Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. GC/MS Analyses
2.3. Antioxidant Activity in Vitro
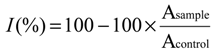
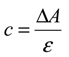
2.4. Antioxidant Activity in Vivo
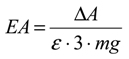
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Juniper Berry Oil
| Substance | Retention Index | % Area |
|---|---|---|
| toluene | 771 | trace |
| hexanal | 800 | trace |
| α-thujene | 933 | 0.9 |
| α-pinene | 943 | 51.4 |
| α-fenchene | 957 | 0.2 |
| camphene | 959 | 0.8 |
| thuja-2,4(10)-diene | 964 | 0.2 |
| sabinene | 981 | 5.8 |
| β-pinene | 988 | 5.0 |
| myrcene | 992 | 8.3 |
| δ-3-carene | 1019 | 0.2 |
| α-terpinene | 1024 | 0.1 |
| p-cymene | 1032 | 0.9 |
| limonene | 1037 | 5.1 |
| β-phellandrene | 1038 | 0.5 |
| (E)-β-ocimene | 1050 | 0.1 |
| γ-terpinene | 1065 | 0.2 |
| cis-sabinene hydrate | 1075 | 0.1 |
| terpinolene | 1096 | 0.4 |
| linalool | 1101 | 0.1 |
| perillene | 1104 | 0.1 |
| trans-sabinene hydrate | 1107 | 0.1 |
| α-pinene oxide | 1111 | 0.1 |
| trans-p-menth-2-en-1-ol | 1131 | tr |
| campholen aldehyde | 1136 | 0.1 |
| trans-pinocarveol | 1154 | 0.3 |
| cis-verbenol | 1156 | 0.5 |
| borneol | 1180 | trace |
| terpinen-4-ol | 1189 | 0.9 |
| p-cymen-8-ol | 1193 | 0.2 |
| α-terpineol | 1201 | 0.2 |
