Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review
Abstract
1. Introduction
2. Progesterone as an Antioxidant in Retina Degeneration
Oxidative Stress and Retinitis Pigmentosa: The Role of Progesterone
3. Lipoic Acid: An Antioxidant and Anti-Inflammatory Molecule
3.1. Diabetes Retinopathy and Lipoic Acid
3.2. Retinitis Pigmentosa and Lipoic Acid
4. Sulforaphane as Antioxidant Treatment in Retinal Diseases
4.1. Sulforaphane Overview
4.2. Age Macular Degeneration
4.3. Retinitis Pigmentosa
4.4. Diabetic Retinopathy
4.5. Retinal Ischemia
4.6. Usher Syndrome, Tubby Model Mice
5. Conclusions
Funding
Conflicts of Interest
References
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nölting, R.; López-Pedrajas, R.; Ekström, P.; Bosch-Morell, F.; van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.M.; Johnson, L.E.; Ahuja, S.; Ekstrom, P.A.; Romero, J.; van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; Trachsel-Moncho, L.; López-Pedrajas, R.; Almansa, I.; Romero, F.J.; Miranda, M. Alterations in glutamate cysteine ligase content in the retina of two retinitis pigmentosa animal models. Free Radic. Biol. Med. 2016, 96, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Lamelas, D.T.; Benlloch-Navarro, S.; López-Pedrajas, R.; Gimeno-Hernández, R.; Olivar, T.; Silvestre, D.; Miranda, M. Lipoic Acid and Progesterone Alone or in Combination Ameliorate Retinal Degeneration in an Experimental Model of Hereditary Retinal Degeneration. Front. Pharmacol. 2018, 9, 5954235. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schumann, U.; Ader, M.; Knels, L.; Funk, R.H. Influence of blue light on photoreceptors in a live retinal explant system. Mol. Vis. 2011, 17, 876–884. [Google Scholar] [PubMed]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Jarret, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar]
- Saenz-de-Viteri, M.; Heras-Mulero, H.; Fernández-Robredo, P.; Recalde, S.; Hernández, M.; Reiter, N.; Moreno-Orduña, M.; García-Layana, A. Oxidative stress and histological changes in a model of retinal phototoxicity in rabbits. Oxid. Med. Cell. Longev. 2014, 2014, 637137. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed]
- German, O.L.; Agnolazza, D.L.; Politi, L.E.; Rotstein, N.P. Light, lipids and photoreceptor survival: Live or let die? Photochem. Photobiol. Sci. 2015, 14, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Oshino, N.; Chance, B. The cellular production of hydrogen peroxide. Biochem. J. 1972, 128, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Garbarino, G.; Caicci, F.; Manni, L.; Candiani, S.; Ravera, S.; Morelli, A.; Traverso, C.E.; Panfoli, I. Functional expression of electron transport chain complexes in mouse rod outer segments. Biochimie 2014, 102, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schumann, U.; Ader, M.; Brunssen, C.; Bramke, S.; Morawietz, H.; Funk, R.H. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE 2013, 8, e71570. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 365, 1795–1809. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Carella, G. Introduction to apoptosis in ophthalmology. Eur. J. Ophthalmol. 2003, 3, S5–S10. [Google Scholar] [CrossRef]
- Stone, J.; Maslim, J.; Valter-Kocsi, K.; Mervin, K.; Bowers, F.; Chu, Y.; Barnett, N.; Provis, J.; Lewis, G.; Fisher, S.K.; et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999, 18, 689–735. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vlachantoni, D.; Bramall, A.N.; Murphy, M.P.; Taylor, R.W.; Shu, X.; Tulloch, B.; Van Veen, T.; Turnbull, D.M.; McInnes, R.R.; Wright, A.F. Evidence of severe mitochondrial oxidative stress and a protective effect of low oxygen in mouse models of inherited photoreceptor degeneration. Hum. Mol. Genet. 2011, 20, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32–42. [Google Scholar] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nölting, R.; Díaz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, Z.; Lu, S.; Liu, A.; Liu, X. Naringin attenuates diabetic reinopathy by inhibiting inflammation, oxidative stress and NF-κB activation in vivo and in vitro. Iran. J. Basic Med. Sci. 2017, 20, 813–821. [Google Scholar] [PubMed]
