Non-Mammalian Prdx6 Enzymes (Proteins with 1-Cys Prdx Mechanism) Display PLA2 Activity Similar to the Human Orthologue
Abstract
:1. Introduction
- TaPER1 from Triticum aestivum: its gene is present as a single copy in the plant genome and is expressed mainly in the cytoplasm and nucleus during the development and germination of wheat seeds [20].
- AtPER1 from Arabidopsis thaliana: its gene is also a single copy and its expression is induced by abscisic acid and oxidative stress [21]. AtPER1 also presents nuclear and cytoplasm localization [22]. Although knockout mutants for this gene are viable (unpublished data), overexpression or down-regulation of the AtPER1 gene affect seed germination during abiotic stresses [23].
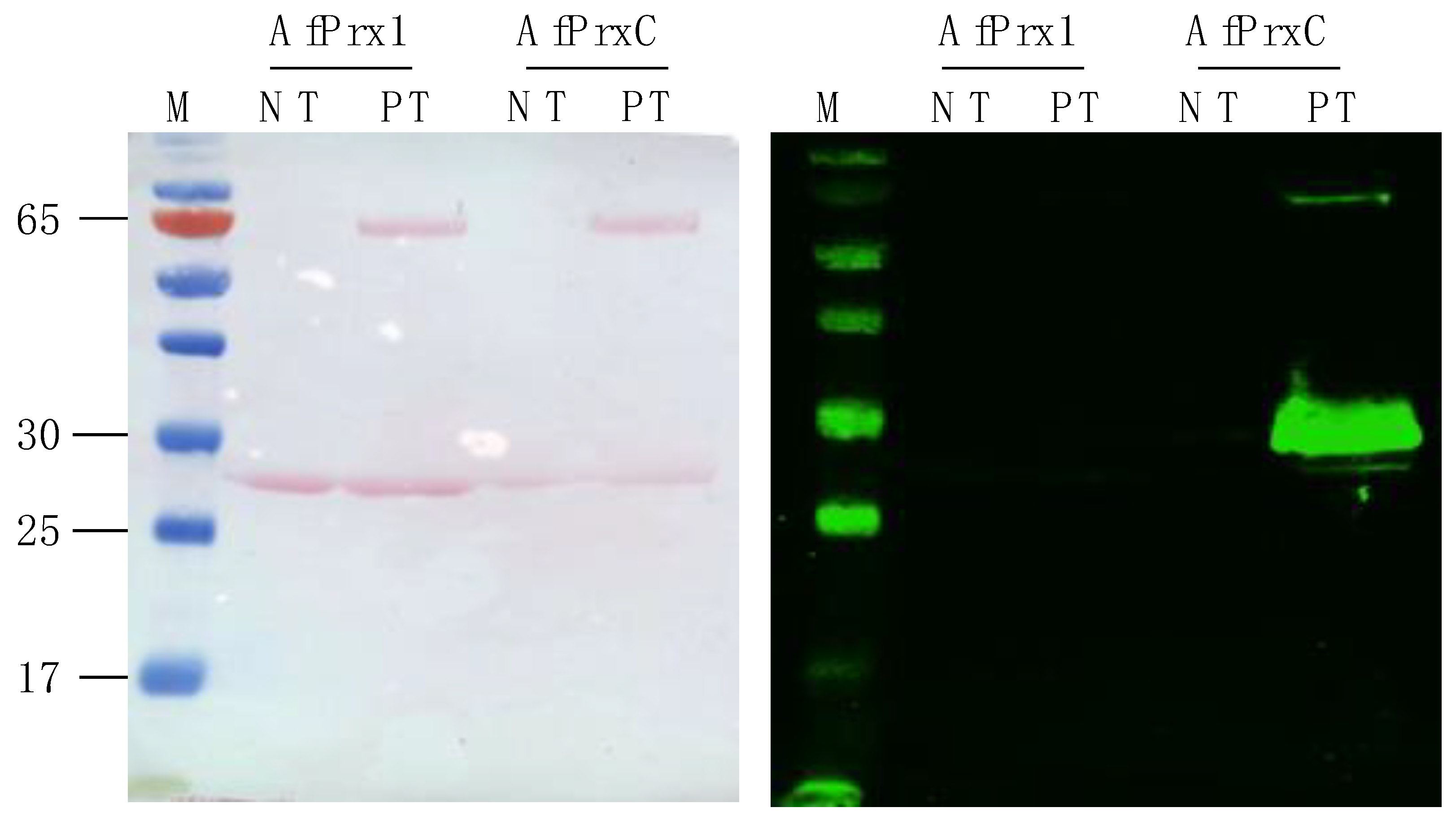
- AfPrx1 from Aspergillus fumigatus is a cytosolic protein that displays high reactivity for H2O2 (k = 2.28 × 107 M−1 s−1) [24]. Moreover, the AfPrx1 is essential for the decomposition of exogenously added H2O2 as shown by the investigation of a knockout strain (Δafprx1) [25]. The strain Δafprx1 is also more sensitive to other stressors’ molecules. Additionally, AfPrx1 plays a role in fungus virulence in neutropenic murine models [24].
