Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Extraction Methods of Goji Leaves
2.3.1. Ultrasound-Assisted Extraction (UAE)
2.3.2. Microwave-Assisted Extraction (MAE)
2.3.3. Maceration (MAC)
2.4. Determination of Total Phenol Content (TPC)
2.5. In Vitro Antioxidant Activities
2.5.1. Free Radical-Scavenging Activity Using DPPH (DPPH Assay)
2.5.2. Free Radical-Scavenging Activity Using ABTS (ABTS Assay)
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. HPLC-DAD Analysis of Phenol Compounds
2.7. UHPLC-MS/MS Analysis of Phenol Compounds
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, A. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Zhang, C.; An, W.; Yin, Y.; Wang, Y.; Cao, Y. Physiological response of four wolfberry (Lycium Linn.) species under drought stress. J. Integr. Agric. 2018, 17, 603–612. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. A simple HPLC-ELSD method for sugar analysis in goji berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Blasi, F.; Montesano, D.; Simonetti, M.S.; Cossignani, L. A simple and rapid extraction method to evaluate the fatty acid composition and nutritional value of Goji berry lipid. Food Anal. Methods 2017, 10, 970–979. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Cossignani, L.; Lucini, L.; Simonetti, M.S.; Blasi, F. Italian Lycium barbarum L. berry: Chemical characterization and nutraceutical value. Nat. Prod. Commun. 2018, 13, 1151–1156. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Blasi, F.; Rocchetti, G.; Montesano, D.; Lucini, L.; Chiodelli, G.; Ghisoni, S.; Baccolo, G.; Simonetti, M.S.; Cossignani, L. Changes in extra-virgin olive oil added with Lycium barbarum L. carotenoids during frying: Chemical analyses and metabolomic approach. Food Res. Int. 2018, 105, 507–516. [Google Scholar] [CrossRef]
- Bertoldi, D.; Cossignani, L.; Blasi, F.; Perini, M.; Barbero, A.; Pianezze, S.; Montesano, D. Characterisation and geographical traceability of Italian goji berries. Food Chem. 2019, 275, 585–593. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.; Bischin, C.; Hanganu, D.; Gheldiu, A.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Pires, T.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018, 122, 574–581. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Lin, S.; AL-Wraikat, M.; Niu, L.; Zhou, F.; Zhang, Y.; Wang, M.; Ren, J.; Fan, J.; Zhang, B.; Wang, L. Degradation enhances the anticoagulant and antiplatelet activities of polysaccharides from Lycium barbarum L. leaves. Int. J. Biol. Macromol. 2019, 133, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, J.; Hu, C.; Shen, B.; Chen, T.; Chang, Y.; Shih, C.; Yang, D. Phenolic composition and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvest at various times. Food Chem. 2017, 215, 284–291. [Google Scholar] [CrossRef]
- Lopatriello, A.; Previtera, R.; Pace, S.; Werner, M.; Rubino, L.; Werz, O.; Taglialatela-Scafati, O.; Forino, M. NMR-based identification of the major bioactive molecules from an Italian cultivar of Lycium barbarum. Phytochemistry 2017, 144, 52–57. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Lycium chinense leaves extract ameliorates diabetic nephropathy by suppressing hyperglycemia mediated renal oxidative stress and inflammation. Biomed. Pharmacother. 2018, 102, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C.C. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Maras, M.; Barba, F.J.; Granato, D.; Roohinejad, S.; Mallikarjunan, K.; Montesano, D.; Lorenzo, J.M.; Putnik, P. Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem. 2018, 268, 513–521. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimisation of phenol extraction from wine using layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Blasi, F.; Urbani, E.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Urbani, E.; Blasi, F.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Investigation on secondary metabolite content and antioxidant activity of commercial saffron powder. Eur. Food Res. Technol. 2016, 242, 987–993. [Google Scholar] [CrossRef]
- Lombardi, G.; Cossignani, L.; Giua, L.; Simonetti, M.S.; Maurizi, A.; Burini, G.; Coli, R.; Blasi, F. Phenol composition and antioxidant capacity of red wines produced in Central Italy changes after one-year storage. J. Appl. Bot. Food Qual. 2017, 90, 197–204. [Google Scholar] [CrossRef]
- Simeoni, M.; Pellegrini, M.; Sergi, M.; Pittia, P.; Ricci, A.; Compagnone, D. Analysis of polyphenols in the Lamiaceae family by matrix solid-phase dispersion followed by ultra-high-performance liquid chromatography-tandem mass spectrometry determination. ACS Omega 2018, 3, 17610–17616. [Google Scholar] [CrossRef]
- International Conference on Harmonization; Q2B: Validation of Analytical Procedures: Methodology; Availability. Fed. Regist. 1997, 62, 27464–27467. Available online: https://www.govinfo.gov/content/pkg/FR-1997-05-19/pdf/97-13063.pdf (accessed on 15 April 2019).