- Haskins, K.; Bradley, B.; Powers, K.; Fadok, V.; Flores, S.; Ling, X.; Pugazhenthi, S.; Reusch, J.; Kench, J. Oxidative stress in type 1 diabetes. Ann. N. Y. Acad. Sci. 2003, 1005, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987, 31, 291–306. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B.; Fine, S.L. Age-related macular degeneration. Surv. Ophthalmol. 1988, 32, 375–413. [Google Scholar] [CrossRef]
- Rupprecht, R.; Berning, B.; Hauser, C.A.; Holsboer, F.; Reul, J.M. Steroid receptor-mediated effects of neuroactive steroids: Characterization of structure-activity relationship. Eur. J. Pharmacol. 1996, 303, 227–234. [Google Scholar] [CrossRef]
- Garcia-Segura, L.; Melcangi, R. Steroids and glial cell function. Glia 2006, 54, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, P.; Cascio, C.; Russo, D.; D’Agostino, S.; Drago, G.; Galizzi, G.; De Leo, G.; Piccoli, F.; Guarneri, M.; Guarneri, R. Neurosteroids in the retina: Neurodegenerative and neuroprotective agents in retinal degeneration. Ann. N. Y. Acad. Sci. 2003, 1007, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.M.; Zorapapel, N.C.; Rich, K.A.; Wagstaff, R.E.; Lambert, R.W.; Rosenberg, S.E.; Moghaddas, F.; Pirouzmanesh, A.; Aoki, A.M.; Kenney, M.C. Effects of cholesterol and apolipoprotein E on retinal abnormalities in ApoE-deficient mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1891–1900. [Google Scholar]
- Sen, K.; Misra, A.; Kumar, A.; Pandey, R.M. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res. Clin. Pract. 2002, 56, 1–11. [Google Scholar] [CrossRef]
- Pajovic, S.B.; Saicic, Z.S.; Spasic, M.B.; Petrović, V.M.; Martinović, J.V. Effects of progesterone and estradiol benzoate on glutathione dependent antioxidant enzyme activities in the brain of female rats. Gen. Physiol. Biophys. 1999, 18, 35–44. [Google Scholar] [PubMed]
- Roof, R.L.; Hall, E.D. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000, 17, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Pajović, S.; Saicić, Z.S.; Spasić, M.B.; Petrović, V.M.; Martinović, J.V. Effect of progesterone and estradiol benzoate on superoxide dismutase activity in the brain of male rats. Experientia 1996, 52, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G.; Wright, D.W.; Kellermann, A.L. Does progesterone have neuroprotective properties? Ann. Emerg. Med. 2008, 51, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.J.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Cekic, M.; Sayeed, I.; Stein, D.G. Combination Treatment with Progesterone and Vitamin D Hormone May Be More Effective than Monotherapy for Nervous System Injury and Disease. Front. Neuroendocrinol. 2009, 30, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.A.; Gao, J.; Toda, I.; Rocha, E.M.; Ono, M.; Sullivan, D.A. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000, 78, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Swiatek-De Lange, M.; Stampfl, A.; Hauck, S.M.; Zischka, H.; Gloeckner, C.J.; Deeg, C.A.; Ueffing, M. Membrane-initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia 2007, 55, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Komeima, K.; Lee, S.Y.; Jo, Y.J.; Ueno, S.; Rogers, B.S.; Wu, Z.; Shen, J.; Lu, L.; Oveson, B.C.; et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol. Ther. 2009, 17, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Cayouette, M.; Behn, D.; Sendtner, M.; Lachapelle, P.; Gravel, C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J. Neurosci. 1998, 18, 9282–9293. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Yasumura, D.; Matthes, M.T.; Ruiz, A.; Duncan, J.L.; Chappelow, A.V.; Zolutukhin, S.; Hauswirth, W.; LaVail, M.M. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/ peripherin mutation. Exp. Eye Res. 2002, 74, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Rider, V.; Psychoyos, A. Inhibition of progesterone receptor function results in loss of basic fibroblast growth factor expression and stromal cell proliferation during uterine remodelling in the pregnant rat. J. Endocrinol. 1994, 140, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.A. Integration of progesterone receptor action with rapid signaling events in breast cancer models. J. Steroid Biochem. Mol. Biol. 2008, 108, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G. Brain damage, sex hormones and recovery: A new role for progesterone and estrogen? Trends Neurosci. 