Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Whey Production and Conditioning
2.2. Enzymatic Hydrolysis Experimental Design
2.3. Degree of Hydrolysis
2.4. Total and Soluble Protein
2.5. Antioxidant Capacity by DPPH Method
2.6. Reverse-Phase HPLC of Hydrolyzed Whey Protein
2.7. Angiotensin-Converting Enzyme Inhibition Assay (ACE)
2.8. Statistical Analysis
3. Results
3.1. Analysis of Variance (ANOVA)
3.2. Correlation Analysis
4. Discussion
4.1. Antioxidant Capacity
4.2. ACEI Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valencia Denicia, E.; Ramírez Castillo, M.L. La industria de la leche y la contaminación del agua. Elementos 2009, 73, 27–31. [Google Scholar]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-lactoglobulin and α-lactalbumin—Technological implications for processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Livestock Processed. Available online: http://www.fao.org/faostat/en/#data/QP (accessed on 4 April 2019).
- SAGARPA. Panorama de la lechería en México (page 12). Available online: http://infosiap.siap.gob.mx/opt/boletlech/Brochure%20leche_Septiembre2016.pdf (accessed on 4 April 2019).
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef]
- Espejo-Carpio, F.J.; De Gobba, C.; Guadix, A.; Guadix, E.M.; Otte, J. Angiotensin I-converting enzyme inhibitory activity of enzymatic hydrolysates of goat milk protein fractions. Int. Dairy J. 2013, 32, 175–183. [Google Scholar] [CrossRef]
- Fitzgerald, R.J.; Murray, B.A. Bioactive peptides and lactic fermentations. Int. J. Dairy Technol. 2006, 59, 118–125. [Google Scholar] [CrossRef]
- Choi, J.; Sabikhi, L.; Hassan, A.; Anand, S. Bioactive peptides in dairy products. Int. J. Dairy Technol. 2012, 65, 1–12. [Google Scholar] [CrossRef]
- Abubakar, A.; Saito, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Structural Analysis of New Antihypertensive Peptides Derived from Cheese Whey Protein by Proteinase K Digestion. J. Dairy Sci. 1998, 81, 3131–3138. [Google Scholar] [CrossRef]
- Didelot, S.; Bordenave-Juchereau, S.; Rosenfeld, E.; Fruitier-Arnaudin, I.; Piot, J.-M.; Sannier, F. Preparation of angiotensin-I-converting enzyme inhibitory hydrolysates from unsupplemented caprine whey fermentation by various cheese microflora. Int. Dairy J. 2006, 16, 976–983. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive components of ovine and caprine cheese whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Ferraretto, A.; Arranz, E.; Stuknytė, M.; Bottani, M.; O’Connor, P.M.; Kelly, P.M.; De Noni, I.; Buckin, V.; Giblin, L. Bovine whey peptides transit the intestinal barrier to reduce oxidative stress in muscle cells. Food Chem. 2019, 288, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009, 113, 196–201. [Google Scholar] [CrossRef]
