Potential of Smoke-Water and One of Its Active Compounds (karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Chemicals and Plant Materials
2.2. In Vitro Propagation and Acclimatization Design
2.3. Ultra-High Performance Liquid Chromatography: Tandem Mass Spectrometry (UHPLC-MS/MS) Analysis of Phytochemicals
2.4. Plant Extraction and Antioxidant Activity Evaluation
2.5. Data Analysis
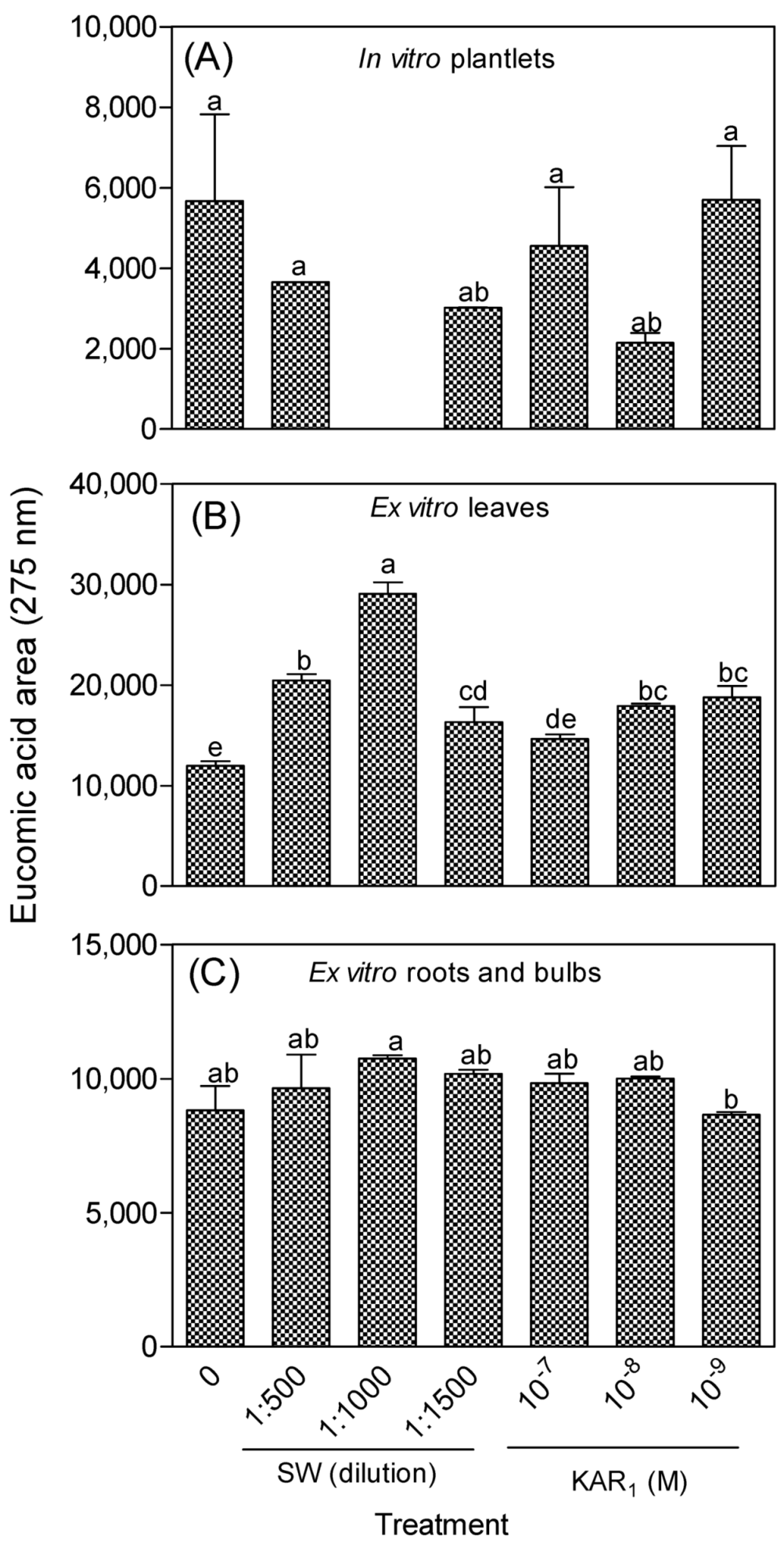
3. Results
3.1. Phytochemical Profiles of In Vitro Regenerants and Greenhouse-Acclimatized Plants
3.1.1. Eucomic Acid in In Vitro and Greenhouse-Acclimatized Plants
3.1.2. Hydroxybenzoic Acid Derivatives In Vitro and Greenhouse-Acclimatized Plants
3.1.3. Hydroxycinnamic Acid Derivatives in Vitro and Greenhouse-Acclimatized Plants
3.1.4. Flavonoids in Vitro and Greenhouse-Acclimatized Plants
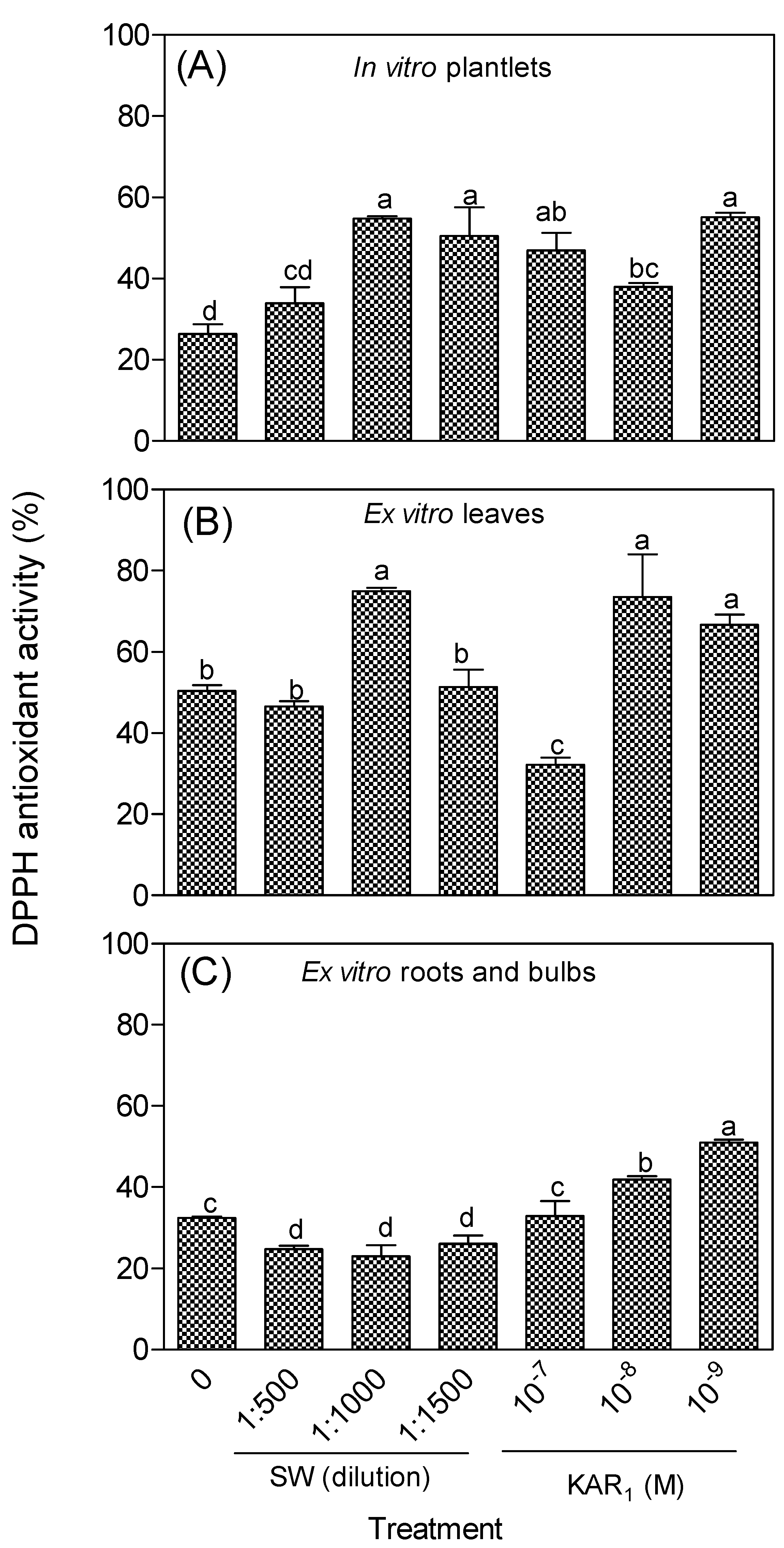
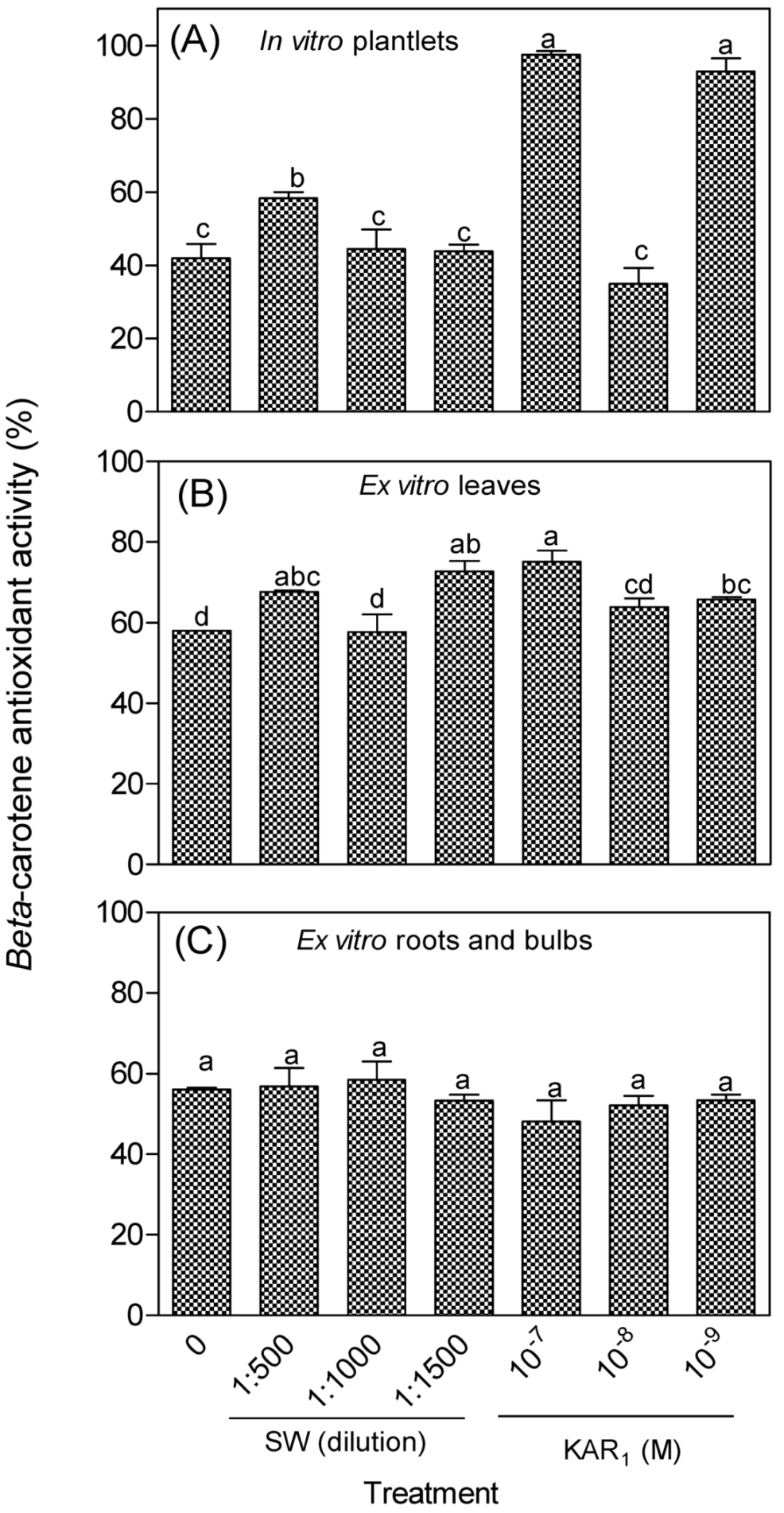
3.2. Antioxidant Activity of In Vitro Regenerants and Greenhouse-Acclimatized Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Lange, J.H.; Brown, N.A.C.; Van Staden, J. Perspectives on the contributions by South African researchers in igniting global research on smoke-stimulated seed germination. S. Afr. J. Bot. 2018, 115, 219–222. [Google Scholar] [CrossRef]
- Flematti, G.R.; Dixon, K.W.; Smith, S.M. What are karrikins and how were they ‘discovered’ by plants? BMC Biol. 2015, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Light, M.E.; Van Staden, J. Plant-derived smoke: Old technology with possibilities for economic applications in agriculture and horticulture. S. Afr. J. Bot. 2011, 77, 972–979. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, J.; Jäger, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Hrdlička, J.; Gucký, T.; Novák, O.; Kulkarni, M.; Gupta, S.; van Staden, J.; Doležal, K. Quantification of karrikins in smoke water using ultra-high performance liquid chromatography–tandem mass spectrometry. Plant Methods 2019, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Light, M.E.; Daws, M.I.; Van Staden, J. Smoke-derived butenolide: Towards understanding its biological effects. S. Afr. J. Bot. 2009, 75, 1–7. [Google Scholar] [CrossRef]