- AfPrxC from Aspergillus fumigatus is a mitochondrial protein that also reacts rapidly with H2O2 (k = 2.28 × 107 M−1 s−1) [24].
2. Materials and Methods
2.1. Sequence and Structural Analysis
2.2. Cloning, Expression and Purification of Recombinant Proteins
2.2.1. Expression Procedures
2.2.2. Purification
2.3. Preparation of Unilamellar Liposomes
2.4. Evaluation of PLA2 Activity
2.5. Phosphorylation Treatments of Non-Mammalian Prdx6
2.6. Reduction, Alkylation and Tryptic Digestion for MS
2.7. Evaluation of Thr Phosphorylation by MS
2.8. Evaluation of Prdx Phosphorylation by Western Blot
3. Results
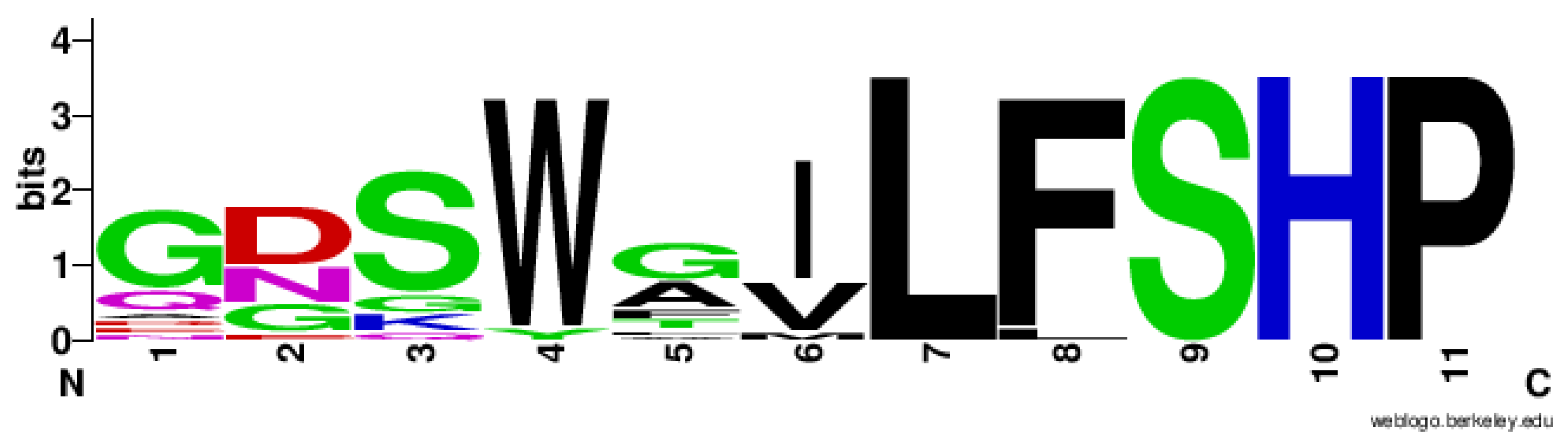
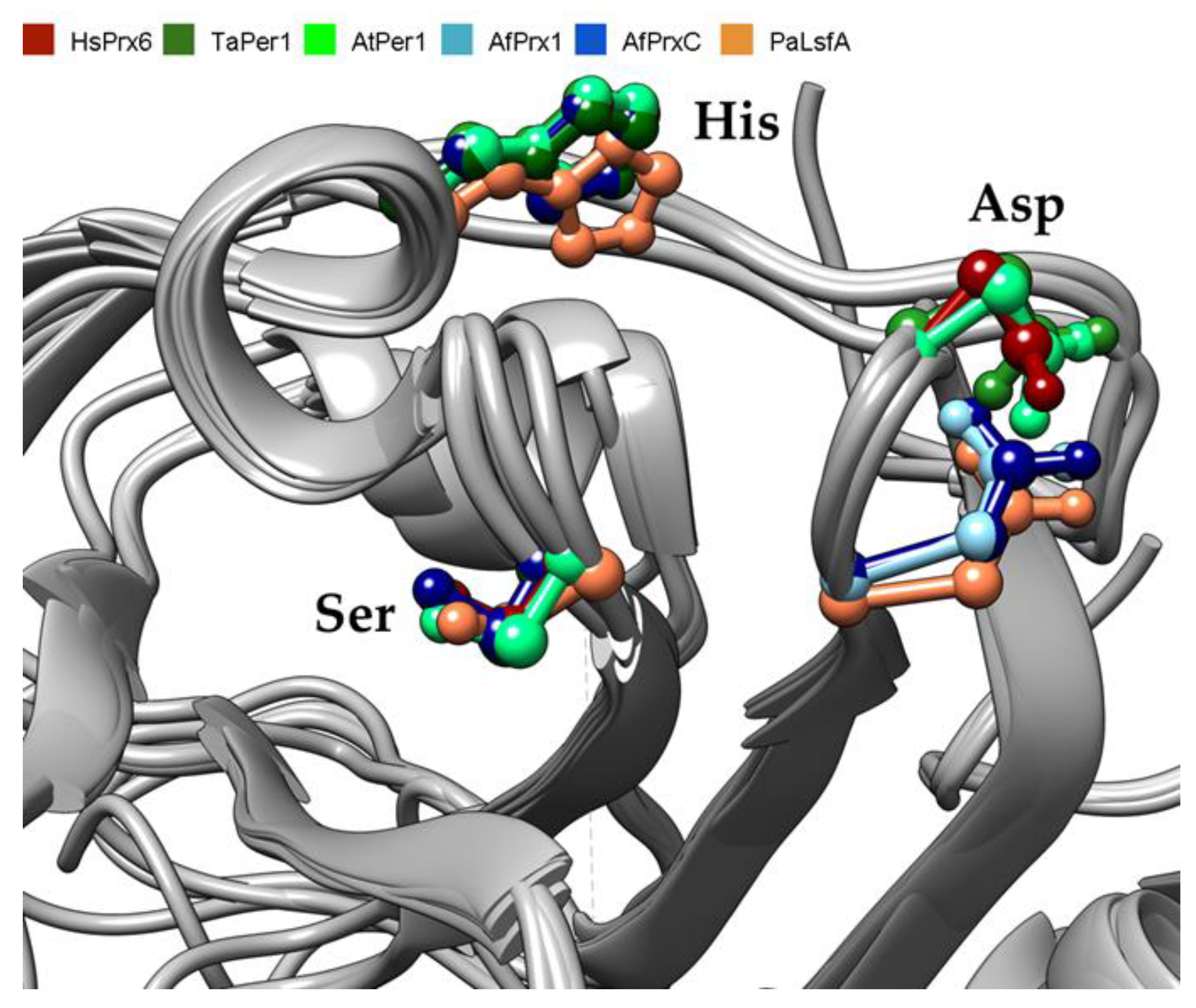
3.1. Conservation of PLA2 Catalytic Triad in Non-Mammalian Prdx6
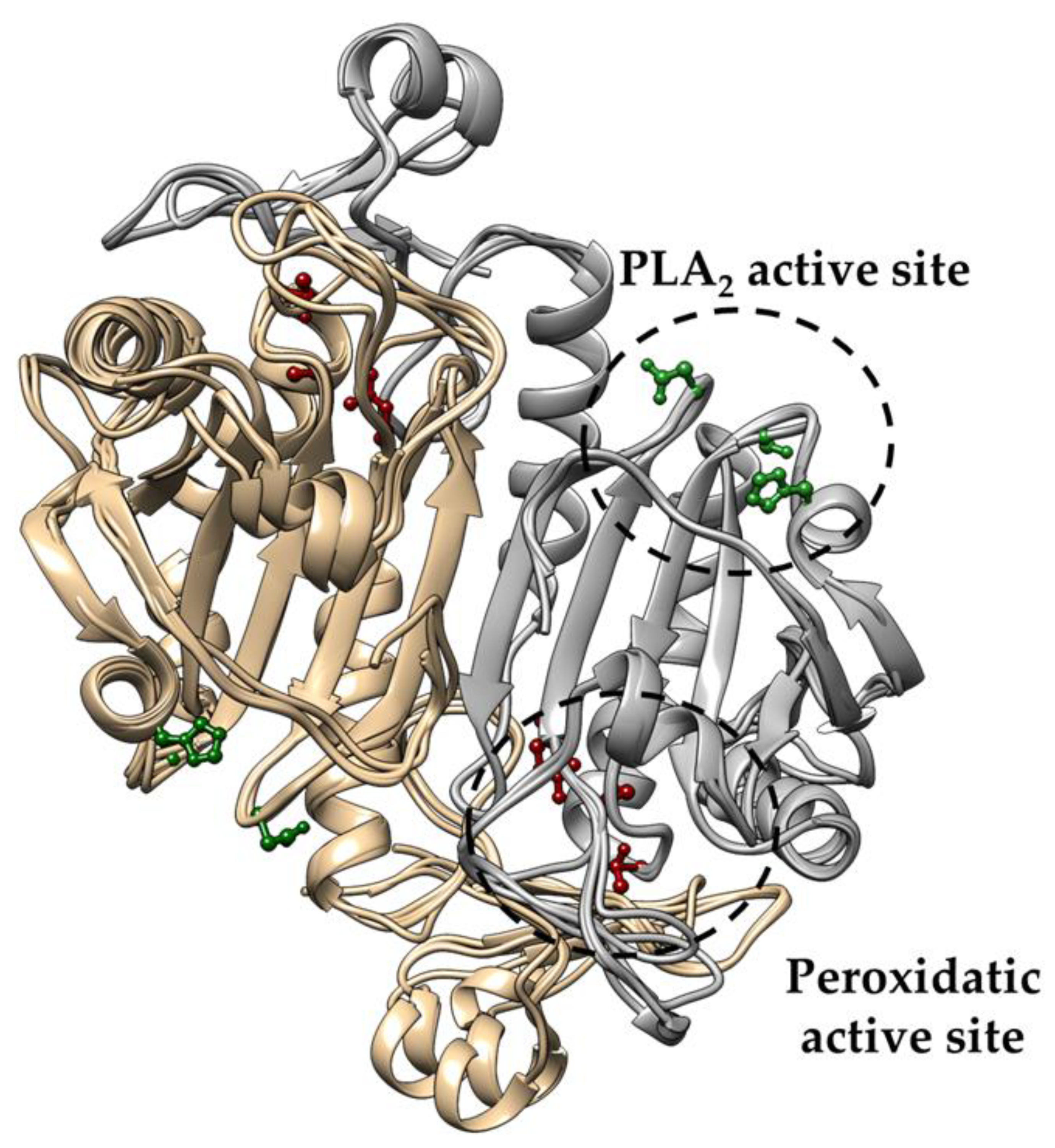
3.2. Structural Conservation of Prdx6 Motifs in TaPER1, AtPER1, AfPrx1, AfPrxC, and PaLsfA
3.3. Acidic, Ca2+-Independent PLA2 (aiPLA2) Activity of Non-Mammalian Prdx6
3.4. Phosphorylation at the C-terminal Thr Is Also Conserved Among Non-Mammalian Prdx6
3.5. Influence of Phosphorylation and Inhibition with MJ33 on PLA2 Activity of AfPrx1 and AfPrxC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perkins, A.; Poole, L.B.; Karplus, P.A. Tuning of peroxiredoxin catalysis for various physiological roles. Biochemistry 2014, 53, 7693–7705. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.Z.; Robison, K.; Poole, L.B.; Church, G.; Storz, G.; Rhee, S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: Alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 1994, 91, 7017–7021. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Toppo, S.; Cozza, G.; Ursini, F. A Comparison of Thiol Peroxidase Mechanisms. Antioxid. Redox Signal. 2011, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J. Mol. Biol. 2010, 402, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Soito, L.; Williamson, C.; Knutson, S.T.; Fetrow, J.S.; Poole, L.B.; Nelson, K.J. PREX: PeroxiRedoxin classification indEX, a database of subfamily assignments across the diverse peroxiredoxin family. Nucleic Acids Res. 2011, 39, D332–D337. