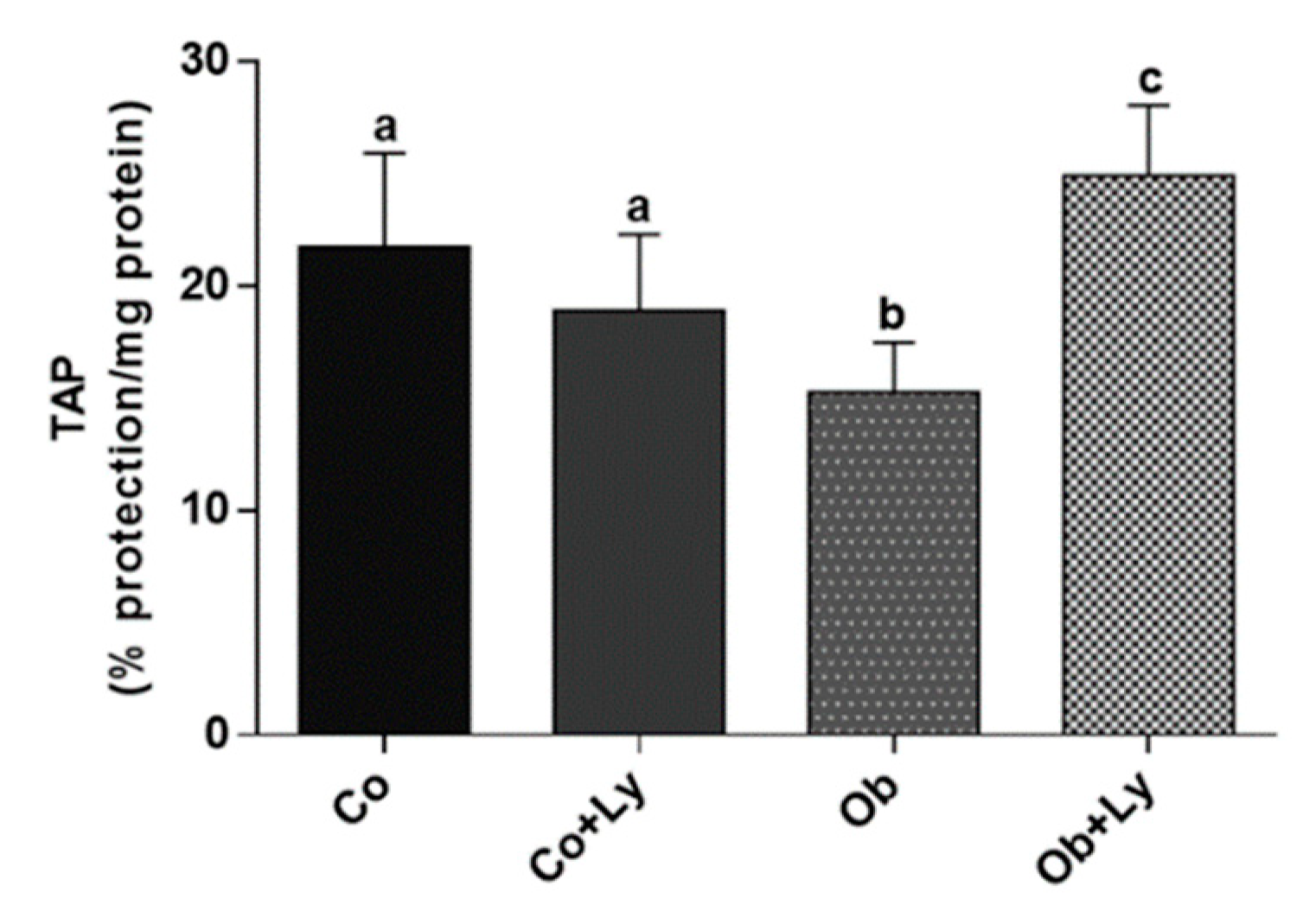
3.8. Lycopene Improves Total Antioxidant Capacity in Hepatic Tissue of Obese Rats