- Chiwocha, S.D.S.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Jain, N.; Stirk, W.A.; Van Staden, J. Cytokinin-and auxin-like activity of a butenolide isolated from plant-derived smoke. S. Afr. J. Bot. 2008, 74, 327–331. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtová, M.; Doležal, K.; Van Staden, J. Plant regeneration and biochemical accumulation of hydroxybenzoic and hydroxycinnamic acid derivatives in Hypoxis hemerocallidea organ and callus cultures. Plant Sci. 2014, 227, 157–164. [Google Scholar] [CrossRef]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Plant growth regulator induced phytochemical and antioxidant variations in micropropagated and acclimatized Eucomis autumnalis subspecies autumnalis (Asparagaceae). Acta Physiol. Plant. 2014, 36, 2467–2479. [Google Scholar] [CrossRef]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. Shoot proliferation and rooting treatments influence secondary metabolite production and antioxidant activity in tissue culture-derived Aloe arborescens grown ex vitro. Plant Growth Regul. 2013, 70, 115–122. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Finnie, J.F.; Van Staden, J. Stimulatory role of smoke-water and karrikinolide on the photosynthetic pigment and phenolic contents of micropropagated ‘Williams’ bananas. Plant Growth Regul. 2012, 67, 271–279. [Google Scholar] [CrossRef]
- Soós, V.; Sebestyén, E.; Juhász, A.; Szalai, G.; Tandori, J.; Light, M.E.; Kohout, L.; Van Staden, J.; Balázs, E. Transcriptome analysis of germinating maize kernels exposed to smoke-water and the active compound KAR1. BMC Plant Biol. 2010, 10, 236. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Moyo, M.; Aremu, A.O.; Van Staden, J. Medicinal plants: An invaluable, dwindling resource in sub-Saharan Africa. J. Ethnopharmacol. 2015, 174, 595–606. [Google Scholar] [CrossRef]
