Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Preparation of the Water Extract (VVWE)
2.3. Cell Culture
2.4. Characterization of Grapevine Extract by High Performance Liquid Chromatography (HPLC)
2.5. IL-8 Release
2.6. Transient Transfection Assays
2.7. NF-κB Nuclear Translocation
2.8. UVB Radiation
2.9. VEGF Release
2.10. MMP-9 Release
2.11. Cytotoxicity Assay
2.12. In Vitro Skin Permeation Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of VVWE
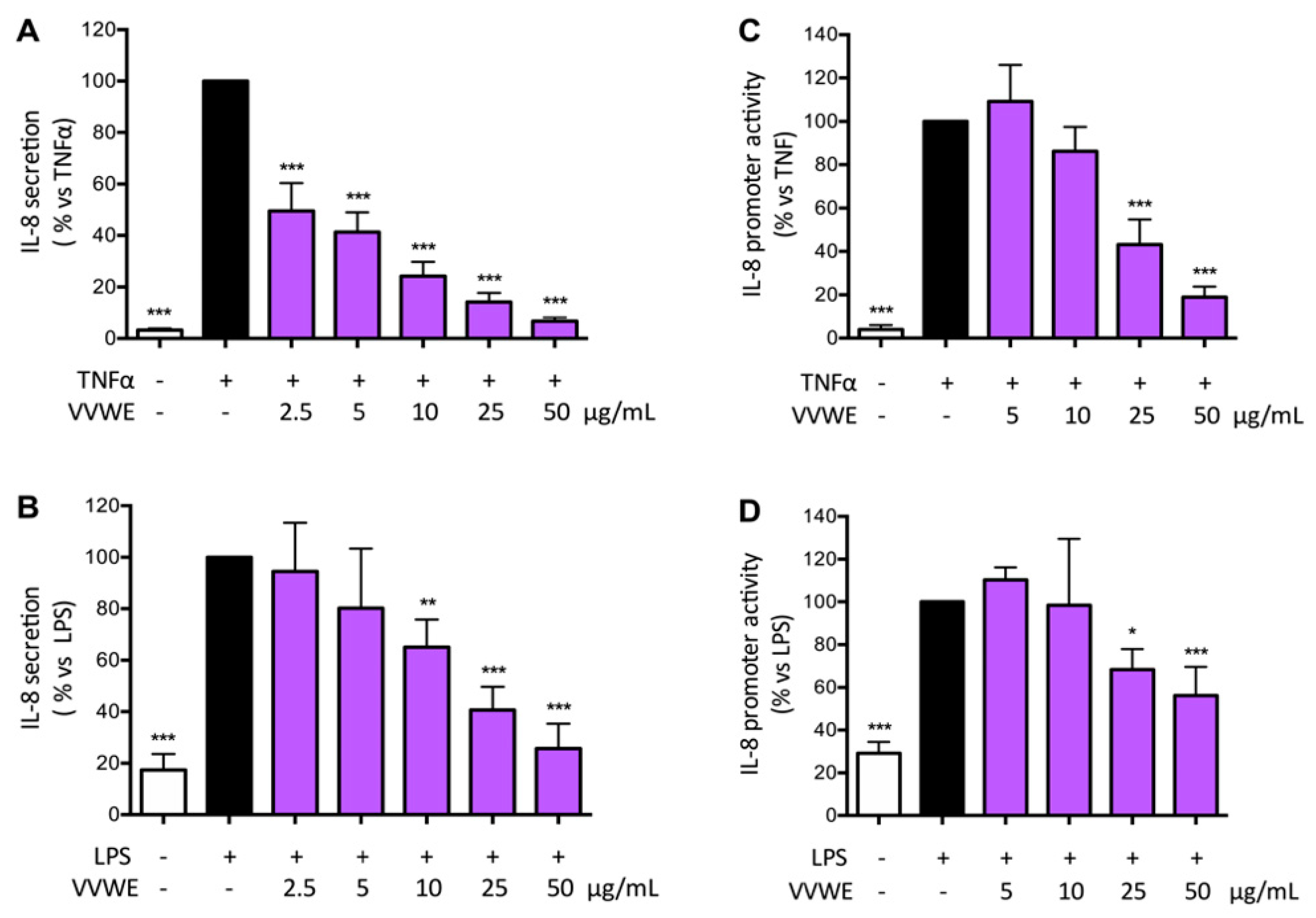
3.2. VVWE Reduces IL-8 Release and Promoter Activity Induced by Pro-Inflammatory Mediators
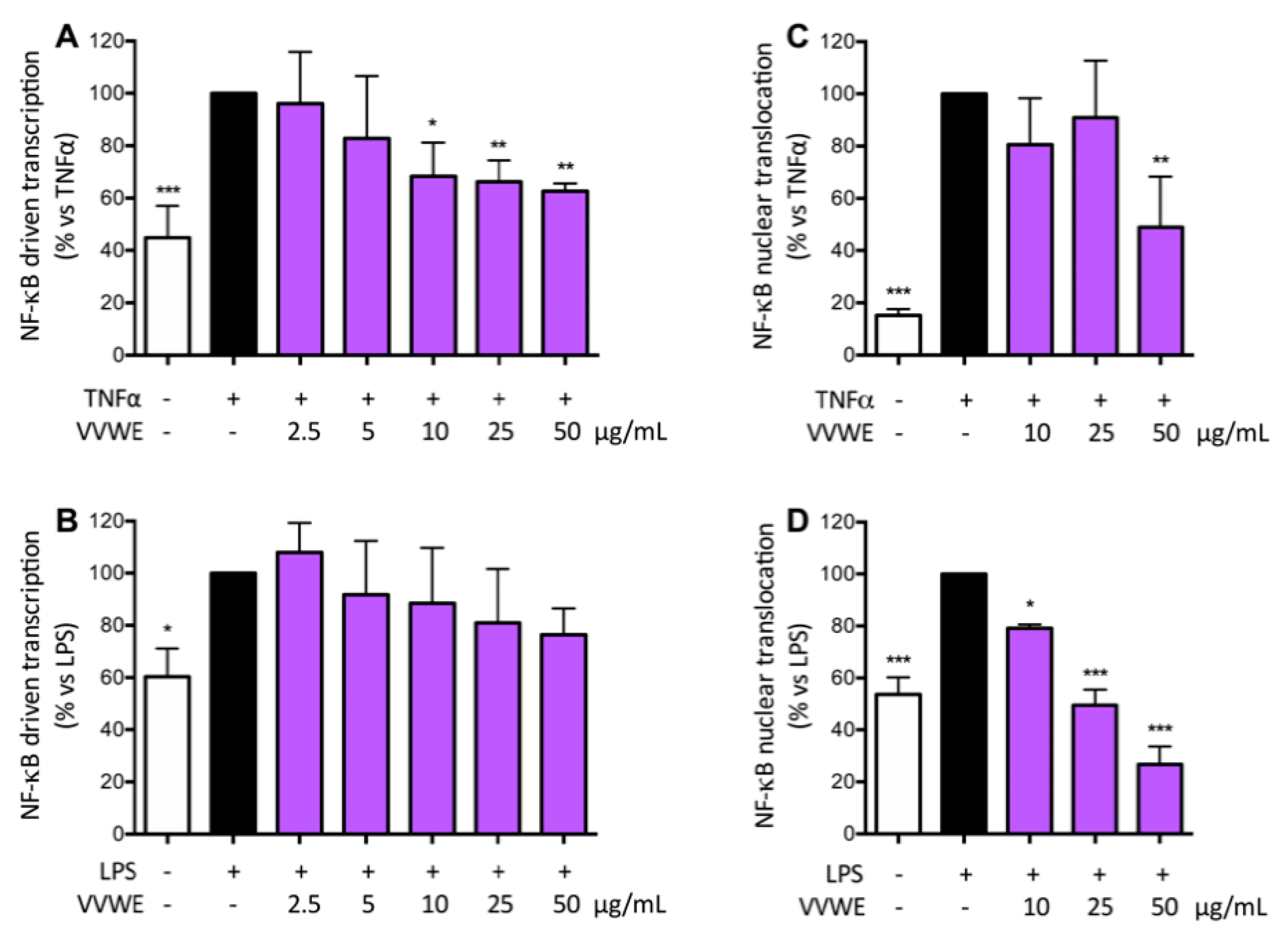
3.3. VVWE Impairs the NF-κB Pathway Acting on Transcription and Nuclear Translocation
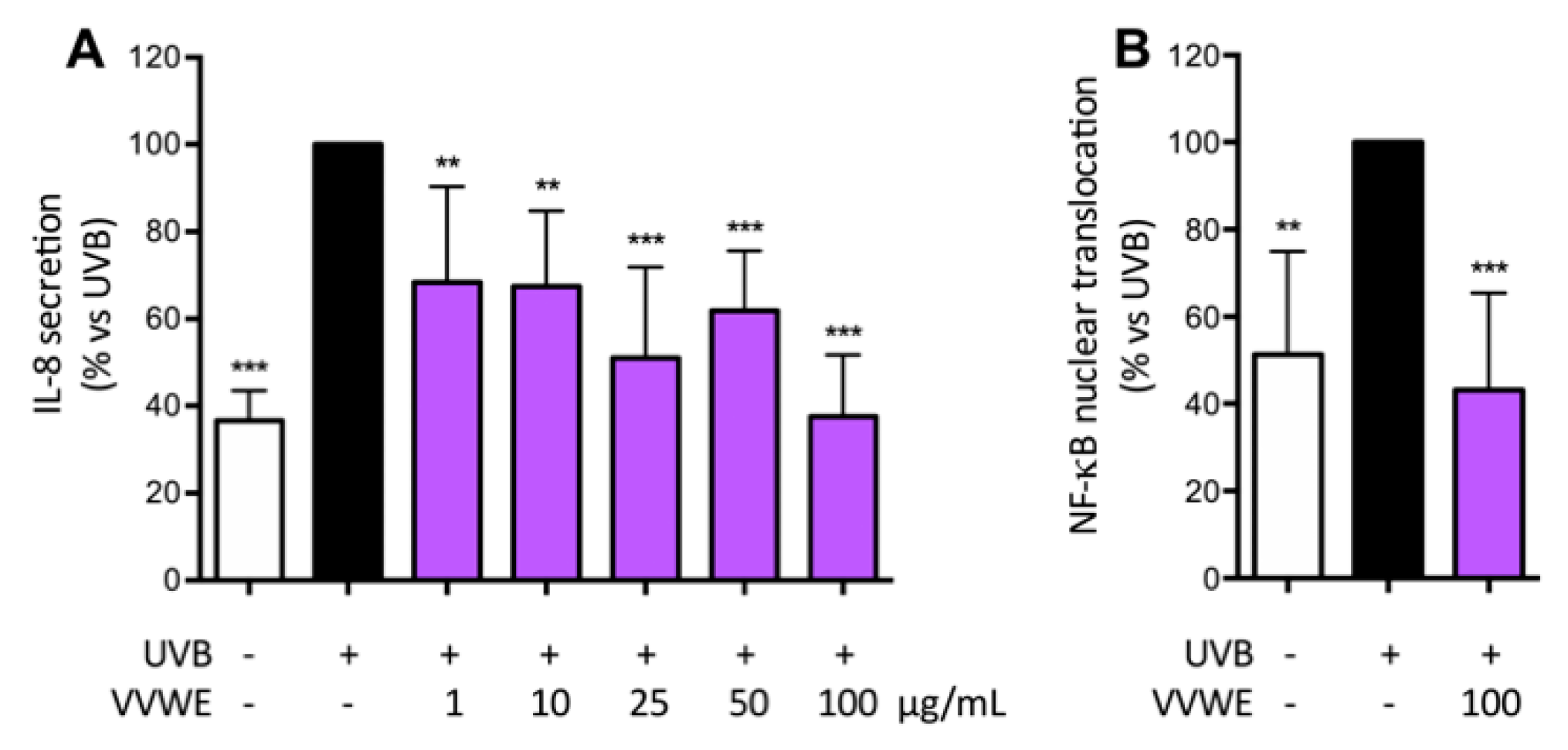
3.4. VVWE Reduces IL-8 Release and NF-κB Nuclear Translocation Induced by UV-B Radiations
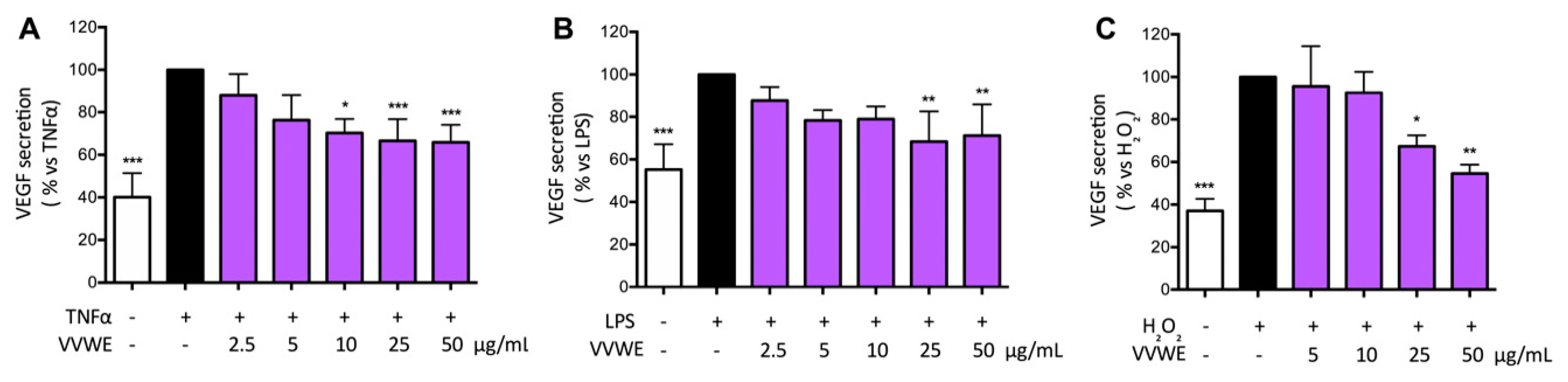
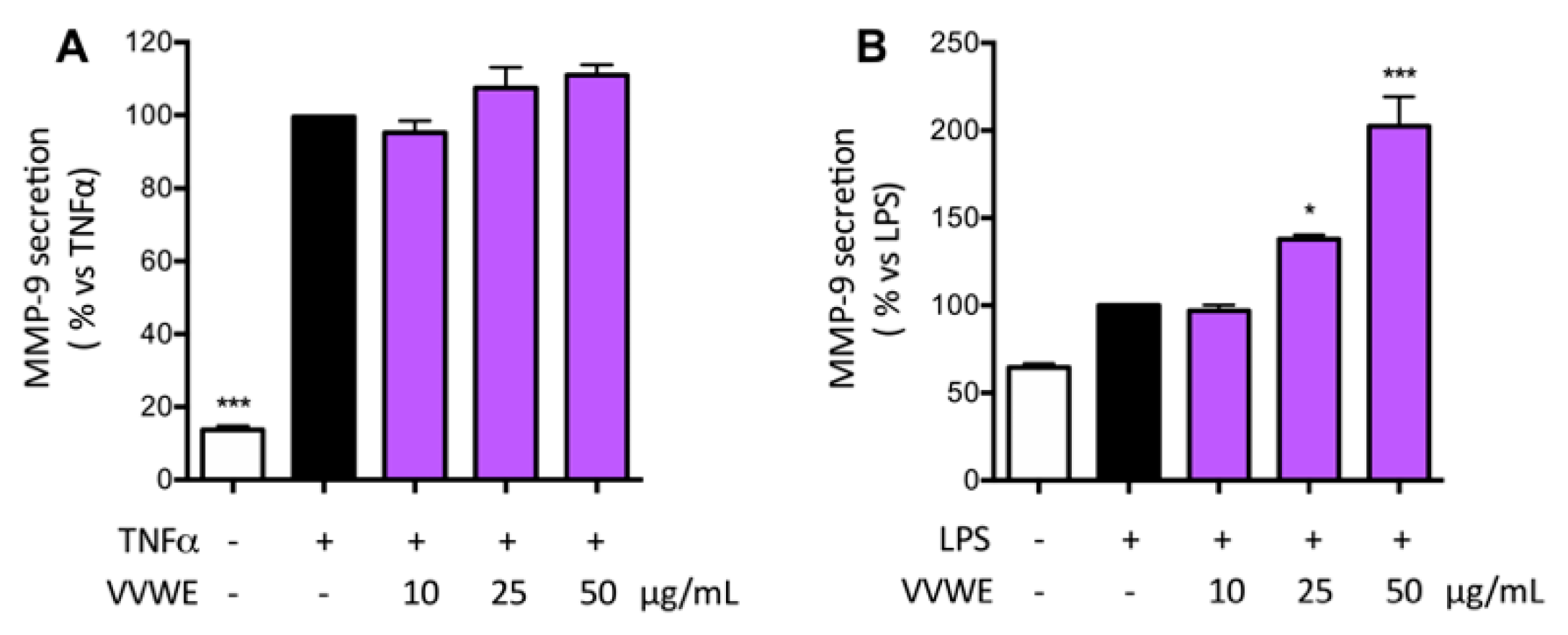
3.5. Effects of VVWE on VEGF and MMP-9 Release
3.6. Franz Diffusion Cell Method
3.7. Effects of Pure Compounds on IL-8 Secretion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- de Bruin Weller, M.S.; Knulst, A.C.; Meijer, Y.; Bruijnzeel-Koomen, C.A.; Pasmans, S.G. Evaluation of the child with atopic dermatitis. Clin. Exp. Allergy 2012, 42, 352–362. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef]