2001, 24, 386–391. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Pinilla, I.; Martín-Nieto, J.; Cuenca, N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Investih. Ophthalmol. Vis. Sci. 2011, 52, 4998–5008. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, J.M.; Martins, A.; Cruz, R.; Rodrigues, C.M.; Ambrósio, A.F.; Santiago, A.R. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience 2013, 253, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Usui, S.; Zafar, A.B.; Oveson, B.C.; Jo, Y.J.; Lu, L.; Masoudi, S.; Campochiaro, P.A. N-Acetylcysteine promotes long-term survival of cones in a model of retinitis pigmentosa. J. Cell. Physiol. 2011, 226, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Oveson, B.C.; Jo, Y.J.; Lauer, T.W.; Usui, S.; Komeima, K.; Xie, B.; Campochiaro, P.A. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox Signal. 2009, 11, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; O’Driscoll, C.; Kenna, P.; Cotter, T.G. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J. Neurochem. 2011, 118, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Navarro, S.; Trachesel-Moncho, L.; Fernandez-Carbonell, A.; Olivar, T.; Soria, J.M.; Almansa, I.; Miranda, M. Progesterone anti-inflamatory properties in hereditary retinal degeneration. J. Steroid Biochem. Mol. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wyse-Jackson, A.C.; Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Progesterone analogue protects stressed photoreceptors via bFGF-mediated calcium influx. Eur. J. Neurosci. 2016, 44, 3067–3079. [Google Scholar] [CrossRef] [PubMed]
- Chollangi, S.; Wang, J.; Martin, A.; Quinn, J.; Ash, J.D. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis. 2009, 34, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Ruiz-Lopez, A.M.; Roche, S.L.; Moloney, J.N.; Wyse-Jackson, A.C.; Cotter, T.G. The synthetic progestin norgestrel modulates Nrf2 signaling and acts as an antioxidant in a model of retinal degeneration. Redox Biol. 2016, 10, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, S.L. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018, 66, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.S.; Olsen, T.W.; Sayeed, I.; Cale, H.A.; Morrison, K.C.; Oumarbaeva, Y.; Lucaciu, I.; Boartright, J.H.; Pardue, M.T.; Stein, D.G. Progesterone Treatment in Two Rat Models of Ocular Ischemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Lipoic acid: A multifunctional antioxidant. Biofactors 2003, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Bramanti, V.; Tomassoni, D.; Bronzi, D.; Malfa, G.; Traini, E.; Napoli, M.; Renis, M.; Amenta, F.; Avola, R. Effect of lipoic acid and α-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J. Neurosci. Res. 2014, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Haenen, G.R.; Bast, A. Scavenging of hypochlorous acid by lipoic acid. Biochem. Pharmacol. 1991, 42, 2244–2246. [Google Scholar] [CrossRef]
- Moini, H.; Packer, L.; Saris, N.E. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Li, X.; Qu, Z.C.; Perriott, L.; Whitesell, R.R.; May, J.M. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002, 33, 83–93. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Dukic-Stefanovic, S.; Gasic-Milenkovic, J.; Schinzel, R.; Wiesinger, H.; Riederer, P.; Münch, G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur. J. Neurosci. 2001, 14, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Albertmann, K.; Bauerle, P.A. Nuclear factor kappa B: An oxidative stress-response transcription factor of eukaryotic cells. Free Rad. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Lodge, L.; Handelman, G.J.; Konishi, T.; Matsugo, S.; Mathur, V.V.; Packer, L. Natural sources of lipoic acid: Determination of lipoyllysine released from protease-digested tissues by high performance liquid chromatography incorporating electrochemical detection. J. Appl. Nutr. 1997, 49, 3–11. [Google Scholar]
- Marangon, K.; Devaraj, S.; Tirosh, O.; Packer, L.; Jialal, I. Comparison of the effect of alpha-lipoic acid and alpha-tocopherol supplementation on measures of oxidative stress. Free Radic. Biol. Med. 1999, 27, 1114–1121. [Google Scholar] [CrossRef]
