Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
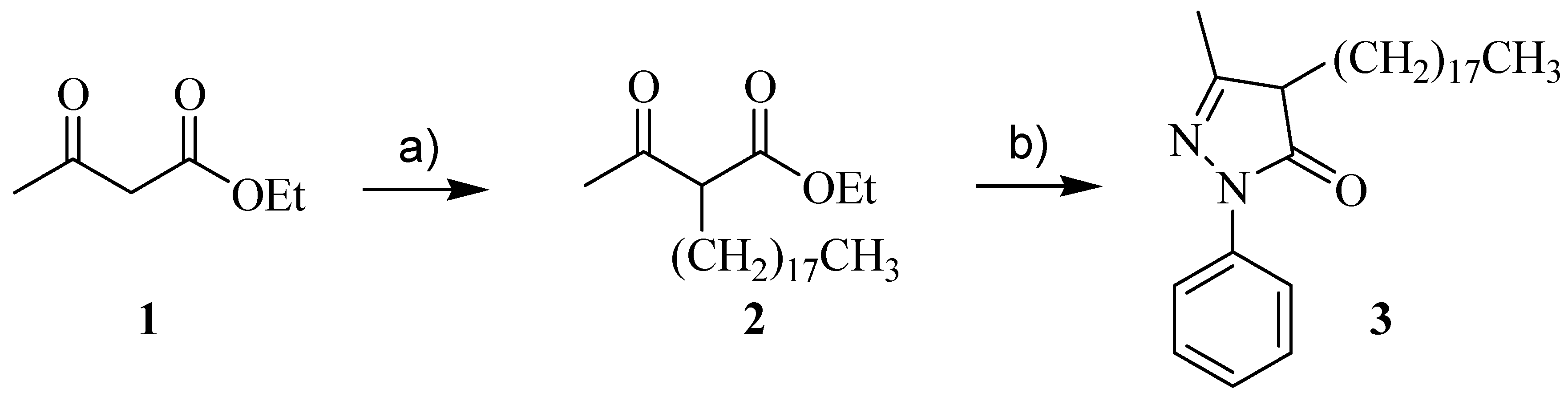
2.2. Synthetic Procedure
2.2.1. Ethyl 3-Oxo-2-Octadecyl-Butanoate (Ethyl 2-Octadecyl-Acetoacetate), 2
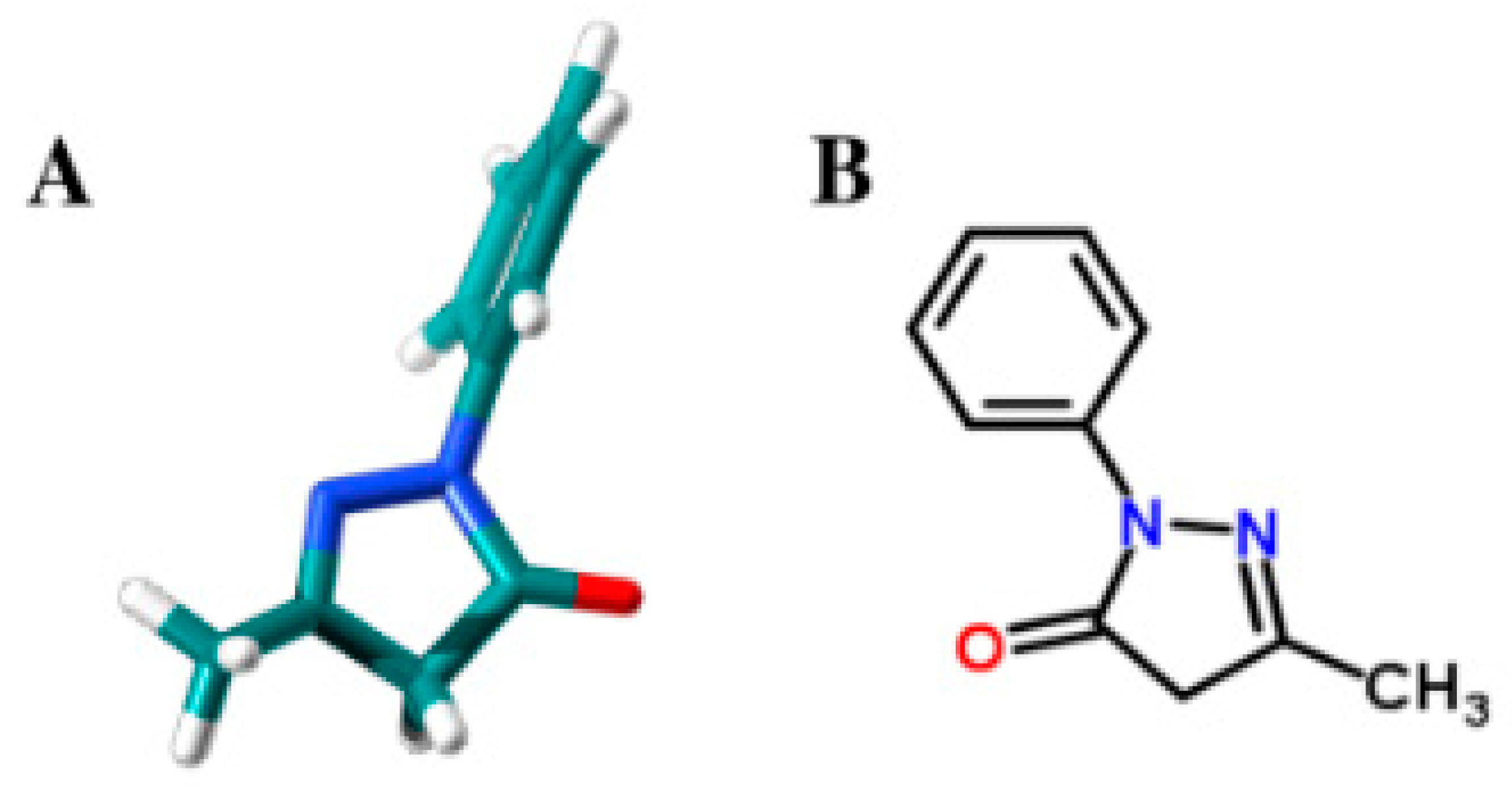
2.2.2. 3-Methyl-4-Octadecyil-1-Phenyl-1H-Pirazol-5(4H)-One (C18-Edaravone)
2.3. Computational Methods
2.4. DPPH Assay
2.5. Liposome Preparation
2.5.1. Multilamellar Vesicles (MLVs)
2.5.2. Large Unilamellar Vesicles (LUVs)
2.6. Peroxidation of PC Liposomes
2.7. Studies on Interaction of Antioxidants with Liposome Cell Membrane Models
2.8. Cell Treatment
2.9. Assay of Mitochondrial Viability (MTT Assay)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of C18-EdV
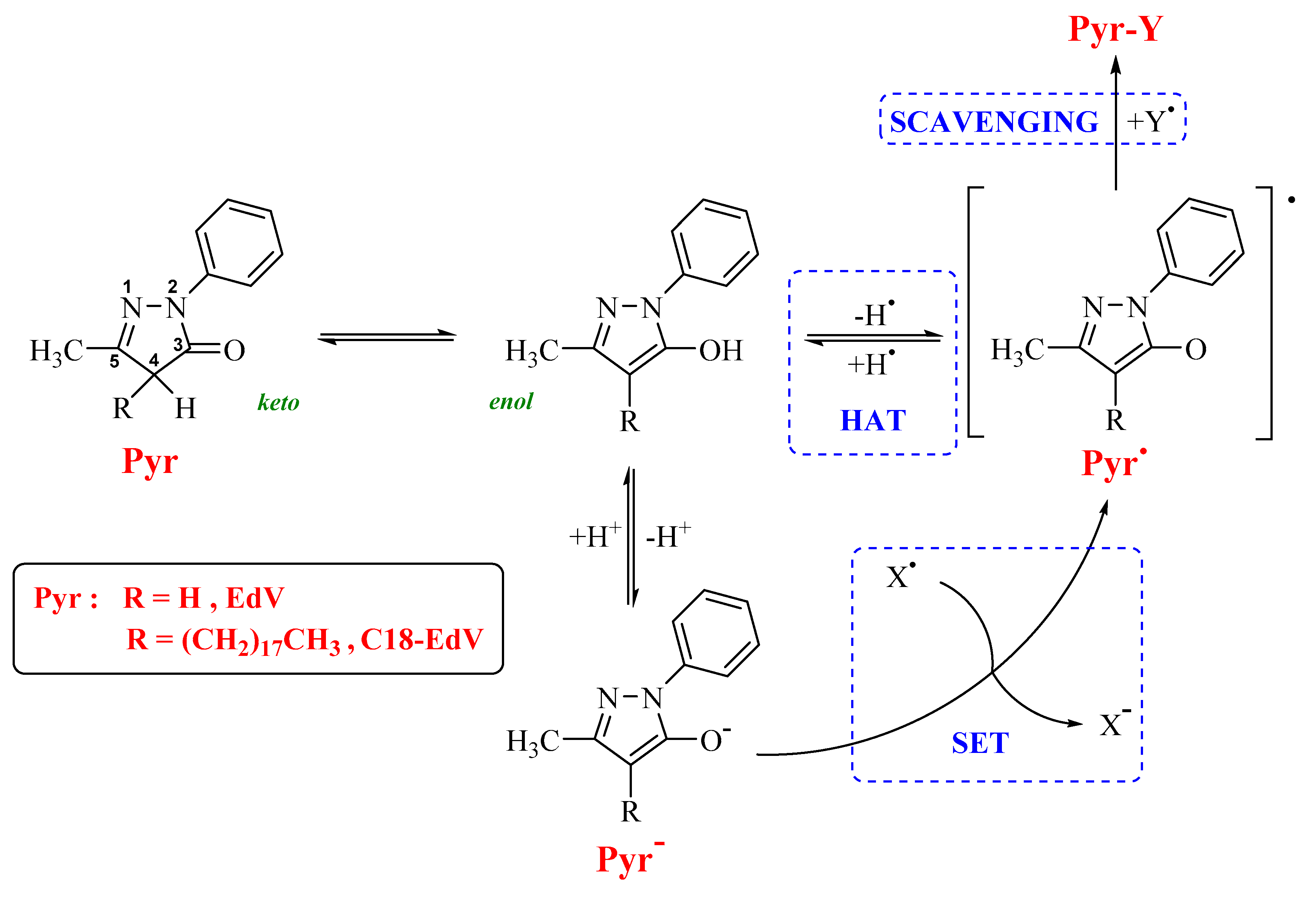