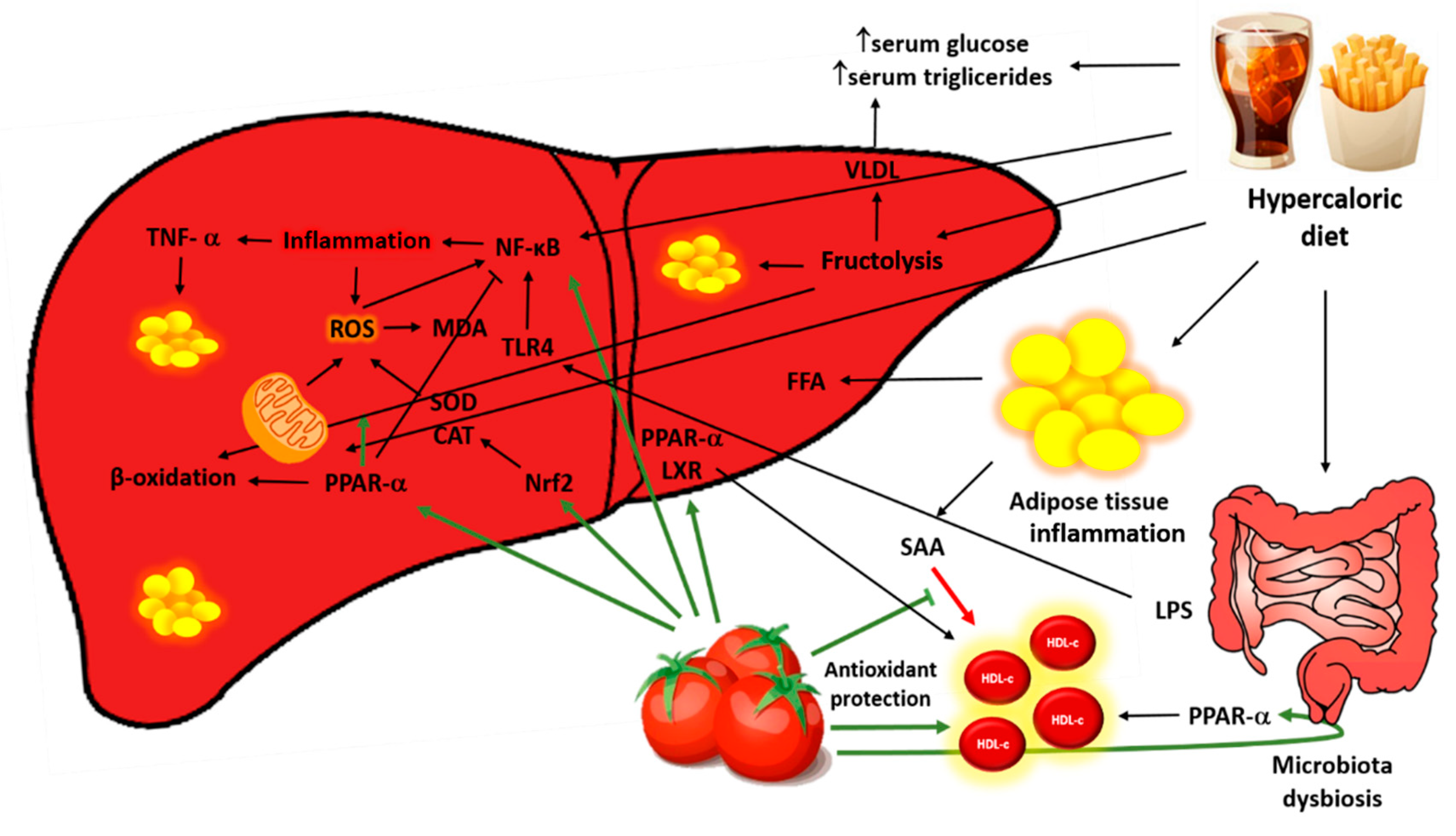
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 December 2018).