- Clare, D.A.; Swaisgood, H.E. Bioactive Milk Peptides: A Prospectus. J. Dairy Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Floris, R.; Recio, I.; Berkhout, B.; Visser, S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr. Pharm. Des. 2003, 9, 1257–1275. [Google Scholar] [CrossRef]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT-Food Sci. Technol. 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Teixeira, F.J.; Santos, H.O.; Howell, S.L.; Pimentel, G.D. Whey protein in cancer therapy: A narrative review. Pharm. Res. 2019, 144, 245–256. [Google Scholar] [CrossRef]
- De Simone, C.; Picariello, G.; Mamone, G.; Stiuso, P.; Dicitore, A.; Vanacore, D. Characterisation and cytomodulatory properties of peptides from Mozzarella di Bufala Campana cheese whey. J. Pept. Sci. 2009, 15, 251–258. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem. 2019, 279, 70–79. [Google Scholar] [CrossRef]
- Ortiz-Chao, P.; Gómez-Ruiz, J.A.; Rastall, R.A.; Mills, D.; Cramer, R.; Pihlanto, A.; Korhonen, H.; Jauregi, P. Production of novel ACE inhibitory peptides from β-lactoglobulin using Protease N Amano. Int. Dairy J. 2009, 19, 69–76. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Mota, M.V.; Tavares, P.; Pereira, A.; Gonçalves, M.P.; Torres, D.; Rocha, C.; Teixeira, J.A. Preparation of ingredients containing an ACE-inhibitory peptide by tryptic hydrolysis of whey protein concentrates. Int. Dairy J. 2007, 17, 481–487. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur Food Res Technol 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Guerra Martínez, J.A.; Montejano, J.G.; Martin del Campo, S.T. Evaluation of proteolytic and physicochemical changes during storage of fresh Panela cheese from Queretaro, Mexico and its impact in texture. CyTA – J. Food 2012, 10, 296–305. [Google Scholar] [CrossRef]
- Nielsen, P.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Gonzalez De Llano, D.; Polo, M.C.; Ramos, M. Study of Proteolysis in Artisanal Cheeses: High Performance Liquid Chromatography of Peptides. J. Dairy Sci. 1995, 78, 1018–1024. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Zhang, Y.; Cai, Z.; Chen, Q.; He, G. A Convenient RP-HPLC Method for Assay Bioactivities of Angiotensin I-Converting Enzyme Inhibitory Peptides. ISRN Biotechnol. 2013, 2013, 453910. [Google Scholar] [CrossRef]
- Havea, P.; Singh, H.; Creamer, L.K. Heat-Induced Aggregation of Whey Proteins: Comparison of Cheese WPC with Acid WPC and Relevance of Mineral Composition. J. Agric. Food Chem. 2002, 50, 4674–4681. [Google Scholar] [CrossRef]