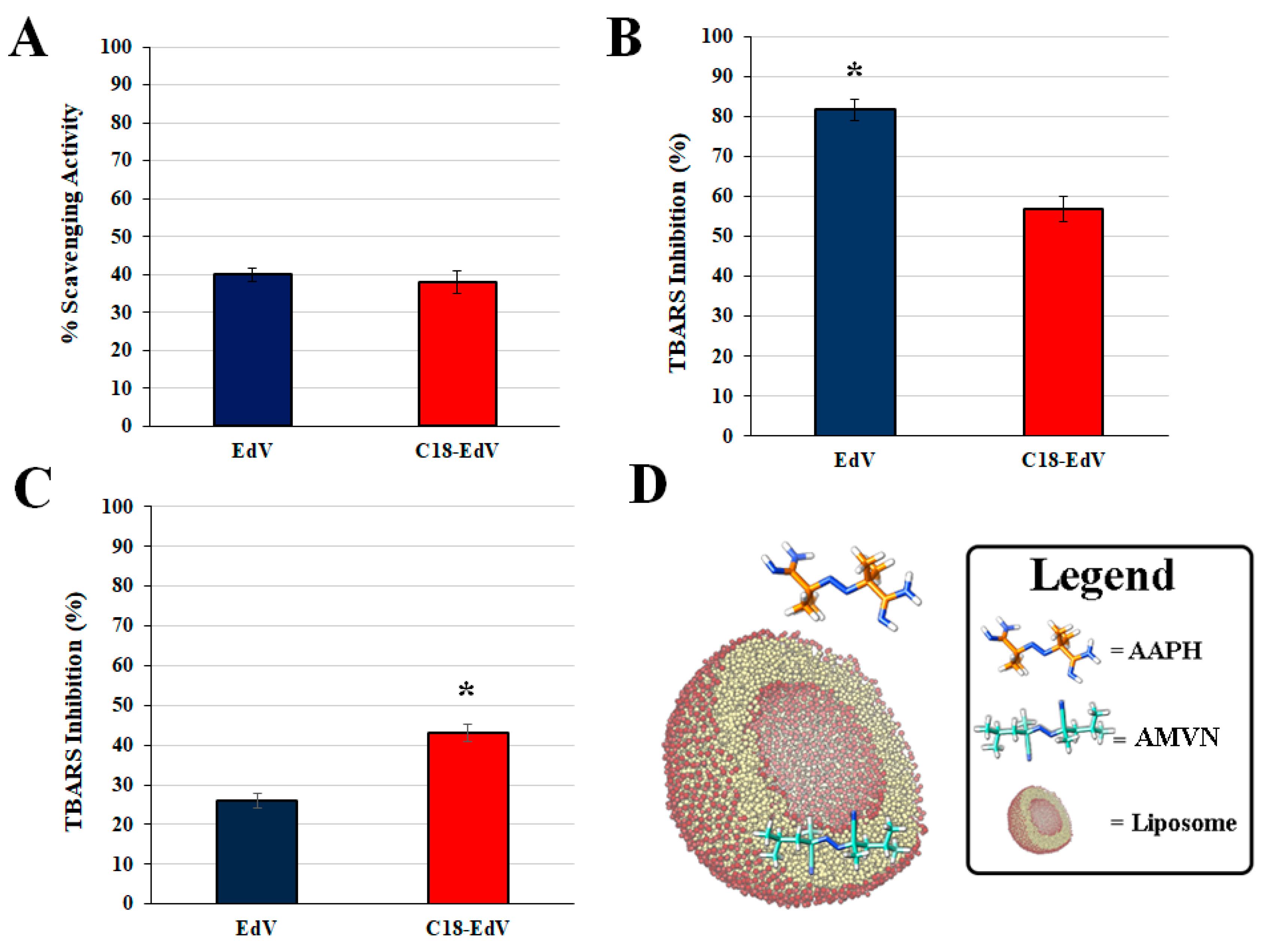
3.2. Scavenging Activity of DPPH Free Radical
3.3. Computational Results
3.4. Protection against Free Radical-Mediated Lipid Peroxidation
3.5. Affinity of EdV and C18-EdV for Lipid Bilayer
3.6. Cellular Experiments
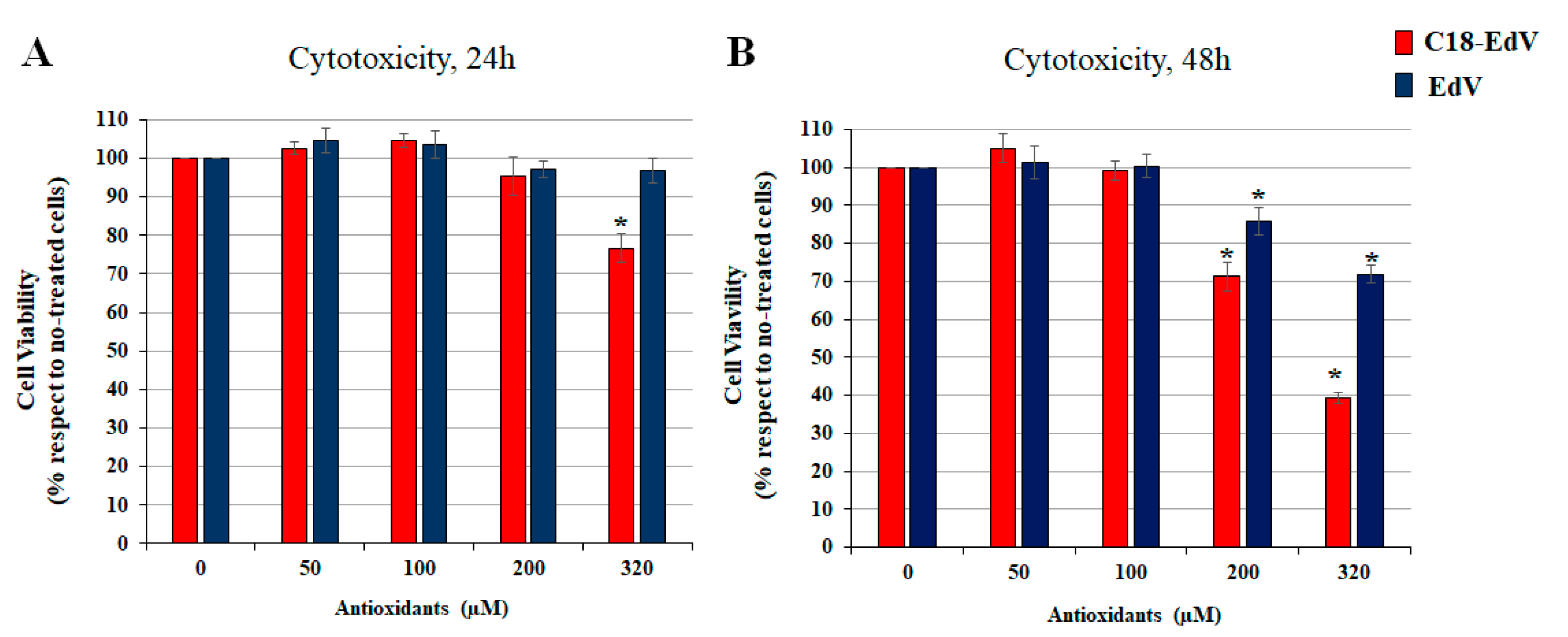
3.6.1. Cytotoxicity in Human Retinal Epithelial Cells
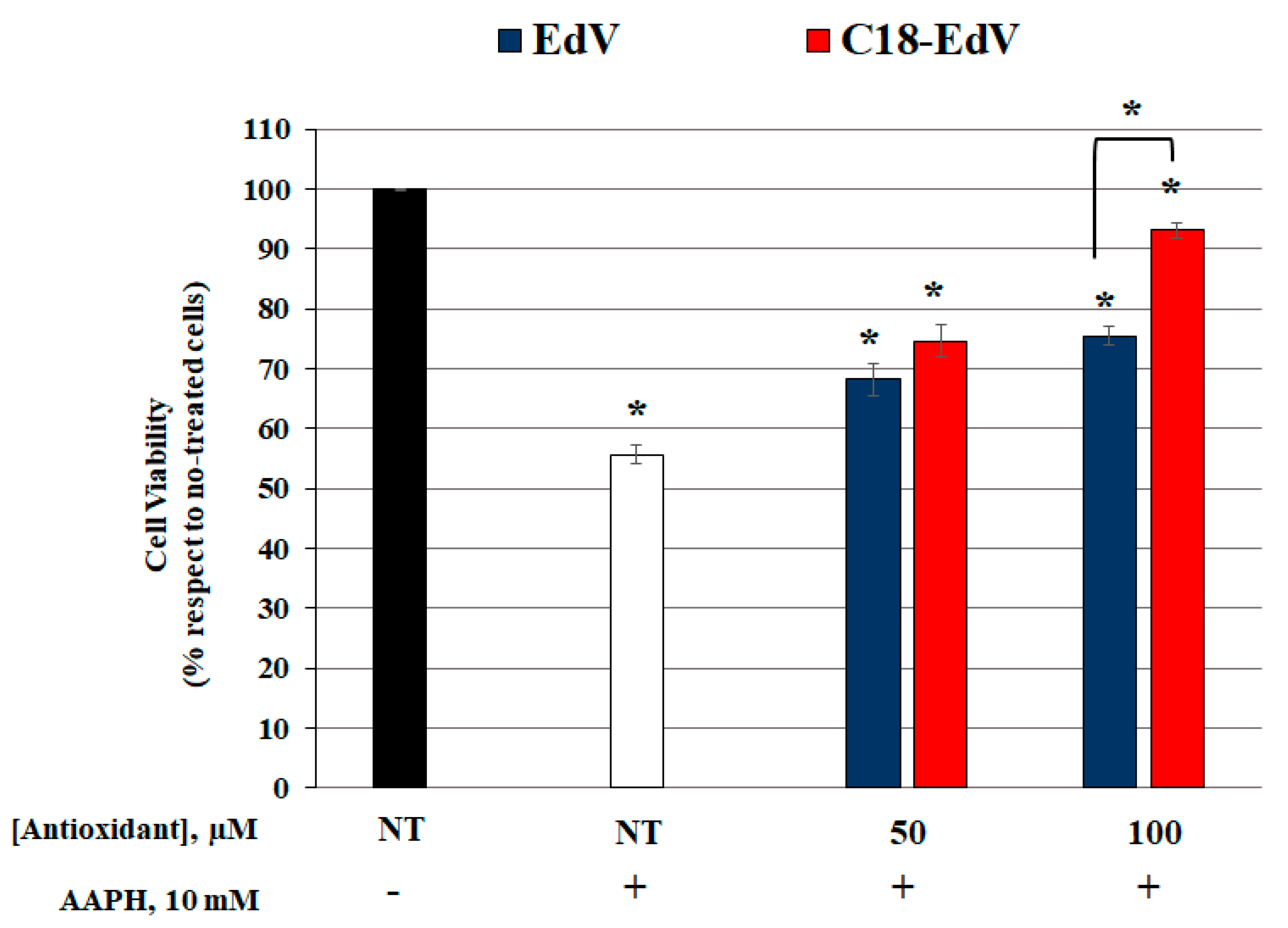
3.6.2. Enhancement of Edaravone Protective Efficacy by C18-Lipid Chain Introduction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.J.; Lee, K.; Park, S.J.; Ahn, B.; Lee, J.C.; Cho, H.; Lee, K.I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Uramaru, N.; Shigematsu, H.; Toda, A.; Eyanagi, R.; Kitamura, S.; Ohta, S. Design, synthesis, and pharmacological activity of nonallergenic pyrazolone-type antipyretic analgesics. J. Med. Chem. 2010, 53, 8727–8733. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, P.; Ravi, T.K.; Gopalakrishnan, S. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur. J. Med. Chem. 2009, 44, 4690–4694. [Google Scholar] [CrossRef] [PubMed]
- Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 2017, 22, 9–68. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kuwahara, T.; Watanabe, K.; Watanabe, K. Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep. 1996, 2, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Namiki, Y.; Fukatsu-Sasaki, K.; Furutani, N.; Tada, N. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006, 12, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Viso, E.; Rodriguez-Ares, M.T.; Gude, F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes eye study). Ophthalmic Epidemiol. 2009, 16, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.L.; Farnsworth, C.C.; Dratz, E.A. A reinvestigation of the fatty acid content of bovine, rat and frog retinal rod outer segments. Exp. Eye Res. 1979, 28, 387–397. [Google Scholar] [CrossRef]
- Chegaev, K.; Cena, C.; Giorgis, M.; Rolando, B.; Tosco, P.; Bertinaria, M.; Fruttero, R.; Carrupt, P.A.; Gasco, A. Edaravone derivatives containing NO-donor functions. J. Med. Chem. 2009, 52, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yuki, S.; Egawa, M.; Nishi, H. Protective effects of MCI-186 on cerebral ischemia: Possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994, 268, 1597–1604. [Google Scholar] [PubMed]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Minnelli, C.; Amici, A.; Massaccesi, L.; Mobbili, G.; Galeazzi, R. Liposomal formulations for an efficient encapsulation of epigallocatechin-3-gallate: An in-silico/experimental approach. Molecules 2018, 23, 441. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision A08; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Oseguera, C.; Alvarez-Idaboy, J.R.; Merino, G.; Galano, A. Rate coefficient and mechanism of the gas phase OH hydrogen abstraction reaction from formic acid: A quantum mechanical approach. J. Phys. Chem. A 2009, 113, 13913–13920. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Macías-Ruvalcaba, N.A.; Campos, O.N.M.; Pedraza-Chaverri, J. Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: A combined theoretical and experimental study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef]
- Galano, A.; Francisco-Marquez, M.; Alvarez-Idaboy, J.R. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Phys. Chem. Chem. Phys. 2011, 13, 11199–11205. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, A.; Galano, A. On the outstanding antioxidant capacity of edaravone derivatives through single electron transfer reactions. J. Phys. Chem. B 2012, 116, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L. Suite 2014; Schrödinger LLC: New York, NY, USA, 2014; Available online: https://www.schrodinger.com/ (accessed on 31 July 2019).