- Farber, E.M.; Nall, M.L. The natural history of psoriasis in 5600 patients. Dermatologica 1974, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J. Skin innate immune system in psoriasis: Friend or foe? J. Clin. Investig. 1999, 104, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Campanati, A.; Ferretti, G.; Simonetti, O.; Liberati, G.; Offidani, A.M. Oxidative stress and psoriasis: The effect of antitumour necrosis factor-alpha inhibitor treatment. Br. J. Dermatol. 2013, 168, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Nestle, F.O.; Papp, K.; Ortonne, J.P.; Evans, R.; Guzzo, C.; Li, S.; Dooley, L.T.; Griffiths, C.E. EXPRESS Study Investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet 2005, 366, 1367–1374. [Google Scholar] [CrossRef]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. Nf-kappab in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar] [CrossRef]
- Wang, W.; Mani, A.M.; Wu, Z.H. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J. Cancer Metastasis Treat. 2017, 3, 45–59. [Google Scholar] [CrossRef]
- Radhiga, T.; Agilan, B.; Muzaffer, U.; Karthikeyan, R.; Kanimozhi, G.; Paul, V.I.; Prasad, N.R. Phytochemicals as modulators of ultraviolet-b radiation induced cellular and molecular events: A review. J. Radiat. Cancer Res. 2016, 7, 2–12. [Google Scholar]