- Masondo, N.A.; Finnie, J.F.; Van Staden, J. Pharmacological potential and conservation prospect of the genus Eucomis (Hyacinthaceae) endemic to southern Africa. J. Ethnopharmacol. 2014, 151, 44–53. [Google Scholar] [CrossRef]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996. [Google Scholar]
- Moyo, M.; Bairu, M.W.; Amoo, S.O.; Van Staden, J. Plant biotechnology in South Africa: Micropropagation research endeavours, prospects and challenges. S. Afr. J. Bot. 2011, 77, 996–1011. [Google Scholar] [CrossRef][Green Version]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.J.M.; Van Staden, J.; Granger, J.E.; Brown, N.A.C. Plant-derived smoke and smoke extracts stimulate seed germination of the fire-climax grass Themeda triandra. Environ. Exp. Bot. 1994, 34, 217–223. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Growth and phytochemical levels in micropropagated Eucomis autumnalis subspecies autumnalis using different gelling agents, explant source, and plant growth regulators. In Vitro Cell. Dev. Biol. Plant 2015, 51, 102–110. [Google Scholar] [CrossRef]
- Gruz, J.; Novák, O.; Strnad, M. Rapid analysis of phenolic acids in beverages by UPLC–MS/MS. Food Chem. 2008, 111, 789–794. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef]
- Aremu, A.O.; Amoo, S.O.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Antioxidant activity, acetylcholinesterase inhibition, iridoid content and mutagenic evaluation of Leucosidea sericea. Food Chem. Toxicol. 2011, 49, 1122–1128. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Koorbanally, C.; Crouch, N.R.; Mulholland, D.A. The phytochemistry and ethnobotany of the southern African genus Eucomis (Hyacinthaceae: Hyacinthoideae). In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 69–85. [Google Scholar]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Van Staden, J. Smoke–water stimulates secondary metabolites during in vitro seedling development in Tulbaghia species. S. Afr. J. Bot. 2014, 91, 49–52. [Google Scholar] [CrossRef]