- Borges, R.S.; Queiroz, A.N.; Mendes, A.P.; Araújo, S.C.; França, L.C.; Franco, E.C.; Leal, W.G.; da Silva, A.B. Density functional theory (DFT) study of edaravone derivatives as antioxidants. Int. J. Mol. Sci. 2012, 13, 7594–7606. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.; Takeshita, K.; Subbarao, N.K.; Hu, L.R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cipollone, M.; Galli, M.C.; Pugnaloni, A.; Biagini, G.; Landi, L. Characterization of large unilamellar vesicles as models for studies of lipid peroxidation initiated by azocompounds. Free Radic. Res. 1994, 21, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990, 186, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pal, S.; Mareddy, J.; Devi, N.S. High speed synthesis of pyrazolones using microwave-assisted neat reaction technology. J. Braz. Chem. Soc. 2008, 19, 1207. [Google Scholar] [CrossRef]
- Damiani, E.; Astolfi, P.; Carloni, P.; Stipa, P.; Greci, L. Antioxidants: How they work. In Oxidants in Biology; Valacchi, G., Davis, P.A., Eds.; Springer Science & Business Media: New York, NY, USA, 2008; pp. 251–266. [Google Scholar]
- Leopoldini, M.; Rondinelli, F.; Russo, N.; Toscano, M. Pyranoanthocyanins: A theoretical investigation on their antioxidant activity. J. Agric. Food Chem. 2010, 58, 8862–8871. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, M.V.; Rutkowski, M.; Ringeissen, S.; Gomar, J.; Frantz, M.C.; Ngom, S.; Adamo, C. Benchmarking the DFT methodology for assessing antioxidant-related properties: Quercetin and edaravone as case studies. J. Mol. Model. 2016, 22, 250. [Google Scholar] [CrossRef]
- Nakayama, T.; Ono, K.; Hashimoto, K. Affinity of antioxidative polyphenols for lipid bilayers evaluated with a liposome system. Biosci. Biotechnol. Biochem. 1998, 62, 1005–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakayama, T.; Niimi, T.; Osawa, T.; Kawakishi, S. The protective role of polyphenols in cytotoxicity of hydrogen peroxide. Mutat. Res. Lett. 1992, 281, 77–80. [Google Scholar] [CrossRef]
- Nakayama, T.; Ogiso, Y.; Osawa, T.; Kawakisin, S. Suppression of hydrogen peroxide-induced cytotoxicity toward Chinese hamster lung fibroblasts by Decylubiquinone, a coenzyme Q homolog. Biosci. Biotech. Biochem. 1994, 58, 1702–1704. [Google Scholar] [CrossRef][Green Version]
- Duan, W.J.; Li, Y.F.; Liu, F.L.; Deng, J.; Wu, Y.P. A SIRT3/AMPK/autophagy network orchestrates protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 the macrophages. Free Radic. Biol. Med. 2016, 95, 230–242. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Yao, X.S.; Li, H.Y.; Dai, Y.; Duan, Y.H. The anti-stress effects of Sarcandra glabra extract on restraint-evoked immunocompromise. Chem. Pharm. Bull. 2009, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Egi, Y.; van Leyen, K.; Lo, E.H.; Arai, K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain Res. 2010, 1307, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, K.; Inokuchi, Y.; Fujisawa, T.; Shimazaki, H.; Akane, M.; Tozuka, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H.; Takeuchi, H. Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur. J. Pharm. Biopharm. 2011, 79, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Galeazzi, R.; Pisani, M.; Crucianelli, E.; Bizzaro, D.; Armeni, T.; Mobbili, G. Selective induction of apoptosis in MCF7 cancer-cell by targeted liposomes functionalised with mannose-6-phosphate. J. Drug Target. 2018, 26, 242–251. [Google Scholar] [CrossRef]