- Cho, J.W.; Lee, K.S.; Kim, C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin e in tnf-alpha-treated HaCat cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007, 19, 469–474. [Google Scholar]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCat cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Detmar, M.; Brown, L.F.; Claffey, K.P.; Yeo, K.T.; Kocher, O.; Jackman, R.W.; Berse, B.; Dvorak, H.F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J. Exp. Med. 1994, 180, 1141–1146. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Sauder, D.N.; Dekoven, J.; Champagne, P.; Croteau, D.; Dupont, E. Neovastat (AE-941), an inhibitor of angiogenesis: Randomized phase I/II clinical trial results in patients with plaque psoriasis. J. Am. Acad. Dermatol. 2002, 47, 535–541. [Google Scholar] [CrossRef]
- Halin, C.; Fahrngruber, H.; Meingassner, J.G.; Bold, G.; Littlewood-Evans, A.; Stuetz, A.; Detmar, M. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am. J. Pathol. 2008, 173, 265–277. [Google Scholar] [CrossRef]
- Cordiali-Fei, P.; Trento, E.; D’Agosto, G.; Bordignon, V.; Mussi, A.; Ardigo, M.; Mastroianni, A.; Vento, A.; Solivetti, F.; Berardesca, E.; et al. Effective Therapy with Anti-TNF-α in Patients with Psoriatic Arthritis Is Associated with Decreased Levels of Metalloproteinases and Angiogenic Cytokines in the