- Zhou, J.; Van Staden, J.; Guo, L.P.; Huang, L.Q. Smoke-water improves shoot growth and indigo accumulation in shoots of Isatis indigotica seedlings. S. Afr. J. Bot. 2011, 77, 787–789. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, M.G.; Amoo, S.O.; Kandari, L.S.; Van Staden, J. Seed germination and phytochemical evaluation in seedlings of Aloe arborescens Mill. Plant Biosyst. 2014, 148, 460–466. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Malik, S.; Sharma, N.; Sharma, U.K.; Singh, N.P.; Bhushan, S.; Sharma, M.; Sinha, A.K.; Ahuja, P.S. Qualitative and quantitative analysis of anthraquinone derivatives in rhizomes of tissue culture-raised Rheum emodi Wall. plants. J. Plant Physiol. 2010, 167, 749–756. [Google Scholar] [CrossRef]
- Aremu, A.O.; Gruz, J.; Šubrtová, M.; Szüčová, L.; Doležal, K.; Bairu, M.W.; Finnie, J.F.; Van Staden, J. Antioxidant and phenolic acid profiles of tissue cultured and acclimatized Merwilla plumbea plantlets in relation to the applied cytokinins. J. Plant Physiol. 2013, 170, 1303–1308. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; El-Demerdash, M.; Saxena, P.K. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef]
- Aremu, A.O.; Plačková, L.; Gruz, J.; Bíba, O.; Novák, O.; Stirk, W.A.; Doležal, K.; Van Staden, J. Seaweed-derived biostimulant (Kelpak) influences endogenous cytokinins and bioactive compounds in hydroponically grown Eucomis autumnalis. J. Plant Growth Regul. 2016, 35, 151–162. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Moyo, M.; Aremu, A.O.; Chukwujekwu, J.C.; Gruz, J.; Skorepa, J.; Doležal, K.; Katsvanga, C.A.T.; Van Staden, J. Phytochemical characterization, antibacterial, acetylcholinesterase inhibitory and cytotoxic properties of Cryptostephanus vansonii, an endemic amaryllid. Phytother. Res. 2017, 31, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Park, S.; Koshizawa, T.; Ueda, M. (R)-Eucomic acid, a leaf-opening factor of the model organism, Lotus japonicus. Tetrahedron 2009, 65, 2136–2141. [Google Scholar] [CrossRef]