- Spain, R.; Powers, K.; Murchison, C.; Heriza, E.; Winges, K.; Yadav, V.; Cameron, M.; Kim, E.; Horak, F.; Simon, J.; et al. Lipoic acid in secondary progressive MS: A randomized controlled pilot trial. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e374. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones 2006, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Estrada, D.E.; Ewart, H.S.; Tsakiridis, T.; Volchuk, A.; Ramlal, T.; Tritschler, H.; Klip, A. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: Participation of elements of the insulin signaling pathway. Diabetes 1996, 45, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Johnsen-Soriano, S.; Garcia-Pous, M.; Arnal, E.; Sancho-Tello, M.; Garcia-Delpech, S.; Miranda, M.; Bosch-Morell, F.; Diaz-Llopis, M.; Navea, A.; Romero, F.J. Early lipoic acid intake protects retina of diabetic mice. Free Radic. Res. 2008, 42, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Gupta, N.; Luczy-Bachman, G.; Limb, G.A.; Kuppermann, B.D.; Kenney, M.C. Protective effects of lipoic acid on chrysene-induced toxicity on Müller cells in vitro. Mol. Vis. 2013, 19, 25–38. [Google Scholar] [PubMed]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. A Clinical Trial about a Food Supplement Containing α-Lipoic Acid on Oxidative Stress Markers in Type 2 Diabetic Patients. Int. J. Mol. Sci. 2016, 17, 1802. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Cheng, W.S.; Chen, J.L.; Chiang, C.H. Potential of nonoral α-lipoic acid aqueous formulations to reduce ocular microvascular complications in a streptozotocin-induced diabetic rat model. J. Ocul. Pharmacol. Ther. 2013, 29, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus(®) Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Marrazzo, G.; Platania, C.B.; Drago, F.; Leggio, G.M.; Salomone, S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J. Diabetes Res. 2013, 2013, 432695. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, C.G.; Yun, I.H.; Hur, D.Y.; Yang, J.W.; Kim, H.W. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin. Exp. Ophthalmol. 2012, 40, e47–e57. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.; Choi, M.Y.; Lee, D.H.; Roh, G.S.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Hong, E.K.; Choi, W.S. Alpha-lipoic acid reduces retinal cell death in diabetic mice. Biochem. Biophys. Res. Commun. 2018, 503, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, C.; Geelen, M.J.; Velasco, G.; Guzman, M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS. Lett. 2001, 489, 149–153. [Google Scholar] [CrossRef]
- Kan, E.; Alici, Ö.; Kan, E.K.; Ayar, A. Effects of alpha-lipoic acid on retinal ganglion cells, retinal thicknesses, and VEGF production in an experimental model of diabetes. Int. Ophthalmol. 2017, 37, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death retinitis pigmentosa. J. Cell. Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Cotter, T.G. Oxidative stress induces caspaseindependent retinal apoptosis in vitro. Cell. Death Differ. 2000, 7, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yan, W.; Beight, C. Lutein and Zeaxanthin Isomers Reduce Photoreceptor Degeneration in the Pde6b rd10 Mouse Model of Retinitis Pigmentosa. Biomed. Res. Int. 2018, 2018, 4374087. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Zheng, C.P. (3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one ameliorates retinal degeneration in Pde6brd10 mice. Cutan. Ocul. Toxicol. 2018, 37, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Emoto, Y.; Yoshizawa, K.; Kinoshita, Y.; Yuri, T.; Yuki, M.; Sayama, K.; Shikata, N.; Tsubura, A. Green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. Graefes. Arch. Clin. Exp. Ophthalmol. 2014, 252, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Brito-García, N.; Del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Coco, R.M.; Rodríguez de la Rúa, E.; Del Cura-González, I.; Serrano-Aguilar, P. Effectiveness and safety of nutritional supplements in the treatment of hereditary retinal dystrophies: A systematic review. Eye 2017, 31, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hughbanks-Wheaton, D.K.; Birch, D.G.; Fish, G.E.; Spencer, R.; Pearson, N.S.; Takacs, A.; Hoffman, D.R. Safety assessment of docosahexaenoic acid in X-linked retinitis pigmentosa: The 4-year DHAX trial. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4958–4966. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Gonzalez-Usano, A.; Agusti, A.; Balzano, T.; Llansola, M.; Felipo, V. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflamm. 2016, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rabaza, V.; Cabrera-Pastor, A.; Taoro-González, L.; Malaguarnera, M.; Agustí, A.; Llansola, M.; Felipo, V. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: Reversal by sulforaphane. J. Neuroinflamm. 2016, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular mechanism of Nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Free radicals, antioxidants and functional foods: Impact on human health. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [PubMed]
- Gao, X.; Dinkova-Kostova, A.T.; Talalay, P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: The indirect antioxidant effects of sulforaphane. Proc. Natl. Acad. Sci. USA 2001, 98, 15221–15226. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Talalay, P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc. Natl. Acad. Sci. USA 2004, 101, 10446–10451. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Masutani, H.; Kim, Y.C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, X.; Cai, B.; Sparrow, J.R. Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuv. Res. 2006, 9, 256–263. [Google Scholar] [CrossRef] [PubMed]
- del V Cano, M.; Reyes, J.M.; Park, C.Y.; Gao, X.; Mori, K.; Chuck, R.S.; Gehlbach, P.L. Demonstration by redox fluorometry that sulforaphane protects retinal pigment epithelial cells against oxidative stress. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yu, T.; Li, Y.; Chen, B.; Zhang, J.; Wen, Z.; Zhang, B.; Zhou, X.; Li, X.; Li, F.; et al. Sulforaphane enhances the ability of human retinal pigment epithelial cell against oxidative stress, and its effect on gene expression profile evaluated by microarray analysis. Oxid. Med. Cell. Longev. 2013, 2013, 413024. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, B.; Zhang, C.; Wang, B.; Wang, H.; Song, X.; Yang, Y.; Ren, X.; Yin, L.; Kong, H.; et al. The therapeutic potential of sulforaphane on light-induced photoreceptor degeneration through antiapoptosis and antioxidant protection. Neurochem. Int. 2016, 100, 52–61. [Google Scholar] [CrossRef] [PubMed]
- O’Mealey, G.B.; Berry, W.L.; Plafker, S.M. Sulforaphane is a Nrf2-independent inhibitor of mitochondrial fission. Redox Biol. 2017, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Dulull, N.K.; Dias, D.A.; Thrimawithana, T.R.; Kwa, F.A.A. L-Sulforaphane Confers Protection against Oxidative Stress in an in Vitro Model of Age-Related Macular Degeneration. Curr. Mol. Pharmacol. 2018, 11, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Pasovic, L.; Eidet, J.R.; Lyberg, T.; Messelt, E.B.; Aabel, P.; Utheim, T.P. Antioxidants Improve the Viability of Stored Adult Retinal Pigment Epithelial-19 Cultures. Ophthalmol. Ther. 2014, 3, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.J.; Dickinson, S.E.; Karlage, K.L.; Bowden, G.T.; Myrdal, P.B. Stability of sulforaphane for topical formulation. Drug Dev. Ind. Pharm. 2014, 40, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Yu, M. Protective effect of sulforaphane against retinal degeneration in the Pde6rd10 mouse model of retinitis pigmentosa. Curr. Eye Res. 2017, 42, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Ferland-McCollough, D.; Slater, S.; Richard, J.; Reni, C.; Mangialardi, G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol. Ther. 2017, 171, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; He, M.; Liu, R.; Brecha, C.; Yu, A.C.; Pu, M. Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS ONE 2014, 9, e114186. [Google Scholar] [CrossRef] [PubMed]