Sera and Skin Lesions. Ann. N. Y. Acad. Sci. 2007, 1110, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Alenius, G.M.; Jonsson, S.; Wallberg Jonsson, S.; Ny, A.; Rantapaa Dahlqvist, S. Matrix metalloproteinase 9 (MMP-9) in patients with psoriatic arthritis and rheumatoid arthritis. Clin. Exp. Rheumatol. 2001, 19, 760. [Google Scholar]
- Scheinfeld, N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J. Dermatolog. Treat. 2004, 15, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Ropke, M.A.; Norsgaard, H. Psoriasis drug discovery: Methods for evaluation of potential drug candidates. Expert Opin. Drug Discov. 2012, 7, 49–61. [Google Scholar] [CrossRef]
- Smith, N.; Weymann, A.; Tausk, F.A.; Gelfand, J.M. Complementary and alternative medicine for psoriasis: A qualitative review of the clinical trial literature. J. Am. Acad. Dermatol. 2009, 61, 841–856. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous sicilian flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human thp-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermatol. 2019, 18, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-inflammatory effects of pomegranate peel extracts on in vitro human intestinal caco-2 cells and ex vivo porcine colonic tissue explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef]
- Deng, S.; May, B.H.; Zhang, A.L.; Lu, C.; Xue, C.C. Plant extracts for the topical management of psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 769–782. [Google Scholar] [CrossRef]
- ESCOP. Escop Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd ed.; Georg Thieme Verlag: New York, NY, USA, 2009. [Google Scholar]
- Orhan, D.D.; Orhan, N.; Ozcelik, B.; Ergun, F. Biological activities of Vitis vinifera L. leaves. Turk. J. Biol. 2009, 33, 341–348. [Google Scholar]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentao, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. Leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Rabe, E.; Stucker, M.; Esperester, A.; Schafer, E.; Ottillinger, B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency-results of a double-blind placebo-controlled study. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 540–547. [Google Scholar] [CrossRef]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. Fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Franze, S.; Minghetti, P.; Casiraghi, A. Emulsion versus nanoemulsion: How much is the formulative shift critical for a cosmetic product? Drug Deliv. Transl. Res. 2018, 8, 414–421. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. Nf-kappab: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Qin, J.Z.; Denning, M.F.; Choubey, D.; Diaz, M.O.; Nickoloff, B.J. Abnormal NF-kappab signaling pathway with enhanced susceptibility to apoptosis in immortalized keratinocytes. J. Dermatol. Sci. 2001, 26, 67–78. [Google Scholar] [CrossRef]