- Ishii, M.; Uemoto, S.; Fujieda, K.; Nonaka, M.; Shoyama, Y.; Miyahara, Y.; Nishioka, I. A new biologically active phenolic from Cattleya trianaei. Phytochemistry 1979, 18, 1211–1213. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Harnly, J. Antioxidant methods. J. Food Compos. Anal. 2017, 64, 145–146. [Google Scholar] [CrossRef]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tissue Organ Cult. 2012, 111, 345–358. [Google Scholar] [CrossRef]
| Plant Stage/Part | Treatment | Protocatechuic Acid | p-Hydroxybenzoic Acid | Vanillic Acid | Syringic Acid |
|---|---|---|---|---|---|
| In vitro | Control | 1.7 ± 0.02 d | 14.5 ± 0.43e | 1.7 ± 0.27a | nd |
| Plantlets | SW 1:500 | 2.1 ± 0.01 c | 21.2 ± 0.19b | 1.8 ± 0.09a | nd |
| SW 1:1000 | nd | nd | nd | nd | |
| SW 1:1500 | 2.6 ± 0.03a | 28.7 ± 0.22a | 1.7 ± 0.01a | nd | |
| KAR1 10−7 | 1.6 ± 0.04e | 18.7 ± 0.41d | 1.4 ± 0.18a | nd | |
| KAR1 10−8 | 1.7 ± 0.01de | 14.6 ± 0.28e | 1.9 ± 0.10a | nd | |
| KAR1 10−9 | 2.3 ± 0.04b | 20.0 ± 0.30c | 1.5 ± 0.20a | nd | |
| Ex vitro | Control | 0.6 ± 0.02c | 0.5 ± 0.01b | nd | nd |
| Leaves | SW 1:500 | 1.0 ± 0.03b | 0.3 ± 0.01c | nd | nd |
| SW 1:1000 | 0.4 ± 0.00d | 0.3 ± 0.01c | nd | nd | |
| SW 1:1500 | 0.6 ± 0.01c | 0.5 ± 0.02b | nd | nd | |
| KAR1 10−7 | 1.1 ± 0.01a | 0.8 ± 0.05a | nd | nd | |
| KAR1 10−8 | 0.2 ± 0.00e | 0.3 ± 0.01c | nd | nd | |
| KAR1 10−9 | 0.7 ± 0.05c | 0.6 ± 0.12b | nd | nd | |
| Bulbs + roots | Control | 0.8 ± 0.09c | 0.8 ± 0.01d | nd | 0.2 ± 0.03b |
| SW 1:500 | 0.3 ± 0.03f | 0.8 ± 0.02e | nd | 0.1 ± 0.02c | |
| SW 1:1000 | 0.6 ± 0.02d | 0.9 ± 0.05c | nd | 0.1 ± 0.03b | |
| SW 1:1500 | 0.5 ± 0.02de | 0.7 ± 0.01d | nd | 0.1 ± 0.01abc | |
| KAR1 10−7 | 0.5 ± 0.00e | 0.7 ± 0.00e | nd | 0.2 ± 0.01ab | |
| KAR1 10−8 | 1.0 ± 0.04b | 1.0 ± 0.02b | nd | 0.1 ± 0.01abc | |
| KAR1 10−9 | 1.1 ± 0.02a | 2.7 ± 0.03a | nd | 0.2 ± 0.04a |
| Plant Stage/Part | Treatment | Caffeic Acid | Coumaric Acid | Cinnamic Acid | Ferulic Acid | Isoferulic Acid |
|---|---|---|---|---|---|---|
| In vitro | Control | 0.20 ± 0.030d | 51.55 ± 0.562a | 3.3 ± 0.24d | 11.82 ± 0.246a | 0.9 ± 0.18a |