- Popkin, B.M. Nutrition transition and the global diabetes epidemic. Curr. Diab. Rep. 2015, 15, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, J.; Ganjifar, Z.K. Overweight and obesity: A brief challenge on prevalence, complications and physical activity among men and women. Women’s Health 2018, 7, 19–24. [Google Scholar] [CrossRef][Green Version]
- DiNicolantonio, J.J.; Berger, A. Added sugars drive nutrient and energy deficit in obesity: A new paradigm. Open Heart 2016, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fleur, S.E.; Luijendijk, M.C.M.; van Rozen, A.J.; Kalsbeek, A.; Adan, R.A.H. A free-choice high-fat high-sugar diet induces glucose intolerance and insulin unresponsiveness to a glucose load not explained by obesity. Int. J. Obes. 2010, 35, 595–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segula, D. Complications of obesity in adults: A short review of the literature. Malawi Med. J. 2014, 26, 20–24. [Google Scholar] [PubMed]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The Relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The Role of fructose in the pathogenesis of nafld and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel action of carotenoids on non-alcoholic fatty liver disease: Macrophage polarization and liver homeostasis. Nutrients 2016, 8, 391. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Kim, C.W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Chakravarthy, M.; Previs, S.; et al. Acetyl-CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: A bedside to bench investigation. Cell Metab. 2017, 26, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Simões, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in non-alcoholic fatty liver disease. Int. J. Biochem. Cell Biol. 2018, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carvalho, D.; Freitas, P. Gut microbiota: Association with NAFLD and metabolic disturbances. BioMed Res. Int. 2015, 2015, 979515. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar]
- Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals; CCAC: Ottawa, ON, Canada, 1993; Volume 1, p. 209. [Google Scholar]
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.S.S.; Garcia, J.L.; Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of gamma-oryzanol as therapeutic agent to prevent cardiorenal metabolic syndrome in animals submitted to high sugar-fat diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef]
- Luvizotto, R.A.M.; Nascimento, A.F.; Miranda, N.C.M.; Wang, X.D.; Ferreira, A.L.A. Lycopene-rich tomato oleoresin modulates plasma adiponectin concentration and mRNA levels of adiponectin, SIRT1, and FoxO1 in adipose tissue of obese Rats. Hum. Exp. Toxicol. 2015, 34, 612–619. [Google Scholar] [CrossRef]
- Luvizotto, R.D.A.M.; Nascimento, A.F.; Imaizumi, E.; Pierine, D.T.; Conde, S.J.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013, 110, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Salvadori, D.M.; Nascimento, M.C.; Rocha, N.S.; Correa, C.R.; Pereira, E.J.; Matsubara, L.S.; Matsubara, B.B.; Ladeira, M.S. Tomato-oleoresin supplement prevents doxorubicin-induced cardiac myocyte oxidative DNA damage in rats. Mutat. Res. 2007, 631, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Van Den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Moreto, F.; de Oliveira, E.P.; Manda, R.M.; Burini, R.C. The higher plasma malondialdehyde concentrations are determined by metabolic syndrome-related glucolipotoxicity. Oxid. Med. Cell. Longev. 2014, 2014, 505368. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L. Product of extracellular-superoxide dismutase catalysis. FEBS Lett. 1985, 184, 237–239. [Google Scholar] [CrossRef]