- Richert, S.; Schrader, A.; Schrader, K. Transdermal delivery of two antioxidants from different cosmetic formulations. Int. J. Cosmet. Sci. 2003, 25, 5–13. [Google Scholar] [CrossRef]
- Chen-yu, G.; Chun-fen, Y.; Qi-lu, L.; Qi, T.; Yan-wei, X.; Wei-na, L.; Guang-xi, Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef]
| VVWE Composition: Identified Compounds | Donor Solution Content mg/g a ± SD | Permeated Amount µg/g b ± SD (% Recovery) | Retained Amount in the Epidermidis µg/g b ± SD (% Recovery) |
|---|---|---|---|
| Caftaric acid | 9.99 ± 0.35 | 47.59 ± 27.64 (0.476%) | 10.20 ± 9.00 (0.102%) |
| Rutin | 1.31 ± 0.05 | 0.30 ± 0.35 (0.023%) | 0.80 ± 0.91 (0.061%) |
| Hyperoside | 2.30 ± 0.17 | 0.47 ± 0.68 (0.020%) | 1.34 ± 1.43 (0.058%) |
| Quercetin 3-O-glucoside | 21.68 ± 0.91 | 8.74 ± 9.50 (0.040%) | 13.58 ± 15.88 (0.063%) |
| Quercetin 3-O-glucuronide | 29.14 ± 1.92 | 51.66 ± 46.10 (0.177%) | 28.08 ± 30.33 (0.096%) |
| Kaempferol 3-O-glucoside | 3.77 ± 0.06 | 2.09 ± 1.70 (0.055%) | 3.53 ± 3.90 (0.094%) |
| Delphinidin 3-O-glucoside | 0.95 ± 0.03 | 0.48 ± 0.68 (0.050%) | 0.04 ± 0.08 (0.004%) |