| myrtenol | 1208 | 0.1 |
| myrtenal | 1210 | 0.2 |
| verbenone | 1223 | 0.2 |
| trans-carveol | 1228 | 0.2 |
| carvone | 1256 | 0.1 |
| methyl citronellate | 1260 | 0.1 |
| undecan-2-one | 1294 | 0.1 |
| bornyl acetate | 1298 | 0.3 |
| methyl geranate | 1326 | trace |
| citronellyl acetate | 1352 | 0.1 |
| terpinyl acetate | 1359 | 0.1 |
| α-cubebene | 1366 | 0.6 |
| geranyl acetate | 1382 | trace |
| α-copaene | 1397 | 0.5 |
| β-elemene | 1409 | 0.7 |
| α-gurjunene | 1426 | 0.2 |
| longifolene | 1438 | 0.1 |
| (E)-β-caryopyhllene | 1447 | 2.0 |
| γ-elemene | 1451 | 0.1 |
| (E)-β-farnesene | 1461 | 0.3 |
| α-humulene | 1481 | 1.3 |
| γ-muurolene | 1497 | 0.4 |
| α-amorphene | 1501 | 0.1 |
| germacrene D | 1507 | 1.1 |
| β-selinene | 1515 | 0.2 |
| α-muurolene | 1520 | 0.4 |
| α-selinene | 1521 | 0.2 |
| γ-cadinene | 1538 | 0.5 |
| δ-cadinene | 1543 | 0.8 |
| α-cadinene | 1560 | 0.1 |
| spathulenol | 1607 | 0.3 |
| caryophyllene oxide | 1616 | 0.9 |
| humulene epoxide II | 1643 | 0.5 |
| τ-muurol + τ-cadinol | 1668 | 0.2 |
| α-cadinol | 1681 | 0.1 |
| m-camphorene | 1964 | 0.2 |
| p-camphorene | 2002 | 0.1 |
| sum | 96.0 |
3.2. Antioxidant Activity of Juniper Berry Oil in Vitro
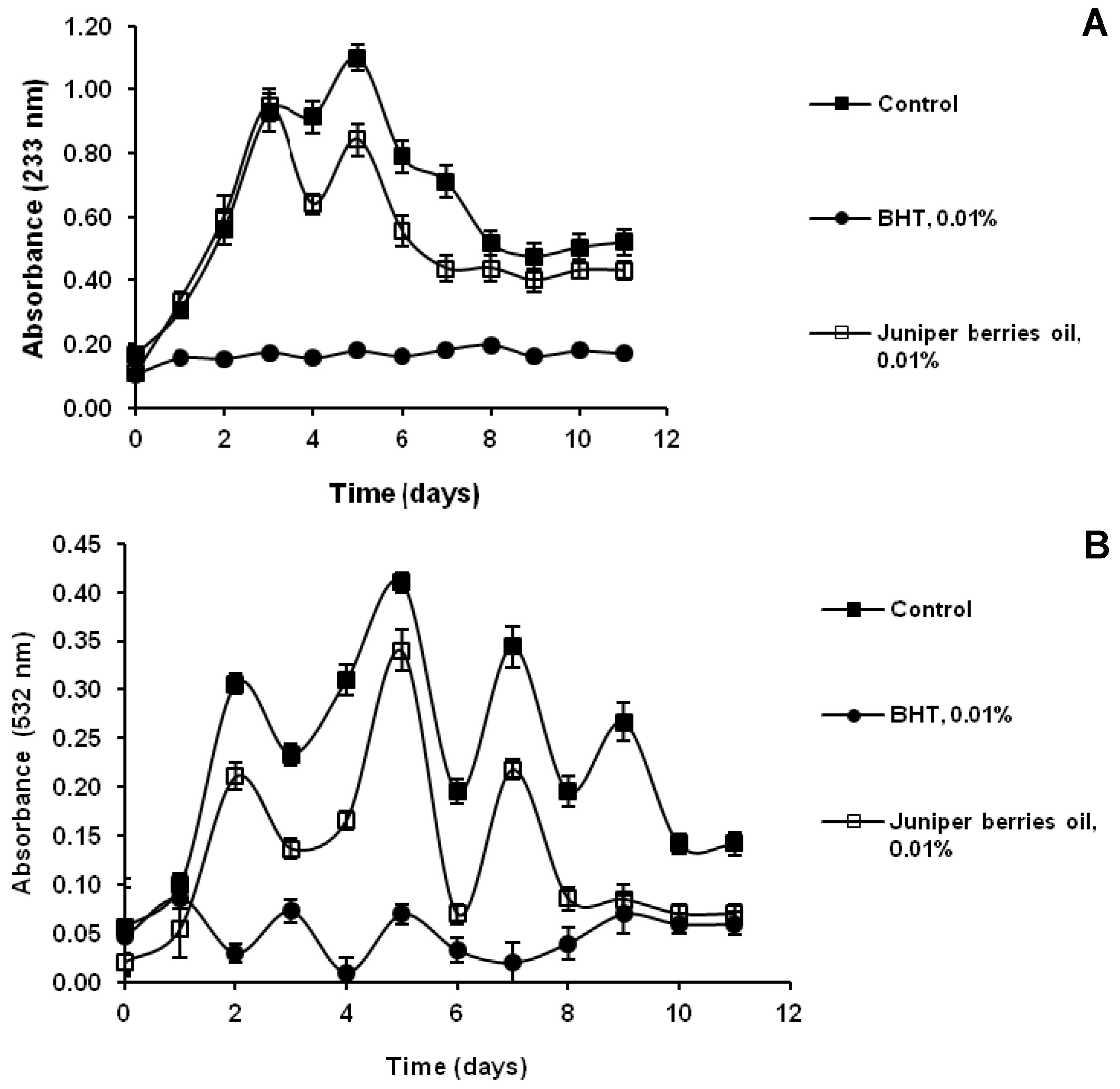
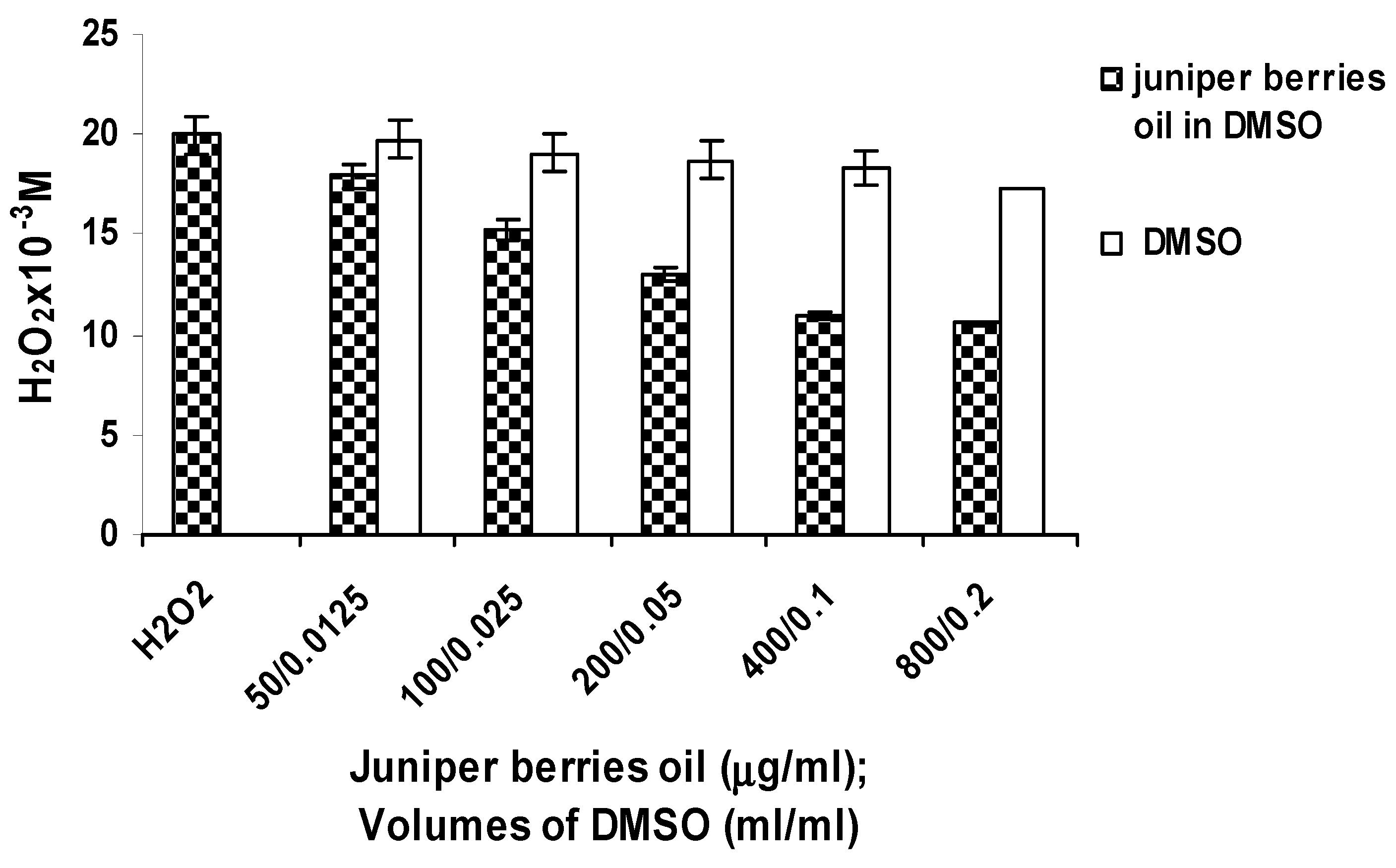