- Aebi, H. Oxygen Radicals in Biological Systems: Preface. Methods Enzymol. 1990, 186, 121–126. [Google Scholar]
- Beretta, G.; Aldini, G.; Facino, R.M.; Russell, R.M.; Krinsky, N.I.; Yeum, K.J. Total antioxidant performance: A validated fluorescence assay for the measurement of plasma oxidizability. Anal. Biochem. 2006, 354, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chang, Y.Y.; Huang, H.C.; Wu, Y.C.; Yang, M.D.; Chao, P.M. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition 2014, 31, 691–696. [Google Scholar] [CrossRef]
- Liu, M.; Liu, F. Transcriptional and post-translational regulation of adiponectin. Biochem. J. 2009, 425, 41–52. [Google Scholar] [CrossRef]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Jafari Anarkooli, I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef]
- Aydin, M.; Çelik, S. Effects of lycopene on plasma glucose, insulin levels, oxidative stress, and body weights of streptozotocin-induced diabetic rats. Turk. J. Med. Sci. 2012, 42, 1406–1413. [Google Scholar]
- Bernal, C.; Martín-Pozuelo, G.; Lozano, A.B.; Sevilla, Á.; García-Alonso, J.; Canovas, M.; Periago, M.J. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem. 2013, 24, 1870–1881. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar]
- Corless, J.K.; Middleton, H.M. Normal liver function. A basis for understanding hepatic disease. Arch. Intern. Med. 1983, 143, 2291–2294. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR the big bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Caris-Veyrat, C.; Garcia, A.L.; Reynaud, E.; Lucas, R.; Aydemir, G.; Rühl, R. A review about lycopene-induced nuclear hormone receptor signalling in inflammation and lipid metabolism via still unknown endogenous Apo-10′-lycopenoids. Int. J. Vitam. Nutr. Res. 2016, 86, 62–70. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 2004, 20, 649–688. [Google Scholar]
- Prawitt, J.; Caron, S.; Van Hul, W.; Lefebvre, P.; Verrijken, A.; Van Gaal, L.; Michielsen, P.; Hubens, G.; Mertens, I.; Taskinen, M.R.; et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Babu, S.; Krishnan, B.; Ravindran, N.C. Non-alcoholic fatty liver disease: A clinical update. J. Clin. Transl. Hepatol. 2017, 5, 384–393. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef]
- Brunt, E.M.; Tiniakos, D.G. Histopathology of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 5286–5296. [Google Scholar] [CrossRef]
- Tandra, S.; Yeh, M.M.; Brunt, E.M.; Vuppalanchi, R.; Cummings, O.W.; Ünalp-Arida, A.; Wilson, L.A.; Chalasani, N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J. Hepatol. 2010, 55, 654–659. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715–732. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic Liver Disease. Nat. Rev. 2018, 4, 109–120. [Google Scholar] [CrossRef]
- Fromenty, B.; Pessayre, D. Impaired mitochondrial function in microvesicular steatosis. J. Hepatol. 1997, 26, 43–53. [Google Scholar] [CrossRef]
- Fromenty, B.; Berson, A.; Pessayre, D. Microvesicular steatosis and steatohepatitis: Role of mitochondrial dysfunction and lipid peroxidation. J. Hepatol. 2005, 26, 13–22. [Google Scholar] [CrossRef]
- Mashek, D.G.; Khan, S.A.; Sathyanarayan, A.; Ploeger, J.M.; Franklin, M.P. Hepatic Lipid droplet biology: Getting to the root of fatty liver. Hepatology 2015, 62, 964–967. [Google Scholar] [CrossRef]
- Martín-Pozuelo, G.; Santaella, M.; Hidalgo, N.; García-Alonso, J.; Periago, M.J.; Navarro-González, I.; Ros, G.; González-Barrio, R.; Gómez-Gallego, C. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur. J. Nutr. 2014, 54, 933–944. [Google Scholar] [CrossRef]