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J. 1-Cysteine peroxiredoxin: A dual-function enzyme with peroxidase and acidic Ca2+-independent phospholipase A2 activities. Biochimie 2010, 92, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Sundaresh, C.S.; Feinstein, S.I.; Dodia, C.; Skach, W.R.; Jain, M.K.; Nagase, T.; Seki, N.; Ishikawa, K.; Nomura, N.; et al. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J. Biol. Chem. 1997, 272, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Dodia, C.; Manevich, Y.; Chen, J.; Feinstein, S. Phospholipid hydroperoxidase are substrates for non-selenium glutathione peroxidase. J. Biol. Chem. 1999, 274, 21326–21334. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Abe, A.; Shayman, J.A. Structure and function of lysosomal phospholipase A2: Identification of the catalytic triad and the role of cysteine residues. J. Lipid Res. 2005, 46, 2441–2447. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E. A Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Dodia, C.; Feinstein, S.I.; Jain, M.K.; Fisher, A.B. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 2000, 275, 28421–28427. [Google Scholar] [CrossRef] [PubMed]
- Manevich, Y.; Reddy, K.S.; Shuvaeva, T.; Feinstein, S.I.; Fisher, A.B. Structure and phospholipase function of peroxiredoxin 6: Identification of the catalytic triad and its role in phospholipid substrate binding. J. Lipid Res. 2007, 48, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, E.M.; Feinstein, S.I.; Milovanova, T.N.; Fisher, A.B. Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L871–L880. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, E.M.; Dodia, C.; Zhou, S.; Tao, J.Q.; Gao, L.; Raabe, T.; Feinstein, S.I.; Fisher, A.B. Mutation of serine 32 to threonine in peroxiredoxin 6 preserves its structure and enzymatic function but abolishes its trafficking to lamellar bodies. J. Biol. Chem. 2016, 291, 9268–9280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Feinstein, S.I.; Manevich, Y.; Chowdhury, I.; Pak, J.H.; Kazi, A.; Dodia, C.; Speicher, D.W.; Fisher, A.B. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A 2 activity. Biochem. J. 2009, 419, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L748–L754. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Feinstein, S.I.; Dodia, C.; Sorokina, E.; Lien, Y.-C.; Nguyen, S.; Debolt, K.; Speicher, D.; Fisher, A.B. Peroxiredoxin 6 Phosphorylation and Subsequent Phospholipase A 2 Activity Are Required for Agonist-mediated Activation of NADPH Oxidase in Mouse Pulmonary Microvascular Endothelium and Alveolar Macrophages. J. Biol. Chem. 2011, 286, 11696–11706. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R.; Ellison, M.A.; Thurman, G.W.; Leto, T.L. Peroxiredoxin 6 translocates to the plasma membrane during neutrophil activation and is required for optimal NADPH oxidase activity. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.-N.; Lee, S.-S.B.; Lee, S.-S.B.; Yoon, S.H.; Kang, G.Y.; Hwang, S.-G.; Um, H.-D. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol. Cancer Ther. 2010, 9, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Pulido, P.; Cazalis, R.; Cejudo, F.J. An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. Plant J. 2009, 57, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Haslekås, C.; Grini, P.E.; Nordgard, S.H.; Thorstensen, T.; Viken, M.K.; Nygaard, V.; Aalen, R.B. ABI3 mediates expression of the peroxiredoxin antioxidant AtPER1 gene and induction by oxidative stress. Plant Mol. Biol. 2003, 53, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Haslekås, C.; Stacy, R.A.; Nygaard, V.; Culiáñez-Macià, F.A.; Aalen, R.B. The expression of a peroxiredoxin antioxidant gene, AtPER1, in Arabidopsis thaliana is seed-specific and related to dormancy. Plant Mol. Biol. 1998, 36, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Haslekas, C. Seed 1-Cysteine Peroxiredoxin Antioxidants Are Not Involved in Dormancy, But Contribute to Inhibition of Germination during Stress. PLANT Physiol. 