3.3. Action of Juniper Berry Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism
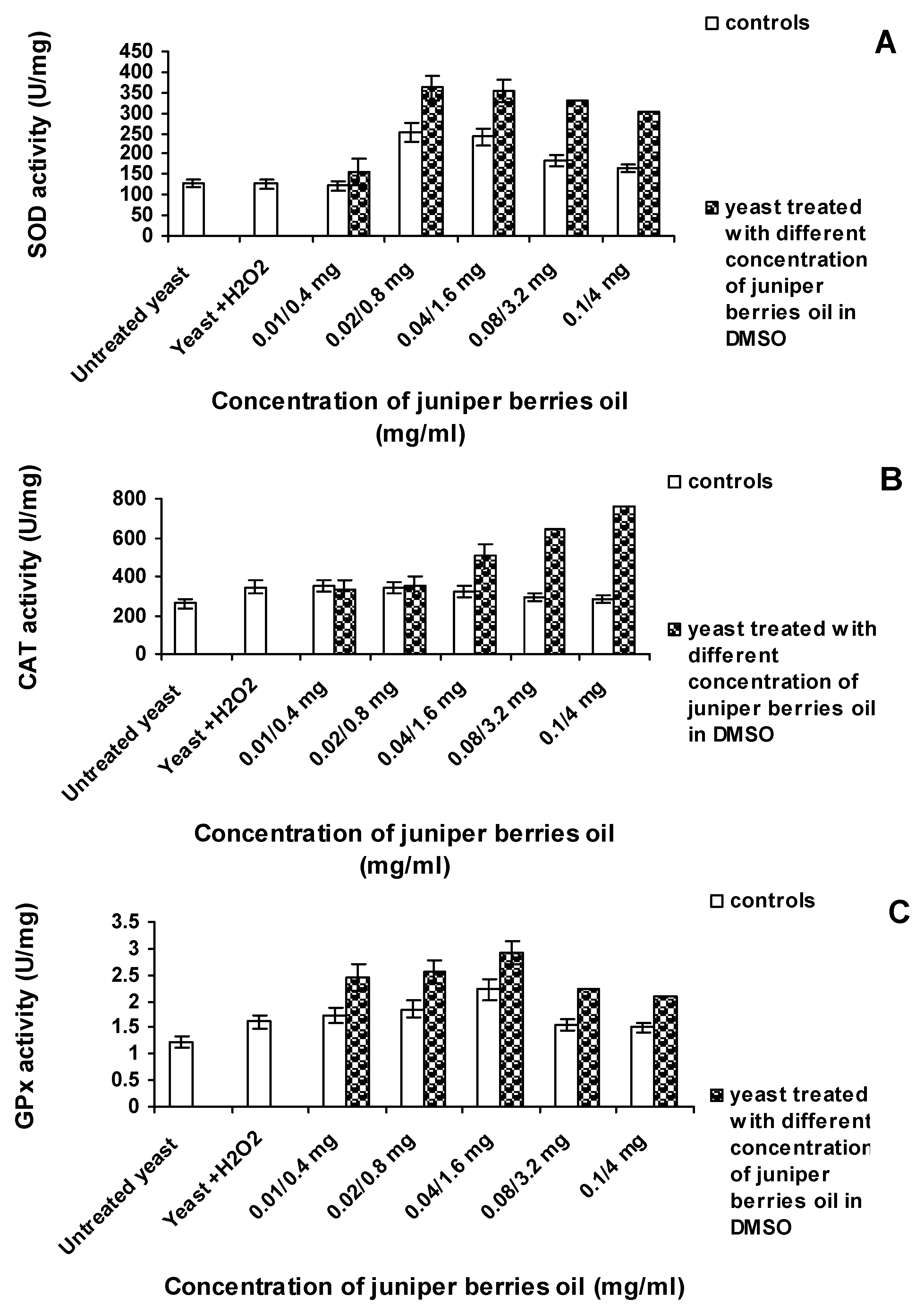
4. Conclusions
Conflicts of Interest
References
- Schopfer, P.; Plachy, C.; Frahry, G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, H.J.; Kwon, Y.M.; Kang, Y.J.; Lee, I.H.; Jin, B.R.; Han, Y.S.; Cheon, H.M.; Ha, N.G.; Seo, S.J. Modulation of MnSOD protein in response to different experimental stimulation in Hyphantria cunea. Comp. Biochem. Physiol. 2010, 157, 343–350. [Google Scholar] [CrossRef]
- Martorell, P.; Forment, J.V.; de Llanos, R.; Montón, F.; Llopis, S.; González, N.; Genovés, S.; Cienfuegos, E.; Monzó, H.; Ramón, D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J. Agric. Food Chem. 2011, 59, 2077–2085. [Google Scholar] [CrossRef]
- Darwin, T. The Scots Herbal: The Plant Lore of Scotland; Birlinn Ltd.: Edinburgh, UK, 2008. [Google Scholar]
- Hiller, K.; Löw, D. Juniperi Pseudo-Fructus. In Teedrogen und Phytopharmaka; Wichtl, M., Ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2009. [Google Scholar]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M.K. Antioxidant activity of the essential oils of different parts of Juniperus communis. subsp. hemisphaerica and Juniperus oblonga. Pharm. Biol. 2007, 45, 769–776. [Google Scholar]