- Hu, M.Y.; Li, Y.-L.; Jiang, C.H.; Liu, Z.Q.; Qu, S.L.; Huang, Y.M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef]
- Lorenz, M.; Fechner, M.; Kalkowski, J.; Fröhlich, K.; Trautmann, A.; Böhm, V.; Liebisch, G.; Lehneis, S.; Schmitz, G.; Ludwig, A.; et al. Effects of lycopene on the initial state of atherosclerosis in New Zealand white (NZW) rabbits. PLoS ONE 2012, 7, e30808. [Google Scholar] [CrossRef]
- Mulkalwar, S.A.; Munjal, N.S.; More, U.K.; More, B.; Chaudhari, A.B.; Dewda, P.R. Effect of purified lycopene on lipid profile, antioxidant enzyme and blood glucose in hyperlipidemic rabbits. Am. J. Pharm. Tech. Res. 2012, 2, 460–470. [Google Scholar]
- Hassan, H.A.; Edrees, G.M. Therapeutic effect of lycopene-rich tomato juice on cardiac disorder in rats fed on fried food in oxidized frying oil. Egypt. J. Hosp. Med. 2004, 14, 115–126. [Google Scholar]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. 2018, 7, 212525. [Google Scholar] [CrossRef]
- Farrer, S. Beyond statins: Emerging evidence for hdl-increasing therapies and diet in treating cardiovascular disease. Adv. Prev. Med. 2018, 2018, 6024747. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Polidorib, M.C.; Li, L.; Weber, D.; Stahlb, W.; Nellese, G.; Gruned, T.; Griffiths, H.R. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J. Alzheimer’s Dis. 2018, 40, 399–408. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Shi, X.; Huang, C.; Liu, J.; Zhou, S.; Heng, C.K. Association between serum amyloid a and obesity: A meta-analysis and systematic review. Inflamm. Res. 2010, 59, 323–334. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves hdl functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Iqbal, J.; Fievet, C.; Timmins, J.M.; Pape, T.D.; Coburn, B.A.; Bissada, N.; Staels, B.; Groen, A.K.; et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Investig. 2006, 116, 1052–1062. [Google Scholar] [CrossRef]
- Colin, S.; Briand, O.; Touche, V.; Wouters, K.; Baron, M.; Pattou, F.; Hanf, R.; Tailleux, A.; Chinetti, G.; Staels, B.; et al. Activation of intestinal peroxisome proliferator-activated receptor- increases high-density lipoprotein production. Eur. Heart J. 2012, 34, 2566–2574. [Google Scholar] [CrossRef]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Xie, M. The role of peroxisome proliferator-activated receptor in the treatment of non-alcoholic fatty liver disease. Acta Pharm. 2017, 67, 1–13. [Google Scholar] [CrossRef]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Kleemann, R.; Kooistra, T.; Drevon, C.A.; Blomhoff, R. Diet-induced obesity increases NF-ΚB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirili, C.; Strazzabosco, M. Stimulation of nuclear receptor ppar-γ limits nf-kb-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2016, 62, 1551–1562. [Google Scholar] [CrossRef]
- Seo, Y.Y.; Cho, Y.K.; Bae, J.C.; Seo, M.H.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Tumor necrosis factor-α as a predictor for the development of nonalcoholic fatty liver disease: A 4-year follow-up study. Endocrinol. Metab. 2013, 28, 41–45. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef]
- Haggag, M.S.; Elsanhoty, R.M.; Ramadan, M.F. Impact of dietary oils and fats on lipid peroxidation in liver and blood of albino rats. Asian Pac. J. Trop. Biomed. 2014, 4, 52–58. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Hayirli, A.; Bilgili, S.; Kucuk, O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016, 95, 1088–1095. [Google Scholar] [CrossRef]
| Parameters | Co | Co+Ly | Ob | Ob+Ly |
|---|---|---|---|---|