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolic compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crops Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Cossignani, L.; Blasi, F.; Bosi, A.; D’Arco, G.; Maurelli, S.; Simonetti, M.S.; Damiani, P. Detection of cow milk in donkey milk by chemometric procedures on triacylglycerol stereospecific analysis results. J. Dairy Res. 2011, 78, 335–342. [Google Scholar] [CrossRef]
- Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty acids and phytosterols to discriminate geographic origin of Lycium barbarum berry. Food Anal. Methods 2018, 11, 1180–1188. [Google Scholar] [CrossRef]
- Blasi, F.; Pollini, L.; Cossignani, L. Varietal authentication of extra virgin olive oils by triacylglycerols and volatiles analysis. Foods 2019, 8, 58. [Google Scholar] [CrossRef]


| Yield | TPC | DPPH | ABTS | FRAP | ||
|---|---|---|---|---|---|---|
| Extraction Solvent | % | mg GAE/g | IC50 * | mg TE/g | μmol Fe+2/g | |
| UAE 1 | MeOH | 26.04 ± 0.13 | 10.02 ± 0.23 | 2.53 ± 0.18 | 20.51 ± 1.62 | 112.65 ± 2.86 |
| UAE 2 | MeOH:H2O, 50:50 | 31.95 ± 0.76 | 8.13 ± 0.06 | 9.45 ± 0.84 | 14.65 ± 0.75 | 92.17 ± 6.64 |
| MAE 1 | MeOH | 22.17 ± 0.79 | 6.65 ± 0.35 | 3.42 ± 0.25 | 17.67 ± 0.81 | 140.74 ± 0.58 |
| MAE 2 | MeOH:H2O, 50:50 | 31.48 ± 1.75 | 5.68 ± 0.38 | 5.68 ± 0.63 | 13.14 ± 0.69 | 140.88 ± 0.61 |
| MAC 1 | MeOH | 24.34 ± 0.86 | 9.52 ± 0.25 | 3.92 ± 0.52 | 14.63 ± 0.92 | 121.23 ± 3.69 |
| MAC2 | MeOH:H2O, 50:50 | 29.82 ± 0.62 | 5.07 ± 0.06 | 23.35 ± 3.27 | 11.68 ± 0.86 | 55.19 ± 0.53 |
| Standard | Range | Regression Equation | R2 | RSD * Intra-Day | RSD * Inter-Day | LOD | LOQ | |
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | |||||||
| μg/mL | % | % | μg/mL | μg/mL | ||||