2003, 133, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.C.; de Godoy, K.F.; Bannitz-Fernandes, R.; Fabri, J.H.T.M.; Barbosa, M.M.F.; de Castro, P.A.; Almeida, F.; Goldman, G.H.; da Cunha, A.F.; Netto, L.E.S.; et al. Analyses of the three 1-Cys Peroxiredoxins from Aspergillus fumigatus reveal that cytosolic Prx1 is central to H2O2 metabolism and virulence. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.S.; Bannitz-Fernandes, R.; Lima, A.S.; Tairum, C.A.; Malavazi, I.; Netto, L.E.S.; Bertotti, M. Monitoring H2O2 inside Aspergillus fumigatus with an integrated microelectrode: The role of peroxiredoxin protein Prx1. Anal. Chem. 2018, 90, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Hummerjohann, J.; Küttel, E.; Quadroni, M.; Ragaller, J.; Leisinger, T.; Kertesz, M.A. Regulation of the sulfate starvation response in Pseudomonas aeruginosa: Role of cysteine biosynthetic intermediates. Microbiology 1998, 144, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Kaihami, G.H.; de Almeida, J.R.F.; dos Santos, S.S.; Netto, L.E.S.; de Almeida, S.R.; Baldini, R.L. Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence. PLoS Pathog. 2014, 10, e1004442. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2014, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.F.; Medeiros, M.H.G.; Di Mascio, P. Lysozyme oxidation by singlet molecular oxygen: Peptide characterization using [18O]-labeling oxygen and nLC-MS/MS. J. Mass Spectrom. 2017, 52, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Chander, A.; Jain, M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. Biochem. J. 1992, 288 Pt 2, 407–411. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C. Role of phospholipase A2 enzymes in degradation of dipalmitoylphosphatidylcholine by granular pneumocytes. J. Lipid Res. 1996, 37, 1057–1064. [Google Scholar] [PubMed]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Kobayashi, J.; Shirai, K.; Saito, Y.; Fukamachi, I.; Hashimoto, H.; Nishida, T.; Shibui, T.; Morimoto, Y.; Yoshida, S. Effects of substitutions of glycine and asparagine for serine 132 on activity and binding of human lipoprotein lipase to very low density lipoproteins. FEBS Lett. 1992, 298, 36–38. [Google Scholar] [CrossRef]