| plantlet | SW 1:500 | 0.36 ± 0.007c | 29.78 ± 0.295c | 13.5 ± 0.05a | 3.39 ± 0.019d | nd |
| SW 1:1000 | nd | nd | nd | nd | nd | |
| SW 1:1500 | 0.56 ± 0.008a | 39.19 ± 1.163b | 10.9 ± 0.06 b | 6.73 ± 0.020b | 0.9 ± 0.05a | |
| KAR1 10−7 | 0.46 ± 0.045b | 31.91 ± 0.215c | 11.5 ± 0.82 b | 4.56 ± 0.082c | nd | |
| KAR1 10−8 | 0.56 ± 0.013a | 23.23 ± 0.528d | 7.8 ± 0.75 c | 3.54 ± 0.150d | nd | |
| KAR1 10−9 | 0.34 ± 0.044c | 38.92 ± 1.665b | 10.3 ± 0.29 b | 4.51 ± 0.242c | 0.5 ± 0.01b | |
| Ex vitro | Control | 0.09 ± 0.005d | 0.76 ± 0.102bc | nd | 0.28 ± 0.010bc | nd |
| leaves | SW 1:500 | 0.16 ± 0.007c | 0.66 ± 0.054cd | nd | 0.36 ± 0.002ab | nd |
| SW 1:1000 | 0.26 ± 0.007a | 0.64 ± 0.049cd | nd | 0.17 ± 0.022d | nd | |
| SW 1:1500 | 0.13 ± 0.010c | 0.89 ± 0.068b | nd | 0.24 ± 0.049cd | nd | |
| KAR1 10−7 | 0.13 ± 0.016c | 1.19 ± 0.071a | nd | 0.34 ± 0.007ab | nd | |
| KAR1 10−8 | 0.16 ± 0.012c | 0.50 ± 0.066de | nd | 0.39 ± 0.048a | nd | |
| KAR1 10−9 | 0.21 ± 0.013b | 0.40 ± 0.015e | nd | 0.18 ± 0.018d | nd | |
| Bulbs + roots | Control | 0.14 ± 0.004a | 0.31 ± 0.007cd | nd | 0.78 ± 0.046f | nd |
| SW 1:500 | 0.07 ± 0.009c | 0.25 ± 0.019e | nd | 0.82 ± 0.010ef | nd | |
| SW 1:1000 | 0.12 ± 0.010ab | 0.32 ± 0.008c | nd | 1.37 ± 0.060b | nd | |
| SW 1:1500 | 0.13 ± 0.002ab | 0.28 ± 0.003de | nd | 0.96 ± 0.035de | nd | |
| KAR1 10−7 | 0.12 ± 0.012ab | 0.27 ± 0.006de | nd | 1.02 ± 0.021cd | nd | |
| KAR1 10−8 | 0.11 ± 0.006b | 0.42 ± 0.023b | nd | 1.14 ± 0.063c | nd | |
| KAR1 10−9 | 0.12 ± 0.001ab | 0.50 ± 0.008a | nd | 1.89 ± 0.067a | nd |
| Plant Stage/Part | Treatment | Hesperetin | Kaempferol | Eriodictyol | Genistein | Pinobaksin | Taxifolin |
|---|---|---|---|---|---|---|---|
| In vitro | Control | nd | nd | nd | nd | nd | nd |
| Plantlet | SW 1:500 | nd | nd | nd | nd | nd | nd |
| SW 1:1000 | nd | nd | nd | nd | nd | nd | |
| SW 1:1500 | nd | 1.7 ± 0.09 | nd | nd | nd | nd | |