| Cyanidin 3-O-glucoside | 2.29 ± 0.04 | 1.63 ± 2.08 (0.071%) | 0.30 ± 0.60 (0.013%) |
| Petunidin 3-O-glucoside | 0.66 ± 0.05 | 0.38 ± 0.53 (0.058%) | 0.10 ± 0.20 (0.015%) |
| Peonidin 3-O-glucoside | 1.91 ± 0.06 | 2.17 ± 2.72 (0.114%) | 0.58 ± 1.17 (0.030%) |
| Malvidin 3-O-glucoside | 1.27 ± 0.07 | 0.92 ± 1.22 (0.072%) | 0.29 ± 0.58 (0.023%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. https://doi.org/10.3390/antiox8050134
Sangiovanni E, Di Lorenzo C, Piazza S, Manzoni Y, Brunelli C, Fumagalli M, Magnavacca A, Martinelli G, Colombo F, Casiraghi A, et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants. 2019; 8(5):134. https://doi.org/10.3390/antiox8050134
Chicago/Turabian StyleSangiovanni, Enrico, Chiara Di Lorenzo, Stefano Piazza, Yuri Manzoni, Cecilia Brunelli, Marco Fumagalli, Andrea Magnavacca, Giulia Martinelli, Francesca Colombo, Antonella Casiraghi, and et al. 2019. "Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases" Antioxidants 8, no. 5: 134. https://doi.org/10.3390/antiox8050134
APA StyleSangiovanni, E., Di Lorenzo, C., Piazza, S., Manzoni, Y., Brunelli, C., Fumagalli, M., Magnavacca, A., Martinelli, G., Colombo, F., Casiraghi, A., Melzi, G., Marabini, L., Restani, P., & Dell’Agli, M. (2019). Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants, 8(5), 134. https://doi.org/10.3390/antiox8050134