- Rahaman, H.; Zhou, S.; Dodia, C.; Feinstein, S.I.; Huang, S.; Speicher, D.; Fisher, A.B. Increased Phospholipase A 2 Activity with Phosphorylation of Peroxiredoxin 6 Requires a Conformational Change in the Protein. Biochemistry 2012, 51, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Green, F.H.Y.; Bachofen, H.; Tn, A. Formation and structure of surface films: Captive bubble surfactometry. Adsorpt. J. Int. Adsorpt. Soc. 1998, 1408, 180–202. [Google Scholar]
- Veldhuizen, E.J.; Haagsman, H.P. Role of pulmonary surfactant components in surface film formation and dynamics. BBA 2000, 1467, 255–270. [Google Scholar] [CrossRef]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta Mol. Basis Dis. 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Shen, D.K.; Noodeh, A.D.; Kazemi, A.; Grillot, R.; Robson, G.; Brugère, J.F. Characterisation and expression of phospholipases B from the opportunistic fungus Aspergillus fumigatus. FEMS Microbiol. Lett. 2004, 239, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Frank, D.W. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 2004, 53, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, M.C.; Kulasekara, B.R.; Liang, X.; Boyd, D.; Wu, K.; Yang, Q.; Miyada, C.G.; Lory, S. Conservation of genome content and virulence determinants among clinical and environmental isolates of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2003, 100, 8484–8489. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.; Vijay, A.K.; Kohli, G.S.; Rice, S.A.; Willcox, M. Association between possession of ExoU and antibiotic resistance in Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0204936. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Gohain, D.; Bora, U.; Tamuli, R. Phospholipases play multiple cellular roles including growth, stress tolerance, sexual development, and virulence in fungi. Microbiol. Res. 2018, 209, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.A.; Brenot, A.; Haas-Stapleton, E.; Agabian, N.; Deva, R.; Nigam, S. Phospholipase A2 and phospholipase B activities in fungi. Biochim. Biophys. Acta 2006, 1761, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Greer, M.S.; Weselake, R.J. Plant phospholipase A: Advances in molecular biology, biochemistry, and cellular function. Biomol. Concepts 2013, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Stacy, R.A.; Nordeng, T.W.; Culiáñez-Macià, F.A.; Aalen, R.B. The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. Plant J. 1999, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, F.; Paciolla, C.; Arrigoni, O. The ascorbate system in recalcitrant and orthodox seeds. Physiol. Plant. 1999, 105, 193–198. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis Seed Development and Germination Is Associated with Temporally Distinct Metabolic Switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef] [PubMed]
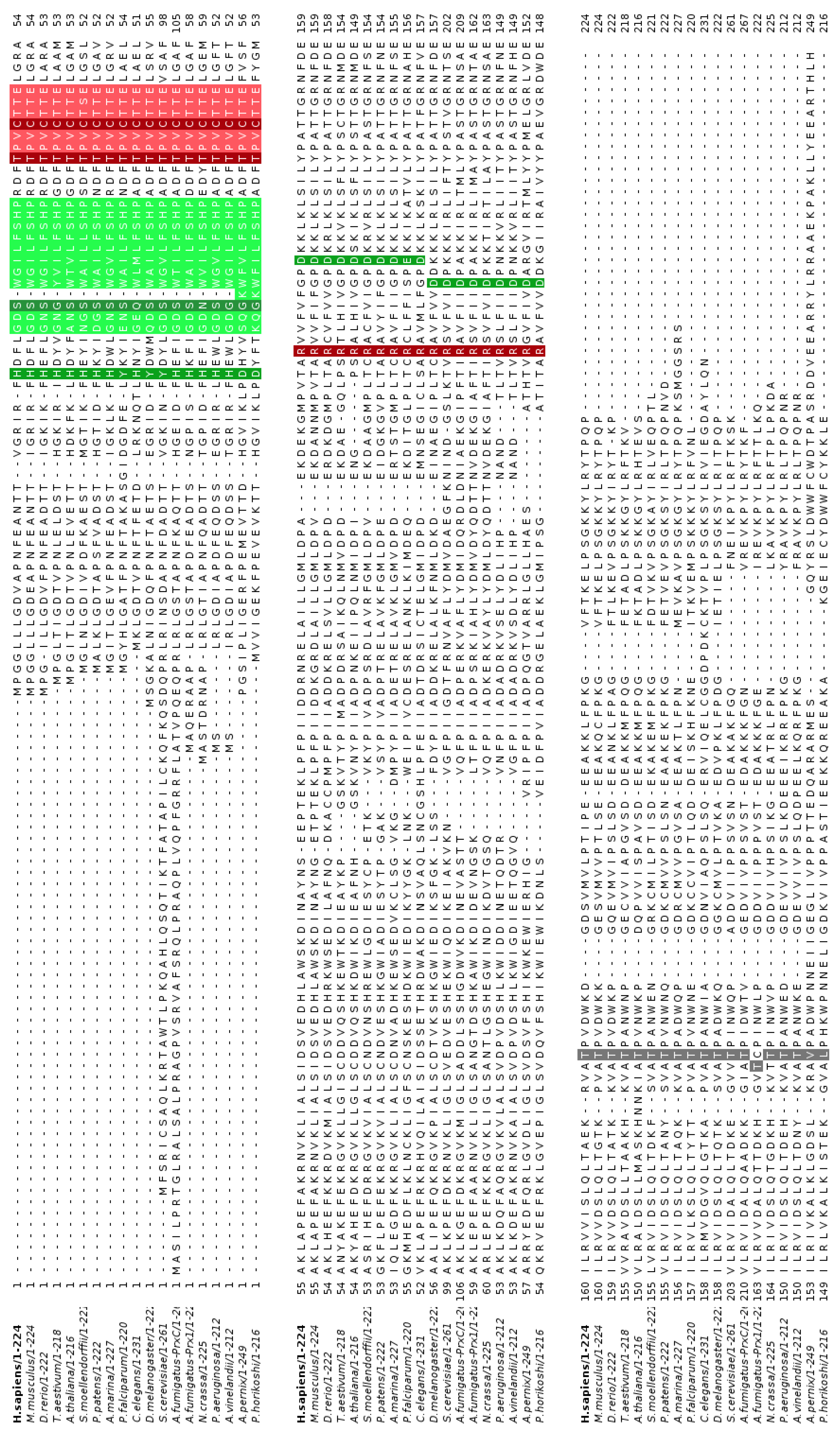
| Organism | Protein | Activity (nmol/min/mg prot.) | |
|---|---|---|---|
| pH 4 | pH 7 | ||
| T. aestivum | TaPER1 | 4.46 ± 0.1 | 0.06 ± 0.01 |
| A. fumigatus | AfPrx1 | 6.09 ± 0.1 | 0.7 ± 0.2 |
| AfPrxC | 4.91 ± 0.2 | 0.5 ± 0.1 | |
| P. aeruginosa | PaLsfA | 3.38 ± 0.1 | 0.04 ± 0.002 |
| Organism | Protein | Fragment Detected after Digestion | Observed Mass | Charge | Score | Error (ppm) |
|---|---|---|---|---|---|---|
| T. aestivum | TaPER1 | HKVAT(+Phospho)PANWNPGECVVIAPGVSDDEAKK | 768.11 | 4 | 61 | 5.53 |
| HKVATPANWNPGECVVIAPGVSDDEAKK | 748.12 | 4 | 85 | 4.89 | ||
| A. thaliana | AtPER1 | ALDSLLMASKHNNKIAT(+Phospho)PVNWKPDQPVVISPAVSDEEAK | 859.43 | 5 | 31 | 5.77 |
| ALDSLLMASKHNNKIATPVNWKPDQPVVISPAVSDEEAK | 843.44 | 5 | 29 | 2.38 | ||
| A. fumigatus | AfPrx1 | VVDALQTTDKHGVTCPINWLPGDDVIIPPPVSTEDAK | 1000.25 | 4 | 42 | 5.96 |