| Tyrosol | 7.31–29.24 | 1.88E + 06 | −9.18E + 05 | 0.9993 | 1.12 | 4.98 | 1.51 | 4.82 |
| Kaempferol-3-O-Glua | 14.80–59.00 | 6.06E + 06 | 7.29E + 06 | 0.9998 | 1.13 | 3.57 | 0.71 | 2.10 |
| Chlorogenic acid | 1.50–117.20 | 3.38E + 06 | −5.20E + 05 | 0.9996 | 0.98 | 3.71 | 0.68 | 2.17 |
| p-Coumaric acid | 1.78–7.10 | 1.89E + 07 | 1.58E + 06 | 0.9999 | 0.79 | 4.80 | 0.06 | 0.19 |
| Ferulic acid | 1.86–7.45 | 1.68E + 07 | 1.20E + 06 | 0.9997 | 1.72 | 4.55 | 0.64 | 2.05 |
| Rutin | 11.10–44.30 | 5.51E + 06 | −1.24E + 06 | 0.9997 | 1.32 | 4.13 | 1.68 | 5.34 |
| UAE 1 | UAE 2 | MAE 1 | MAE 2 | MAC 1 | MAC 2 | |
|---|---|---|---|---|---|---|
| Neochlorogenic acid | 594.04 ± 45.60 | 412.50 ± 23.11 | 462.37 ± 25.74 | 597.91 ± 43.91 | 450.11 ± 58.40 | 21.26 ± 2.34 |
| Tyrosol | 660.61 ± 37.13 | 640.80 ± 30.73 | 476.10 ± 34.91 | 598.19 ± 46.92 | 501.30 ± 6.40 | 577.18 ± 40.42 |
| Chlorogenic acid | 2991.55 ± 46.62 | 1728.83 ± 110.08 | 2656.66 ± 150.23 | 2059.65 ± 104.72 | 2692.46 ± 166.06 | 732.71 ± 35.81 |
| Cryptochlorogenic acid | 375.76 ± 30.55 | 374.88 ± 4.34 | 323.91 ± 24.71 | 346.87 ± 21.94 | 227.69 ± 28.05 | 378.29 ± 20.35 |
| p-Coumaric acid | 83.81 ± 8.26 | 81.20 ± 8.42 | 88.08 ± 7.83 | 80.20 ± 7.41 | 33.14 ± 1.55 | 42.08 ± 3.84 |
| Rutin | 1678.68 ± 61.74 | 1197.55 ± 95.89 | 1328.50 ± 74.91 | 705.06 ± 34.92 | 1330.61 ± 184.12 | 452.41 ± 24.75 |
| Yield | TPC | DPPH | ABTS | FRAP | |
|---|---|---|---|---|---|
| % | mg GAE/g | IC50 * | mg TE/g | μmol Fe+2/g | |
| 1B | 16.67 ± 0.54 | 14.31 ± 0.12 | 1.01 ± 0.06 | 30.18 ± 1.32 | 194.40 ± 6.85 |
| 2B | 23.84 ± 0.84 | 8.95 ± 0.08 | 5.30 ± 0.26 | 21.91 ± 0.45 | 138.08 ± 1.15 |
| 3B | 22.78 ± 0.76 | 6.35 ± 0.14 | 14.95 ± 1.53 | 15.46 ± 0.86 | 76.34 ± 7.92 |