| KAR1 10−7 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−8 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−9 | 3.4 ± 1.22 | nd | nd | nd | nd | nd | |
| Ex vitro | Control | nd | nd | 0.18 ± 0.008a | 0.05 ± 0.008a | nd | nd |
| Leaves | SW 1:500 | nd | nd | 0.11 ± 0.004b | nd | nd | nd |
| SW 1:1000 | nd | nd | 0.07 ± 0.001c | 0.03 ± 0.004b | nd | nd | |
| SW 1:1500 | nd | nd | nd | nd | nd | 0.17 ± 0.039 | |
| KAR1 10−7 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−8 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−9 | nd | nd | nd | nd | nd | nd | |
| Bulbs + roots | Control | nd | nd | 0.23 ± 0.015a | nd | 0.12 ± 0.020a | nd |
| SW 1:500 | nd | nd | 0.13 ± 0.017b | nd | 0.07 ± 0.004b | nd | |
| SW 1:1000 | nd | nd | 0.10 ± 0.019b | nd | nd | nd | |
| SW 1:1500 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−7 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−8 | nd | nd | nd | nd | nd | nd | |
| KAR1 10−9 | nd | nd | nd | nd | nd | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aremu, A.O.; Masondo, N.A.; Gruz, J.; Doležal, K.; Van Staden, J. Potential of Smoke-Water and One of Its Active Compounds (karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis. Antioxidants 2019, 8, 611. https://doi.org/10.3390/antiox8120611
Aremu AO, Masondo NA, Gruz J, Doležal K, Van Staden J. Potential of Smoke-Water and One of Its Active Compounds (karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis. Antioxidants. 2019; 8(12):611. https://doi.org/10.3390/antiox8120611
Chicago/Turabian StyleAremu, Adeyemi Oladapo, Nqobile Andile Masondo, Jiri Gruz, Karel Doležal, and Johannes Van Staden. 2019. "Potential of Smoke-Water and One of Its Active Compounds (karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis" Antioxidants 8, no. 12: 611. https://doi.org/10.3390/antiox8120611
APA StyleAremu, A. O., Masondo, N. A., Gruz, J., Doležal, K., & Van Staden, J. (2019). Potential of Smoke-Water and One of Its Active Compounds (karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis. Antioxidants, 8(12), 611. https://doi.org/10.3390/antiox8120611