| VVDALQTTDKHGVTCPINWLPGDDVIIPPPVSTEDAK | 1000.26 | 4 | 50 | 2.06 | ||
| AfPrxC | VIDALQAADKKGIAT(+Phospho)PIDWTVGEDVIVPPSVSTEDAK | 983.00 | 4 | 19 | 0.31 | |
| VIDALQAADKKGIATPIDWTVGEDVIVPPSVSTEDAK | 963.01 | 4 | 56 | 2.35 | ||
| P. aeruginosa | PaLsfA | VIDSLQLTDEHKVATPANWEDGDEVVIVPSLKDEEEIKR | 1473.07 | 3 | 111 | 8.59 |
| VIDSLQLTDEHKVATPANWEDGDEVVIVPSLKDEEEIKR | 1473.07 | 3 | 65 | 7.64 |
| Treatment | AfPrx1 | AfPrxC | ||
|---|---|---|---|---|
| pH 4 | pH 7 | pH 4 | pH 7 | |
| No treatment | 6.09 ± 0.1 | 0.7 ± 0.2 | 4.91 ± 0.2 | 0.5 ± 0.1 |
| Erk2 | 21.85 ± 0.5 | 20.96 ± 0.3 | 18.69 ± 0.2 | 18.47 ± 0.1 |
| MJ33 | 0.85 ± 0.05 | ND | 0.89 ± 0.01 | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannitz-Fernandes, R.; Aleixo-Silva, R.; Silva, J.P.; Dodia, C.; Vazquez-Medina, J.P.; Tao, J.-Q.; Fisher, A.; Netto, L. Non-Mammalian Prdx6 Enzymes (Proteins with 1-Cys Prdx Mechanism) Display PLA2 Activity Similar to the Human Orthologue. Antioxidants 2019, 8, 52. https://doi.org/10.3390/antiox8030052
Bannitz-Fernandes R, Aleixo-Silva R, Silva JP, Dodia C, Vazquez-Medina JP, Tao J-Q, Fisher A, Netto L. Non-Mammalian Prdx6 Enzymes (Proteins with 1-Cys Prdx Mechanism) Display PLA2 Activity Similar to the Human Orthologue. Antioxidants. 2019; 8(3):52. https://doi.org/10.3390/antiox8030052
Chicago/Turabian StyleBannitz-Fernandes, Renata, Rogério Aleixo-Silva, João Paulo Silva, Chandra Dodia, Jose Pablo Vazquez-Medina, Jian-Qin Tao, Aron Fisher, and Luis Netto. 2019. "Non-Mammalian Prdx6 Enzymes (Proteins with 1-Cys Prdx Mechanism) Display PLA2 Activity Similar to the Human Orthologue" Antioxidants 8, no. 3: 52. https://doi.org/10.3390/antiox8030052
APA StyleBannitz-Fernandes, R., Aleixo-Silva, R., Silva, J. P., Dodia, C., Vazquez-Medina, J. P., Tao, J.-Q., Fisher, A., & Netto, L. (2019). Non-Mammalian Prdx6 Enzymes (Proteins with 1-Cys Prdx Mechanism) Display PLA2 Activity Similar to the Human Orthologue. Antioxidants, 8(3), 52. https://doi.org/10.3390/antiox8030052