- Chelulei Cheison, S.; Brand, J.; Leeb, E.; Kulozik, U. Analysis of the effect of temperature changes combined with different alkaline pH on the beta-lactoglobulin trypsin hydrolysis pattern using MALDI-TOF-MS/MS. J. Agric. Food Chem. 2011, 59, 1572–1581. [Google Scholar] [CrossRef]
- Şanlıdere Aloğlu, H.; Öner, Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Önay-Uçar, E.; Arda, N.; Pekmez, M.; Yılmaz, A.M.; Böke-Sarıkahya, N.; Kırmızıgül, S.; Yalçın, A.S. Comparison of antioxidant capacity, protein profile and carbohydrate content of whey protein fractions. Food Chem. 2014, 150, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007, 102, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of sheep cheese whey as a source of bioactive peptides with antioxidant and angiotensin-converting enzyme inhibitory activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Contreras, M.; Hernández-Ledesma, B.; Amigo, L.; Martín-Álvarez, P.J.; Recio, I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology. LWT-Food Sci. Technol. 2011, 44, 9–15. [Google Scholar] [CrossRef]
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; FitzGerald, R.J. Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chem. 2016, 199, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bai, L.; Zhou, Y.; Tong, R.; Zeng, M.; Li, X.; Shi, J. Polysaccharides from Chinese herbal medicine for anti-diabetes recent advances. Int. J. Biol. Macromol. 2019, 121, 1240–1253. [Google Scholar] [CrossRef]

| Parameter | Factor (p-Value) a | Mean | Minimum | Maximum | |
|---|---|---|---|---|---|
| pH | Temperature | ||||
| % Hydrolysis | 0.002 ** | 0.230 | 66.67 | 32.82 | 100.34 |
| Soluble Protein b | 0.010 ** | 0.000 *** | 0.28 | 0.05 | 0.46 |
| Antioxidant capacity c | 0.035 * | 0.000 *** | 12.04 | 4.76 | 26.20 |
| Δ ACE inhibition d | 0.000 *** | 0.246 | 0.79 | 0.68 | 0.89 |
| AA fraction | 0.438 | 0.171 | 25,412.87 | 13,200.70 | 35,041.30 |
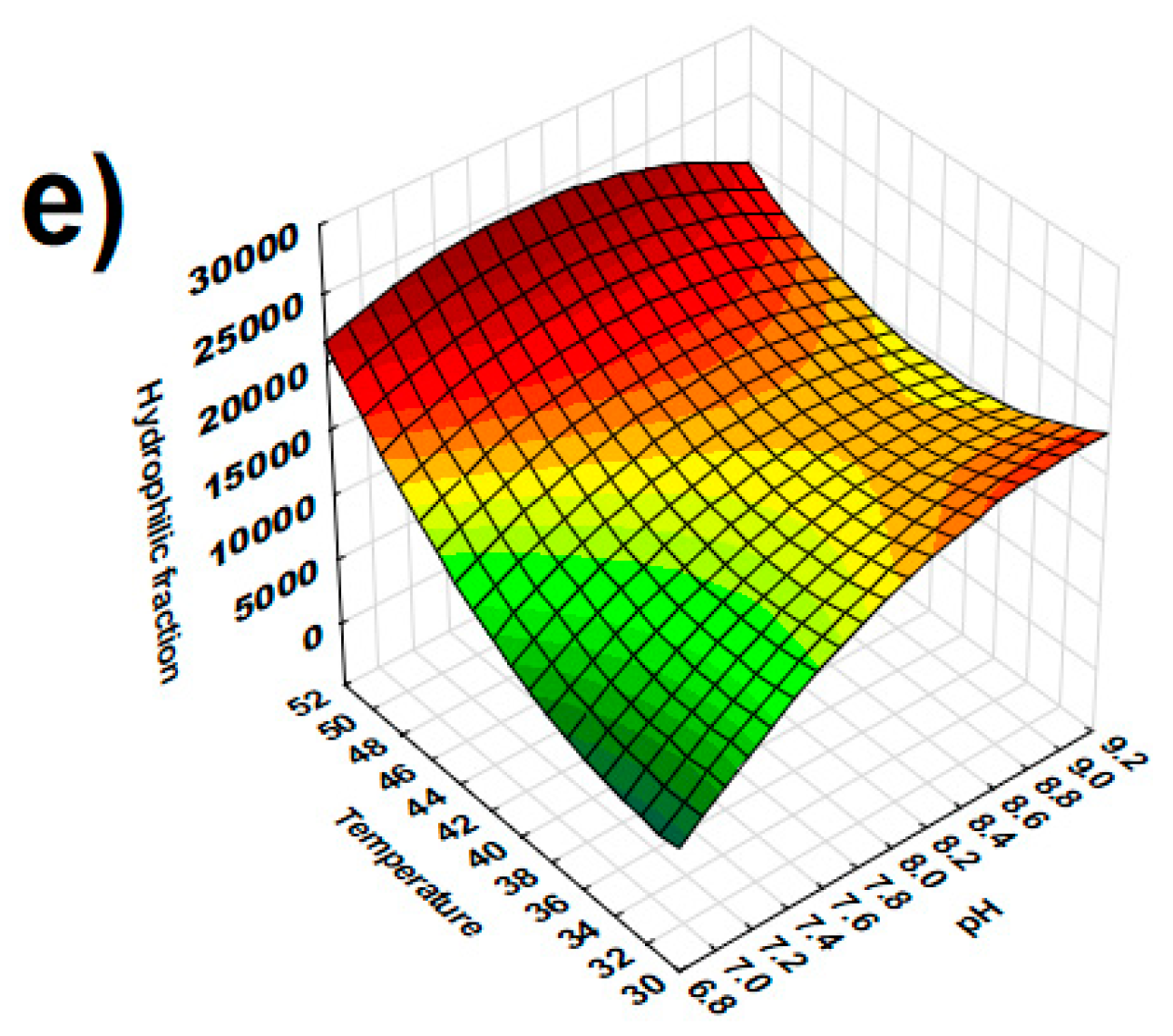
| Hydrophilic fraction (HI) | 0.161 | 0.000 *** | 15,602.86 | 7364.70 | 27,454.90 |
| Hydrophobic fraction (HO) | 0.890 | 0.563 | 23,738.38 | 409.50 | 372,127.00 |
| Ratio HI:HO | 0.139 | 0.071 | 2.94 | 0.03 | 20.12 |
| Variable a | RT | Antioxidant Capacity b | Δ ACE Inhibition c | Variable | RT | Antioxidant Capacity b | Δ ACE Inhibition c |
|---|---|---|---|---|---|---|---|
| Δ ACE inhibition c | 0.516 * | P61 | 22.603 | 0.667 ** | 0.518 * | ||
| AA fraction | 0.333 | 0.204 | P62 | 22.899 | −0.290 | −0.547 * | |
| Hydrophilic fraction (HI) | 0.770 *** | 0.706 ** | P73 | 26.790 | 0.541 * | 0.512 * | |
| Hydrophobic fraction (HO) | −0.231 | 0.005 | P74 | 27.345 | 0.745 *** | 0.374 | |
| Ratio HI:HO | −0.069 | −0.516 * | P75 | 27.623 | −0.526 * | −0.008 | |