| Chow intake (g/d) | 24 ± 0.8 a | 24.4 ± 0.4 a | 12.8 ± 0.6 b | 11.8 ± 0.4 b |
| Water intake (mL/d) | 24.1 ± 0.6 a | 27 ± 1.4 a | 23.9 ± 0.6 a | 23.7 ± 0.4 a |
| Caloric intake (kcal/d) | 89.9 ± 2.9 a | 88.9 ± 0.9 a | 95 ± 3.9 a | 94.2 ± 1.8 a |
| Initial Weight (g) | 192 ± 28.4 a | 209 ± 14.3 a | 212 ± 21.4 a | 198 ± 11.6 a |
| Final Weight (g) | 490 ± 43.7 a | 470 ± 59.6 a | 568.5 ± 75.2 b | 552 ± 42.5 b |
| Weight Gain (g) | 297 ± 33.7 a | 260 ± 52.0 a | 356 ± 72.9 b | 354 ± 34.2 b |
| Parameters | Co | Co+Ly | Ob | Ob+Ly |
|---|---|---|---|---|
| Liver (µg/100 g tissue) | ND | 47.32 ± 5.95 a | ND | 25 ± 2.91 b |
| Plasma (µg/mL) | ND | 3.18 ± 0.586 a | ND | 5.22 ± 2.31 a |
| Parameters | Co | Co+Ly | Ob | Ob+Ly |
|---|---|---|---|---|
| Urea (mg/dL) | 54.2 ± 8.8 a | 51.5 ± 3.9 a | 48.0 ± 33.6 a | 42.8 ± 20.9 a |
| Creatinine (mg/dL) | 0.432 ± 0.034 a | 0.433 ± 0.057 a | 0.568 ± 0.291 a | 0.504 ± 0.092 a |
| Uric Acid (mg/dL) | 0.544 ± 0.103 a | 0.693 ± 0.307 a | 0.695 ± 0.122 a | 0.800 ± 0.166 a |
| AST (U/L) | 149 (100–230) a | 123 (113–152) a | 112 (87–213) a | 141 (135–179) a |
| ALT (U/L) | 44.5 (38.7–161) a | 50.5 (35.5–106) a | 51 (32.7–133) a | 38.5 (32.5–95.7) a |
| Albumin (g/dL) | 2.7 ± 0.1 a | 2.6 ± 0.1 a | 2.7 ± 0.1 a | 2.7 ± 0.1 a |
| Total Proteins (g/dL) | 5.6 ± 0.1 a | 5.6 ± 0.3 a | 5.9 ± 0.2 a | 5.9 ± 0.3 a |
| Parameters | Co | Co+Ly | Ob | Ob+Ly |
|---|---|---|---|---|
| Fasting Blood Glucose (mg/dL) | 74.2 ± 7.56 a | 91.6 ± 16.6 a | 102 ± 21.4 b | 104 ± 7.50 b |
| Triglycerides (mg/dL) | 62.0 ± 20.3 a | 77.3 ± 32.5 a | 113 ± 41.8 b | 93.9 ± 12.4 a |
| Hepatic Triglycerides (mg/dL) | 20.6 ± 4.29 a | 20.6 ± 2.92 a | 33.2 ± 7.9 b | 29.3 ± 5.59 a,b |
| Total Cholesterol (mg/dL) | 51.5 ± 8.80 a | 60.0 ± 12.1 a. b | 56.9 ± 14.1 a | 71.7 ± 10.8 b |
| HDL Cholesterol (mg/dL) | 18.0 ± 3.25 a | 23.5 ± 3.94 b | 19.9 ± 5.43 a | 29.0 ± 2.69 c |
| Non-HDL Cholesterol (mg/dL) | 33.5 ± 5.95 a | 36.4 ± 9.09 a | 37.0 ± 9.86 a | 42.6 ± 8.87 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Róvero Costa, M.; Leite Garcia, J.; Cristina Vágula de Almeida Silva, C.; Junio Togneri Ferron, A.; Valentini Francisqueti-Ferron, F.; Kurokawa Hasimoto, F.; Schmitt Gregolin, C.; Henrique Salomé de Campos, D.; Roberto de Andrade, C.; dos Anjos Ferreira, A.L.; et al. Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants 2019, 8, 276. https://doi.org/10.3390/antiox8080276
Róvero Costa M, Leite Garcia J, Cristina Vágula de Almeida Silva C, Junio Togneri Ferron A, Valentini Francisqueti-Ferron F, Kurokawa Hasimoto F, Schmitt Gregolin C, Henrique Salomé de Campos D, Roberto de Andrade C, dos Anjos Ferreira AL, et al. Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants. 2019; 8(8):276. https://doi.org/10.3390/antiox8080276
Chicago/Turabian StyleRóvero Costa, Mariane, Jéssica Leite Garcia, Carol Cristina Vágula de Almeida Silva, Artur Junio Togneri Ferron, Fabiane Valentini Francisqueti-Ferron, Fabiana Kurokawa Hasimoto, Cristina Schmitt Gregolin, Dijon Henrique Salomé de Campos, Cleverton Roberto de Andrade, Ana Lúcia dos Anjos Ferreira, and et al. 2019. "Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats" Antioxidants 8, no. 8: 276. https://doi.org/10.3390/antiox8080276
APA StyleRóvero Costa, M., Leite Garcia, J., Cristina Vágula de Almeida Silva, C., Junio Togneri Ferron, A., Valentini Francisqueti-Ferron, F., Kurokawa Hasimoto, F., Schmitt Gregolin, C., Henrique Salomé de Campos, D., Roberto de Andrade, C., dos Anjos Ferreira, A. L., Renata Corrêa, C., & Moreto, F. (2019). Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats. Antioxidants, 8(8), 276. https://doi.org/10.3390/antiox8080276