- Laudadio, E.; Mobbili, G.; Minnelli, C.; Massaccesi, L.; Galeazzi, R. Salts influence cathechins and flavonoids encapsulation in liposomes: A molecular dynamics investigation. Mol. Inform. 2017, 36, 11. [Google Scholar] [CrossRef]
- Galeazzi, R.; Laudadio, E.; Falconi, E.; Massaccesi, L.; Ercolani, L.; Mobbili, G.; Minnelli, C.; Scirè, A.; Cianfruglia, L.; Armeni, T. Protein-protein interactions of human glyoxalase II: Findings of a reliable docking protocol. Org. Biomol. Chem. 2018, 16, 5167–5177. [Google Scholar] [CrossRef]
- Mangiaterra, G.; Laudadio, E.; Cometti, M.; Mobbili, G.; Minnelli, C.; Massaccesi, L.; Biavasco, F.; Citterio, B.; Galeazzi, R. Inhibitors of multidrug efflux pumps of Pseudomonas aeruginosa from natural sources: An in silico high-throughput virtual screening and in vitro validation. Med. Chem. Res. 2017, 26, 414–430. [Google Scholar] [CrossRef]
- Fedeli, D.; Montani, M.; Bordoni, L.; Galeazzi, R.; Nasuti, C.; Correia-Sá, L.; Domingues, V.F.; Jayant, M.; Brahmachari, V.; Massaccesi, L.; et al. In vivo and in silico studies to identify mechanisms associated with nurr1 modulation following early life exposure to permethrin in rats. Neuroscience 2017, 340, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, R.; Bruni, P.; Crucianelli, E.; Laudadio, E.; Marini, M.; Massaccesi, L.; Mobbili, G.; Pisani, M. Liposome-based gene delivery systems containing a steroid derivative: Computational and small angle X-ray diffraction study. RSC Adv. 2015, 5, 54070. [Google Scholar] [CrossRef]
- Galeazzi, R.; Laudadio, E.; Massaccesi, L. Recent advances in computational simulations of lipid bilayer based molecular systems. Front. Comput. Chem. 2015, 2, 326–388. [Google Scholar] [CrossRef]

| Compound | BDHCH (1) | ΔEiso (1) | BDECH (2) | ΔEiso (2) |
|---|---|---|---|---|
| EdV | 82.36 kcal/mol | 0 | 84.4 kcal/mol | 0 |
| 3.57 eV | 3.66 eV | |||
| C18-EdV | 83.90 kcal/mol | 1.17 kcal/mol | 85.34 kcal/mol | 0.90 kcal/mol |
| 3.64 eV | 0.050 eV | 3.70 eV | 0.039 eV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnelli, C.; Laudadio, E.; Galeazzi, R.; Rusciano, D.; Armeni, T.; Stipa, P.; Cantarini, M.; Mobbili, G. Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone. Antioxidants 2019, 8, 258. https://doi.org/10.3390/antiox8080258
Minnelli C, Laudadio E, Galeazzi R, Rusciano D, Armeni T, Stipa P, Cantarini M, Mobbili G. Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone. Antioxidants. 2019; 8(8):258. https://doi.org/10.3390/antiox8080258
Chicago/Turabian StyleMinnelli, Cristina, Emiliano Laudadio, Roberta Galeazzi, Dario Rusciano, Tatiana Armeni, Pierluigi Stipa, Mattia Cantarini, and Giovanna Mobbili. 2019. "Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone" Antioxidants 8, no. 8: 258. https://doi.org/10.3390/antiox8080258
APA StyleMinnelli, C., Laudadio, E., Galeazzi, R., Rusciano, D., Armeni, T., Stipa, P., Cantarini, M., & Mobbili, G. (2019). Synthesis, Characterization and Antioxidant Properties of a New Lipophilic Derivative of Edaravone. Antioxidants, 8(8), 258. https://doi.org/10.3390/antiox8080258