| 4B | 27.34 ± 0.95 | 19.12 ± 0.26 | 0.40 ± 0.02 | 34.27 ± 1.19 | 272.26 ± 4.94 |
| 1C | 21.12 ± 0.86 | 12.68 ± 0.51 | 2.21 ± 0.22 | 25.62 ± 0.23 | 165.60 ± 2.36 |
| 2C | 21.36 ± 0.98 | 14.37 ± 0.24 | 1.93 ± 0.12 | 26.79 ± 0.87 | 222.57 ± 3.82 |
| 3C | 16.32 ± 0.72 | 13.54 ± 0.18 | 1.33 ± 0.09 | 24.23 ± 0.64 | 210.19 ± 8.38 |
| 4C | 19.07 ± 0.92 | 10.78 ± 0.23 | 2.05 ± 0.21 | 21.41 ± 1.21 | 158.89 ± 0.75 |
| Rt (min) | λmax (nm) | [M+H]+ | MS Fragments (m/z) | |
|---|---|---|---|---|
| Neochlorogenic acid | 8.1 | 296sh; 324 | 377[M+Na]+ | 191 [M-H-caffeoyl]−; 179 [M-H-quinic]−; 707 [2M-H]− |
| Tyrosol | 8.9 | 231; 275 | 137 | 137 [M-H]−; 93 [M-H-CO2]− |
| Quercetin-3-O-Rut-7-O-Glua | 12.3 | 255; 266sh; 354 | 773 | 611 [M-H-glucose]+; 465 [M-H-rutinose]+; |
| Quercetin-3-O-Soph-7-O-Rhab | 12.8 | 255; 266sh; 354 | 773 | 627 [M-H-rhamnose]+; 465 [M-H-rhamnose; M-H-sophorose]+; |
| Kaempferol-3-O-Rut-7-O-Gluc | 13.3 | 265; 340 | 757 | 611 [M-H-glucose]+; 449 [M-H-rutinose]+ |
| Chlorogenic acid | 13.8 | 244; 296sh; 320 | 355 | 191 [M-H-caffeoyl]−; 179 [M-H-quinic]−; 707 [2M-H]− |
| Cryptochlorogenic acid | 14.3 | 244; 296sh; 326 | 377[M+Na]+ | 191 [M-H-caffeoyl]−; 179 [M-H-quinic]−; 707 [2M-H]− |
| p-Coumaric acid | 20.2 | 312 | 163 | 147 [M-H-H2O]−; 119 [M-H-CO2]− |
| Ferulic acid | 23.7 | 238; 290sh; 322 | 193 | 193 [M-H]−; 178 [M-H-CH3]− |
| Rutin | 26.6 | 256; 266sh; 354 | 611 | 303 [M-H-rutinose]+; 1243 [2M+Na]+ |
| 1B | 2B | 3B | 4B | |
| µg/g | µg/g | µg/g | µg/g | |
| Neochlorogenic acid | 466.43 ± 9.72 | 8655.31 ± 266.61 | 324.82 ± 3.11 | 508.14 ± 8.88 |
| Tyrosol | 513.51 ± 18.71 | 1921.88 ± 19.14 | 1105.65 ± 24.58 | 716.23 ± 60.40 |
| Quercetin-3-O-Rut-7-O-Glu a | nd | nd | nd | nd |
| Quercetin-3-O-Soph-7-O-Rhab | nd | nd | nd | nd |