- Misharina, T.A.; Samusenko, A.L. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Prikl. Biokhimiia Mikrobiol. 2008, 44, 482–486. [Google Scholar]
- Misharina, T.A.; Terenina, M.B.; Krikunova, N.I. Antioxidant properties of essential oils. Prikl. Biokhimiia Mikrobiol. 2009, 45, 710–716. [Google Scholar]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Bua-in, S.; Paisooksantivatana, Y. Essential oil and antioxidant activity of Cassumunar ginger (Zingiberaceae: Zingiber montanum (Koenig) Link ex Dietr.) collected from various parts of Thailand. Kasetsart J. Nat. Sci. 2009, 43, 467–475. [Google Scholar]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [PubMed]
- Karioti, A.; Hadjipavlou-Litina, D.; Mensah, M.L.K.; Fleischer, T.C.; Skaltsa, H. Composition and antioxidant activity of the essential oils of Xylopia aethiopica (Dun) A. Rich. (Annonaceae) leaves, stem bark, root bark, and fresh and dried fruits, growing in Ghana. J. Agric. Food Chem. 2004, 52, 8094–8098. [Google Scholar] [CrossRef]
- Van Lieshout, E.M.; Posner, G.H.; Woodard, B.T.; Peters, W.H. Effects of the sulforaphane analog compound 30, indole-3-carbinol, d-limonene or relafen on glutathione S-transferases and glutathione peroxidase of the rat digestive tract. Biochim. Biophys. Acta 1998, 1379, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sepici-Dincel, A.; Açikgöz, S.; Cevik, C.; Sengelen, M.; Yeşilada, E. Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J. Ethnopharmacol. 2007, 110, 498–503. [Google Scholar] [CrossRef]
- Jakubowski, W.; Biliński, T.; Bartosz, G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 2000, 28, 659–664. [Google Scholar] [CrossRef]
- Manfredini, V.; Roehrs, R.; Peralba, M.C.R.; Henriques, J.A.P.; Saffi, J.; Ramos, A.L.L.P.; Benfato, M.S. Glutathione peroxidase induction protects Saccharomyces cerevisiae sod1deltasod2delta double mutants against oxidative damage. Braz. J. Med. Biol. Res. 2004, 37, 159–165. [Google Scholar] [CrossRef]
- Longo, V.D.; Gralla, E.B.; Valentine, J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996, 271, 12275–12280. [Google Scholar] [CrossRef]
- Tsuzi, D.; Maeta, K.; Takatsume, Y.; Izawa, S.; Inoue, Y. Regulation of the yeast phospholipid hydroperoxide glutathione peroxidase GPX2 by oxidative stress is mediated by Yap1 and Skn7. FEBS Lett. 2004, 565, 148–154. [Google Scholar] [CrossRef]
- Inoue, Y.; Matsuda, T.; Sugiyama, K.; Izawa, S.; Kimura, A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 27002–27009. [Google Scholar] [CrossRef] [PubMed]
- FINNIGAN Xcalibur; release 1.2; ThermoQuest Corporation: San Jose, CA, USA, 2000.