| P2 | 2.045 | 0.379 | 0.513 * | P76 | 28.114 | 0.631 ** | 0.501 * |
| P3 | 2.432 | −0.789 *** | −0.257 | P78 | 28.704 | 0.563 * | 0.121 |
| P8 | 3.759 | 0.346 | 0.576 * | P80 | 29.626 | 0.498 * | 0.363 |
| P10 | 4.103 | 0.720 *** | 0.484 * | P81 | 29.734 | 0.546 * | 0.243 |
| P11 | 4.857 | 0.746 *** | 0.573 * | P82 | 30.868 | 0.636 ** | 0.575 * |
| P12 | 5.689 | 0.446 | 0.601 ** | P86 | 32.838 | 0.641 ** | 0.464 |
| P13 | 6.133 | 0.535 * | 0.565 * | P87 | 33.339 | 0.647 ** | 0.533 * |
| P18 | 8.316 | 0.524 * | 0.496 * | P89 | 34.293 | 0.471 * | 0.711 *** |
| P19 | 8.943 | 0.591 ** | 0.616 ** | P90 | 34.590 | −0.271 | 0.022 |
| P22 | 10.204 | 0.548 * | 0.324 | P91 | 34.848 | 0.390 | 0.590 ** |
| P23 | 10.547 | 0.720 *** | 0.498 * | P92 | 35.479 | 0.611 ** | 0.633 ** |
| P27 | 12.367 | 0.359 | 0.475 * | P98 | 38.279 | 0.580 * | −0.014 |
| P28 | 13.195 | 0.389 | 0.514 * | P99 | 38.950 | 0.481 * | 0.018 |
| P31 | 14.097 | 0.392 | 0.569 * | P100 | 39.193 | 0.035 | 0.476 * |
| P34 | 14.893 | 0.612 ** | 0.051 | P102 | 39.876 | 0.590 * | 0.348 |
| P35 | 15.050 | 0.048 | 0.537 * | P106 | 41.778 | 0.241 | 0.494 * |
| P37 | 15.698 | 0.568 * | 0.426 | P107 | 42.052 | 0.605 ** | 0.126 |
| P39 | 16.190 | 0.519 * | 0.216 | P109 | 43.430 | 0.729 *** | 0.563 ** |
| P45 | 17.733 | 0.796 *** | 0.561 * | P110 | 44.162 | 0.771 *** | 0.168 |
| P46 | 18.131 | 0.555 * | 0.450 | P111 | 44.881 | 0.692 ** | 0.250 |
| P48 | 18.768 | 0.520 * | 0.593 ** | P114 | 46.743 | 0.831 *** | 0.590 ** |
| P51 | 19.534 | 0.580 * | 0.595 ** | P116 | 48.529 | 0.290 | 0.503 * |
| P54 | 20.964 | 0.466 | 0.687 ** | P119 | 53.330 | 0.344 | 0.646 ** |
| P56 | 21.355 | 0.422 | 0.475 * | P120 | 58.930 | 0.335 | 0.506 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8, 158. https://doi.org/10.3390/antiox8060158
Martín-del-Campo ST, Martínez-Basilio PC, Sepúlveda-Álvarez JC, Gutiérrez-Melchor SE, Galindo-Peña KD, Lara-Domínguez AK, Cardador-Martínez A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants. 2019; 8(6):158. https://doi.org/10.3390/antiox8060158
Chicago/Turabian StyleMartín-del-Campo, Sandra Teresita, Pablo César Martínez-Basilio, Juan Carlos Sepúlveda-Álvarez, Susana Estela Gutiérrez-Melchor, Karla Deniss Galindo-Peña, Ana Karen Lara-Domínguez, and Anaberta Cardador-Martínez. 2019. "Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production" Antioxidants 8, no. 6: 158. https://doi.org/10.3390/antiox8060158
APA StyleMartín-del-Campo, S. T., Martínez-Basilio, P. C., Sepúlveda-Álvarez, J. C., Gutiérrez-Melchor, S. E., Galindo-Peña, K. D., Lara-Domínguez, A. K., & Cardador-Martínez, A. (2019). Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants, 8(6), 158. https://doi.org/10.3390/antiox8060158