| Kaempferol-3-O-Rut-7-O-Gluc | 610.30 ± 38.4 | 108.25 ± 2.66 | 99.45 ± 8.51 | nd |
| Chlorogenic acid | 6354.36 ± 204.81 | 3048.82 ± 13.93 | 1353.13 ± 12.24 | 3139.02 ± 132.54 |
| Cryptochlorogenic acid | 492.43 ± 65.23 | 230.46 ± 2.35 | 161.93 ± 1.17 | 429.92 ± 6.80 |
| p-Coumaric acid | nd | 49.49 ± 0.23 | 24.55 ± 0.27 | 585.47 ± 8.80 |
| Ferulic acid | nd | nd | 14.58 ± 0.44 | 10.53 ± 1.80 |
| Rutin | 5756.65 ± 340.5 | 1808.75 ± 19.37 | 743.50 ± 4.13 | 5233.17 ± 264.88 |
| 1C | 2C | 3C | 4C | |
| µg/g | µg/g | µg/g | µg/g | |
| Neochlorogenic acid | 439.58 ± 13.80 | 325.54 ± 10.91 | 432.58 ± 7.24 | 423.96 ± 9.50 |
| Tyrosol | 596.37 ± 29.25 | 2057.51 ± 30.74 | 118.40 ± 1.54 | 1303.28 ± 43.07 |
| Quercetin-3-O-Rut-7-O-Glua | 195.39 ± 2.33 | 939.49 ± 21.03 | 268.94 ± 11.07 | 205.60 ± 3.04 |
| Quercetin-3-O-Soph-7-O-Rhab | 1946.70 ± 38.95 | 1011.92 ± 27.54 | 344.02 ± 5.58 | 1271.42 ± 16.21 |
| Kaempferol-3-O-Rut-7-O-Gluc | 380.72 ± 3.07 | 91.77 ± 4.83 | 170.21 ± 10.04 | 366.13 ± 10.04 |
| Chlorogenic acid | 3811.85 ± 41.41 | 7721.47 ± 130.84 | 6056.74 ± 149.80 | 2153.11 ± 187.22 |
| Cryptochlorogenic acid | nd | nd | nd | nd |
| p-Coumaric acid | nd | nd | nd | nd |
| Ferulic acid | 670.72 ± 16.53 | 198.96 ± 5.39 | 451.57 ± 12.85 | 1201.89 ± 24.12 |
| Rutin | 506.21 ± 8.47 | 432.63 ± 6.40 | 1029.45 ± 57.52 | 375.60 ± 14.95 |
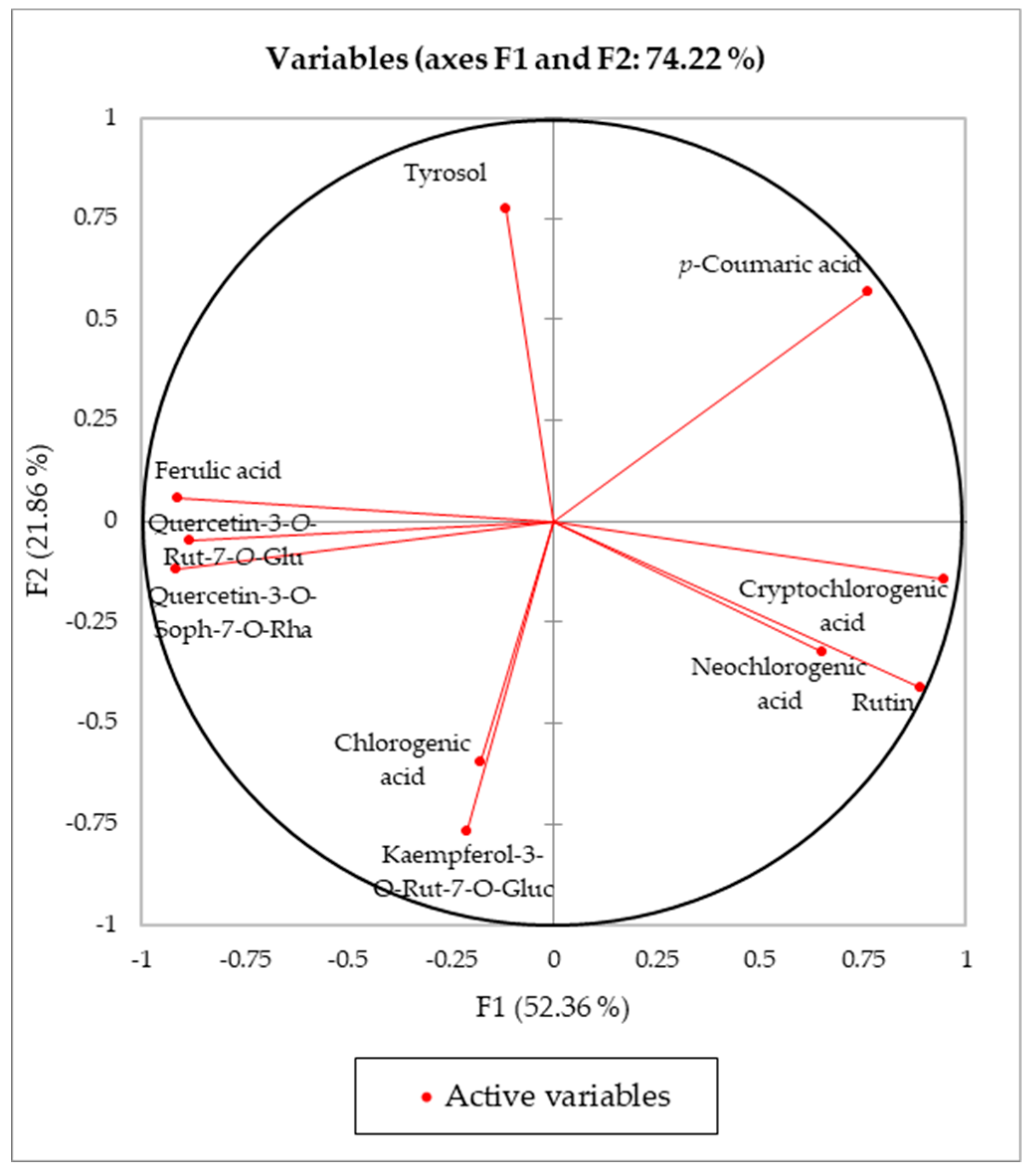
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
|---|---|---|---|---|---|---|---|
| Eigenvalue | 5.235536 | 2.186099 | 1.160795 | 0.671911 | 0.579965 | 0.115526 | 0.050168 |
| Variability % | 52.35536 | 21.86099 | 11.60795 | 6.719115 | 5.799649 | 1.155262 | 0.50168 |
| Cumulative % | 52.35536 | 74.21635 | 85.82429 | 92.54341 | 98.34306 | 99.49832 | 100 |