- Ambrecht, L.A.; Perlman, J.I.; McDonnell, J.F.; Zhai, Y.; Qiao, L.; Bu, P. Protection of retinal function by sulforaphane following retinal ischemic injury. Exp. Eye Res. 2015, 138, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tanito, M.; Huang, Z.; Li, F.; Zhou, X.; Zaharia, A.; Yodoi, J.; McGinnis, J.F.; Cao, W. Delay of photoreceptor degeneration in tubby mouse by sulforaphane. J. Neurochem. 2007, 101, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Federici, M.; Rusciano, D.; Evangelista, M.; Pescosolido, N. Oxidative stress in preretinopathic diabetes subjects and antioxidants. Diabetes Technol. Ther. 2012, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.D.; Dong, Y.D.; Fan, R.; Zhai, L.L.; Bai, Y.L.; Jia, L.H. Effect of (R)-α-lipoic acid supplementation on serum lipids and antioxidative ability in patients with age-related macular degeneration. Ann. Nutr. Metab. 2012, 60, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Jiang, P.; Wei, Y.; Wang, P.; Sun, X.; Wang, H. α-Lipoic Acid Treatment Improves Vision-Related Quality of Life in Patients with dry Age-related macular degeneration. Tohoku J. Exp. Med. 2016, 240, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Haritoglou, C.; Gerss, J.; Hammes, H.P.; Kampik, A.; Ulbig, M.W.; RETIPON Study Group. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica 2011, 226, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Abd El-Twab, S.M.; Abdel-Reheim, E.S. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: The underlying mechanism. Eur. J. Nutr. 2017, 56, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Thiraphatthanavong, P.; Thukham-Mee, W.; Muchimapura, S.; Wannanond, P.; Tong-Un, T. Anticataractogenesis and Antiretinopathy Effects of the Novel Protective Agent Containing the Combined Extract of Mango and Vietnamese Coriander in STZ-Diabetic Rats. Oxid. Med. Cell. Longev. 2017, 2017, 5290161. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
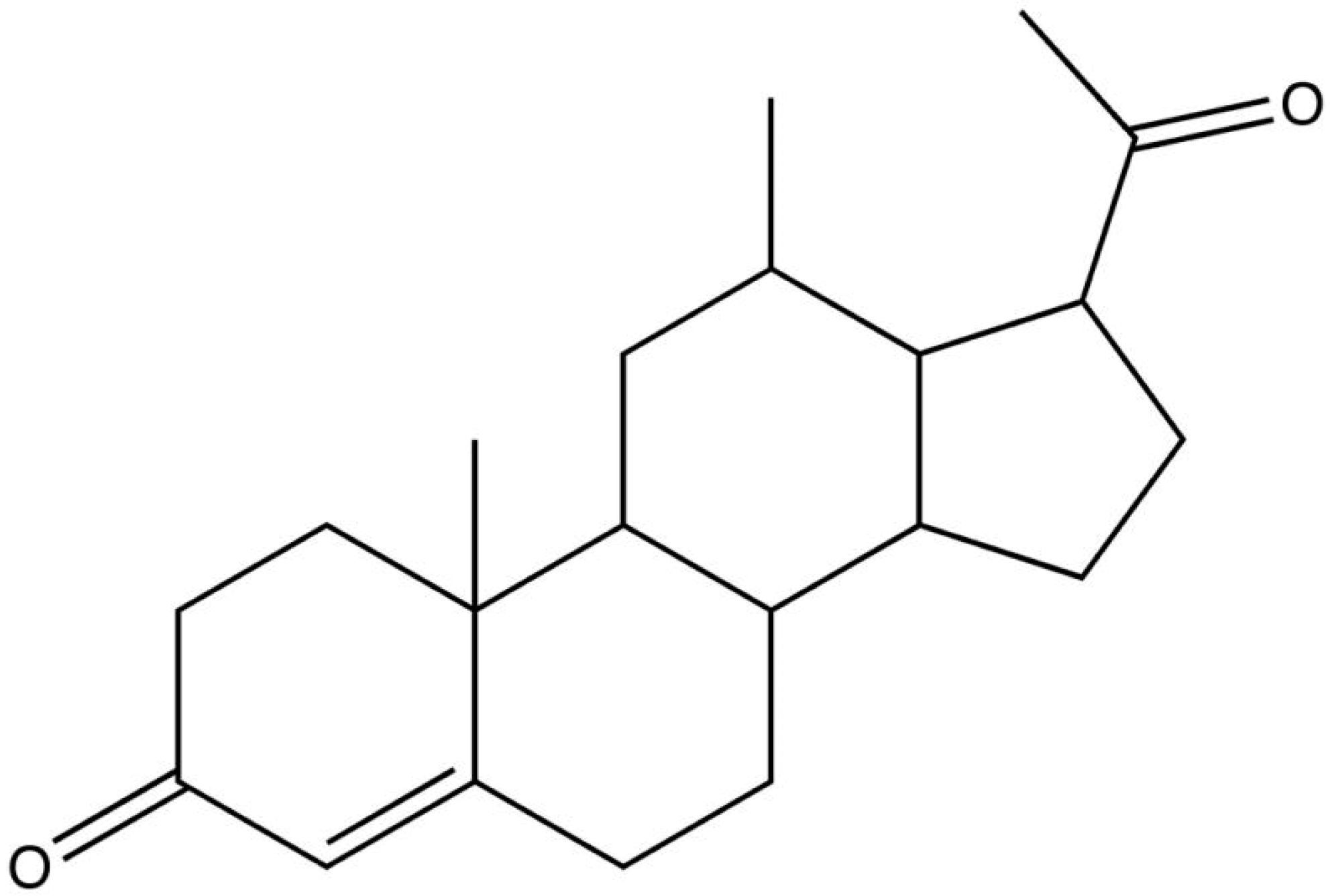

| Antioxidant: Progesterone | ||
|---|---|---|
| Retinitis Pigmentosa | ||
| Authors | Study Type and Treatments | Results |
| Doonan, F. et al., 2011. [55] | The light damage model and the rd10 model. Treatment: 100 mg/kg Norgestrel, i.p. Treatment was on alternate days commencing at P18. | Decreased photoreceptors apoptosis and improved electroretinogram |
| Sánchez-Vallejo, V. et al. 2015. [41] | rd1 model Treatment: 100 mg/kg progesterone, o.a. Treatment was on alternate days starting at P7. | Decreased cell death Reduced glial reaction Decreased oxidative stress |
| Wyse-Jackson, A.C. et al. 2016. [57] | Retina explants and photoreceptor derived 661W cell line. Treatment: 20 µM Norgestrel in vitro studies. | Increased in the quantity of PGRMC1. Cytoprotective effect |
| Byrne, A.M. et al. 2016. [59] | Light damage model Treatment:100 mg/kg Norgestrel, i.p | Rescue of photoreceptor cells from light-induced ROS production and cell death. Reduced morphological damage. Increased expression of Nrf2 and SOD2 |
| Roche, S.L. et al. 2017 [60] | rd10 model Treatment: Norgestrel was added to the chow at a concentration of 0.05% (500 ppm). | Decreased microglial activity and Müller cell gliosis |
| Benlloch-Navarro, S. et al. 2019 [56] | rd10 model Treatment: 100 mg/kg progesterone, o.a. | Decreased retinal malondialdehyde, a lipid peroxidation product. Decreased photoreceptors cell death and reactive gliosis. |
| Ocular Ischemia | ||
| Authors | Study Type and Treatments | Results |
| Allen, R.S. et al. 2015. [61] | Middle cerebral artery occlusion model. Treatment: 8 mg/kg progesterone i.p. at 1 h post injury, and then s.c. at 6, 24, and 48 h. | Reduced ERG deficits, upregulation of glutamine synthetase and GFAP, and cytoprotection (retinal ganglion). |
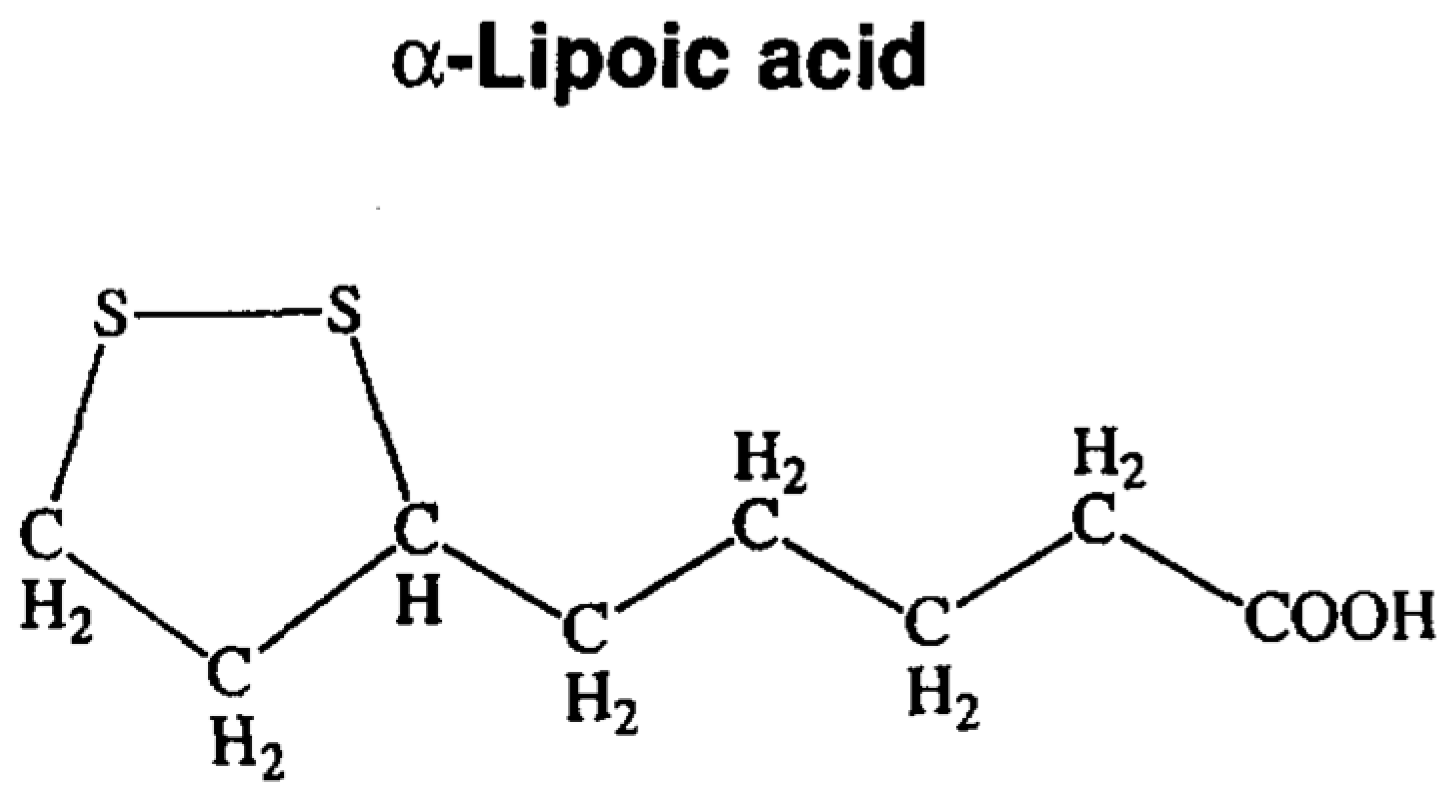
| Antioxidant: Lipoic Acid | ||
|---|---|---|
| Diabetes Retinopathy | ||
| Authors | Study Type and Treatments | Results |
| Lee, S.G., et al., 2012. [88] | Streptozotocin-induced diabetic rats LA Treatment: 10 mg/kg o.a. | LA oral: Suppresses superoxide formation and increases NADPH oxidase. Reduces expression of VEGF, erythropoietin and angiopoietin 2. |
| Chen, C.L., et al., 2013. [85] | Streptozotocin-induced diabetic rats Treatment: 30 mg/kg/day LA i.p. 1% LA eyedrop 1% LA 1% HA intravitreal 1% LA 3% HA intravitreal | Decrease in fluorescein leakage from ocular vascular vessels and decrease in vascular lesion after LA administration (i.p.). |
| Bucolo, C. et al., 2013. [87] | Streptozotocin-induced diabetic rats LA Treatment: Fortified extract—containing LA (300mg/kg) among others (i.p.) | Treatment with fortified extract reduces retinal TNF-α and VEGF and suppresses lipid peroxidation in the plasma, in diabetic rats. |