- NIST/EPA/NIH Mass Spectral Library; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Pub. Corp.: Carol Stream, IL, USA, 2001. [Google Scholar]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils. Available online: http://massfinder.com/wiki/Terpenoids_Library (accessed on 12 February 2014).
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20 M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chang, Y.-C.; Su, S.-W. Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem. 2003, 83, 49–54. [Google Scholar] [CrossRef]
- Zainol, M.K.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. 2003, 81, 575–581. [Google Scholar] [CrossRef]
- Romero, A.M.; Doval, M.M.; Sturla, M.A.; Judis, M.A. Antioxidant properties of polyphenol-containing extract from soybean fermented with Saccharomyces cerevisiae. Eur. J. Lipid Sci. Technol. 2004, 106, 424–431. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Carrillo, M.C.; Kanai, S.; Nokubo, M.; Kitani, K. (−) Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 1991, 48, 517–521. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- MATLAB, version 6.5.1.; MathWorks Inc.: Natick, MA, USA, 2003.
- Orav, A.; Koel, M.; Kailas, T.; Müürisepp, M. Comparative analysis of the composition of essential oils and supercritical carbon dioxide extracts from the berries and needles of Estonian juniper (Juniperus communis L.). Procedia Chem. 2010, 2, 161–167. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Srinivasan, C.; Liba, A.; Imlay, J.A.; Valentine, J.S.; Gralla, E.B. Yeast lacking superoxide dismutase(s) show elevated levels of “free iron” as measured by whole cell electron paramagnetic resonance. J. Biol. Chem. 2000, 275, 29187–29192. [Google Scholar] [CrossRef]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.-T. α-Terpinene, an antioxidant in tea tree oil, autoxidizes rapidly to skin allergens on air exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef]
- Elmastaş, İ.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What role do they play in physical activity and health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [PubMed]
- Schüller, C.; Brewster, J.L.; Alexander, M.R.; Gustin, M.C.; Ruis, H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994, 13, 4382–4389. [Google Scholar] [PubMed]
- Jamieson, D.J.; Rivers, S.L.; Stephen, D.W. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology 1994, 140, 3277–3283. [Google Scholar] [CrossRef]
- Avery, A.M.; Avery, S.V. Saccharomyces cerevisiae expresses three phospholipid hydroperoxide glutathione peroxidases. J. Biol. Chem. 2001, 276, 33730–33735. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (Acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Parveen, M.; Hasan, M.K.; Takahashi, J.; Murata, Y.; Kitagawa, E.; Kodama, O.; Iwahashi, H. Response of Saccharomyces cerevisiae to a monoterpene: Evaluation of antifungal potential by DNA microarray analysis. J. Antimicrob. Chemother. 2004, 54, 46–55. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81-98. https://doi.org/10.3390/antiox3010081
Höferl M, Stoilova I, Schmidt E, Wanner J, Jirovetz L, Trifonova D, Krastev L, Krastanov A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants. 2014; 3(1):81-98. https://doi.org/10.3390/antiox3010081
Chicago/Turabian StyleHöferl, Martina, Ivanka Stoilova, Erich Schmidt, Jürgen Wanner, Leopold Jirovetz, Dora Trifonova, Lutsian Krastev, and Albert Krastanov. 2014. "Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism" Antioxidants 3, no. 1: 81-98. https://doi.org/10.3390/antiox3010081
APA StyleHöferl, M., Stoilova, I., Schmidt, E., Wanner, J., Jirovetz, L., Trifonova, D., Krastev, L., & Krastanov, A. (2014). Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants, 3(1), 81-98. https://doi.org/10.3390/antiox3010081