| Observation | Prior | Posterior | Pr(B) | Pr(C) | F1 | D2(B) | D2(C) |
|---|---|---|---|---|---|---|---|
| Obs1 | B | B | 1.000 | 0.000 | 6.134 | 4.188 | 125.126 |
| Obs2 | B | B | 1.000 | 0.000 | 4.793 | 2.181 | 96.680 |
| Obs3 | B | B | 1.000 | 0.000 | 4.565 | 3.633 | 93.645 |
| Obs4 | B | B | 1.000 | 0.000 | 4.225 | 3.258 | 86.562 |
| Obs5 | C | C | 0.000 | 1.000 | −4.626 | 92.706 | 1.487 |
| Obs6 | C | C | 0.000 | 1.000 | −4.347 | 87.470 | 1.758 |
| Obs7 | C | C | 0.000 | 1.000 | −6.613 | 134.879 | 4.499 |
| Obs8 | C | C | 0.000 | 1.000 | −4.131 | 83.530 | 2.087 |
| Classification Results (Training Sample) | ||||
| From/to | B | C | Total | % Correct |
| B | 4 | 0 | 4 | 100.00% |
| C | 0 | 4 | 4 | 100.00% |
| Total | 4 | 4 | 8 | 100.00% |
| Classification Results (Cross-Validation) | ||||
| From/to | B | C | Total | % Correct |
| B | 4 | 0 | 4 | 100.00% |
| C | 0 | 4 | 4 | 100.00% |
| Total | 4 | 4 | 8 | 100.00% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants 2019, 8, 260. https://doi.org/10.3390/antiox8080260
Pollini L, Rocchi R, Cossignani L, Mañes J, Compagnone D, Blasi F. Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants. 2019; 8(8):260. https://doi.org/10.3390/antiox8080260
Chicago/Turabian StylePollini, Luna, Rachele Rocchi, Lina Cossignani, Jordi Mañes, Dario Compagnone, and Francesca Blasi. 2019. "Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques" Antioxidants 8, no. 8: 260. https://doi.org/10.3390/antiox8080260
APA StylePollini, L., Rocchi, R., Cossignani, L., Mañes, J., Compagnone, D., & Blasi, F. (2019). Phenol Profiling and Nutraceutical Potential of Lycium spp. Leaf Extracts Obtained with Ultrasound and Microwave Assisted Techniques. Antioxidants, 8(8), 260. https://doi.org/10.3390/antiox8080260