| Kowrulu, R.A., et al., 2014. [84] | Streptozotocin-induced diabetic rats LA Treatment: Nutritional supplement—each kilogram contains 750 mg among others | Treatment with nutritional supplement: Decreases cell death, decreases VEGF and anti-inflammatory respond. Increases mitochondrial DNA-encoded proteins. Decreases total ROS levels. Prevents ERG alterations induced in diabetic rats. |
| Alvarez-Rivera, F., et al., 2016. [86] | LA ≥ 99% Soluplus® Bovine corneas | LA with Soluplus ® (Ludwigshafen, Germany) increases the LA presence in the bovine cornea. |
| Kan, E., et al., 2017. [91] | Streptozotocin-induced diabetic mice (BALB/C) LA Treatment: 100 mg/kg i.p. | LA i.p.: Increases the thicknesses of total retina, ONL, and inner nuclear layer; the number of retinal ganglion cells and decreases the VEGF expression in diabetic retinas. |
| Kim, Y.S., et al., 2018. [89] | Streptozotocin-induced diabetic mice LA Treatment: 200mg/kg o.a. | LA oral: Decreases 4-hydroxynonenal and increases glutathione peroxidase. Reverses the decreases activation AMP-activated protein kinase. Decreases the levels of O-GlcNAc transferase. Increases proliferator-activated receptor family of nuclear receptors delta and sirtuin3. Decreases cleaved poly adenosine diphosphate Ribose polymerases and cleaved caspase-3. |
| Retinitis Pigmentosa | ||
| Authors | Study Type and Treatments | Results |
| Komeima, K., et al., 2006. [2] | rd1 model Mixture of antioxidants: 100 mg/kg LA among others | Mixture of antioxidants: Decreases acrolein and reductions of carbonyl adducts Increases cones density. Improves the function of the retina measured by ERG—mean b-wave amplitude. LA: Increases cones density. |
| Komeima, K., et al., 2007. [99] | rd10 model and Q344ter model Mixture of antioxidants: 100 mg/kg LA among others | rd10 model: Preservation of cone density. Improves the function of the retina measured by ERG. Slow rod cell death. Q344ter model: Preservation of cone density. |
| Sanz, M.M., et al., 2007. [3] | rd1 model Mixture of antioxidants: 10 mg/kg LA among others | Mixture of antioxidants: Rescues the number of rows. Decreases avidin and TUNEL positive cells in the ONL. |
| Miranda, M., et al., 2010. [1] | rd1 model Mixture of antioxidants: 10 mg/kg LA among others | Mixture of antioxidants: Decreases avidin and TUNEL positive cells in the ONL. Increases glutathione peroxidase activity and GSH concentration. Decreases Cyss concentration. No changes in NADPH-diaphorase, neuronal oxide nitric synthase positive cells and nytrotyrosine concentration. |
| Ramírez-Lamelas, D.T., et al., 2018. [5] | rd1 model LA 100 mg/kg and/or progesterone 100 mg/kg o.a. | Decreases TUNEL-positive cells. Decreases glial fibrillary acidic protein staining. Increases GSH concentration. No changes in glutamate-cysteine ligase catalytic subunit staining. |
| Antioxidant: Sulforaphane | ||
|---|---|---|
| Retinal Pigment Epithelial Cell Damage. Macular Degeneration | ||
| Authors | Study Type and Treatments | Results |
| Gao, X., et al., 2001. [107] | ARPE-19 cell line. Oxidative stressors: chemical SFN treatment: 5mM, final concentration with culture medium 0.16–5.0 μM. In vitro study | Cytoprotective effect (cell death reduction). Induction of phase 2 genes. |
| Gao, X., et al., 2004. [108] | ARPE-19 cell line and mice fibroblast Knockout cells. Oxidative stressors: photooxydation + retinoid SFN treatment: (0–2.5 μM) In vitro study | Antiapoptotic and cytoprotective effects. Induction of phase 2 genes. |
| Tanito, M., et al., 2006. [109] | BALB/c mice Oxidative stressor: photooxydation SFN treatment: 0.1 or 0.5 mg/d, 5 days, i.p. 0.5/25µL 7 days, o.a. In vivo study K-1034 cell line. Oxidative stressors: Chemical (H2O2) SFN treatment: 0–10μM (24 or 48 h) 30 μM (6 h). In vitro study | In vivo: Cytoprotective effect and retinal function recovery. Induction of the Trx system. In vitro: SFN does not produce cellular damage (10 μM-48 h). ARE promoter involvement in Trx regulation. Activation of ARE sequence of Trx gene by the Nrf2, small Maf or c-Jun binding proteins. |
| Zhou, J. et al., 2006. [110] | ARPE-19 cell line. Oxidative stressors: chemical (lipofuscin fluorophore A2E) + photooxidation. (Buthionine sulfoximine treatment: to decrease GSH). SFN treatment: 5-μM sulforaphane for 48 h. In vitro study. | Increased expression and gene activity of enzymes with reductase activity, including GSH, GST, and NQO1. |
| del V Cano, M., et al., 2008. [111] | ARPE-19 cell line. Oxidative stressors: Chemical stressors SFN treatment: 4 μM (24 h). In vitro study | Regulates the redox ratio and increases cell viability against oxidative stress. |
| Ye, L. et al., 2013. [112] | ARPE-19 cell line. Oxidative stressors: chemical (H2O2) SFN treatment:10 μM (12 h) In vitro study | Increase cell viability and antioxidant mechanisms. Identification of several genes induced by SFN, including those related to the response to oxidative stress. An increase of Nrf2 translocation. |
| Kong, L., et al., 2016. [113] | BALB/cJ mice animal model. Oxidative stressors: photooxydation SFN treatment: 18 mg/kg, 7 days, intracardially. In vivo study. | Retinal protection, to cell and functional level. Increased expression of Trx and Nrf2 and their regulatory elements Ras and ERK. Antiapoptotic effect. Reduction of the mitochondrial intrinsic and caspase-3 antiapoptotic pathways. |
| O’Mealey, G.B., et al., 2017. [114] | RPE-1 cell line. Cells transfected with siRNA. Apoptotic inducer: staurospine. SFN treatment: 50 μM (2 h). In vitro study. | SFN induces mitochondrial fusion, but independent of the Keap1-Nrf2-ARE pathway. Mechanism: depletion of the fission machinery. Antiapoptotic effect independent of Nrf2. |
| Dulull, N.K. et al., 2018. [115] | ARPE-19 cell line. Oxidative stressors: chemical (H2O2). In vitro study | Cytoprotective effect. Induction of phase 2 enzymes (Glutathione-S-Transferase gene). Metabolic effect. |
| Retinitis Pigmentosa | ||
| Authors | Study Type and Treatments | Results |
| Kang, K., et al., 2017. [118] | Pde6b rd10 animal model, C57/BL6 wild type. SFN: 35 mg/kg, 15 days (from P6 to P20); i.p. | Improvement of retinal function. Reduction of cell degeneration. |
| Diabetes Retinopathy | ||
| Authors | Study Type and Treatments | Results |
| Maeda, S. et al., S.2014. [122] | Bovine retinal pericytes treated with or without AGE-BSA (Bovine serum albumin). SFN: 0.1 or 0.4 μmol/L sulforaphane. | Reduces the AGE effects on pericytes through the antioxidative mechanisms. Mechanisms: suppression of RAGE expression. |
| Retinal Ischemia Reperfusion | ||
| Authors | Study Type and Treatments | Results |
| Pan, H., et al., 2014. [123] | Male Sprague-Dawley rats. Animal model by increasing the intraocular pressure SFN: 12.5 mg/kg, 7 consecutive days; i.p. In vivo experiment | Cytoprotective (ganglion and amacrine cells), anti-inflammatory and antioxidant effects. Increase in the Nrf2 nuclear translocation and the HO-1 levels. |
| Ambrecht, L.A. et al., 2015. [124] | C57BL/6 mice. Animal model by increasing the intraocular pressure. SFN: 25mg/kg, 5 consecutive day; i.p. In vivo experiment | Cytoprotective (retinal morphology) and functional recovery (changes in ERG responses). |
| Usher Syndrome (Tubby Model Mice) | ||
| Authors | Study Type and Treatments | Results |
| Kong, Li., et al., 2007. [125] | Homozygous tubby mice. C57BL/6J wild type. SFN: 25, 50 or 75 mg/kg. 5 consecutive days (P10–P14) 25 mg/kg from P14 to P34; i.p. Some animals: ERK inhibitor PD98059; i.p. | Cytoprotective effect. Increased Nrf2 retinal level. Reversion of a damaged Trx and TRXR endogenous system. Regulation of the SFN response by external kinases signals. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rabaza, V.; López-Pedrajas, R.; Almansa, I. Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review. Antioxidants 2019, 8, 53. https://doi.org/10.3390/antiox8030053
Hernández-Rabaza V, López-Pedrajas R, Almansa I. Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review. Antioxidants. 2019; 8(3):53. https://doi.org/10.3390/antiox8030053
Chicago/Turabian StyleHernández-Rabaza, Vicente, Rosa López-Pedrajas, and Inmaculada Almansa. 2019. "Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review" Antioxidants 8, no. 3: 53. https://doi.org/10.3390/antiox8030053
APA StyleHernández-Rabaza, V., López-Pedrajas, R., & Almansa, I. (2019). Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review. Antioxidants, 8(3), 53. https://doi.org/10.3390/antiox8030053




