Effect of Vitamin C on the Antioxidant and Immune Response of Male White Shrimp (Penaeus vannamei) Broodstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Dietary Proximal Analysis
2.3. Experimental Procedures
2.4. Sample Collection
2.5. Hemolymph Biochemical Analysis
2.6. Reproductive System Biochemical Analysis
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Spermatic Quantity and Quality
2.9. Statistical Analyses
3. Results
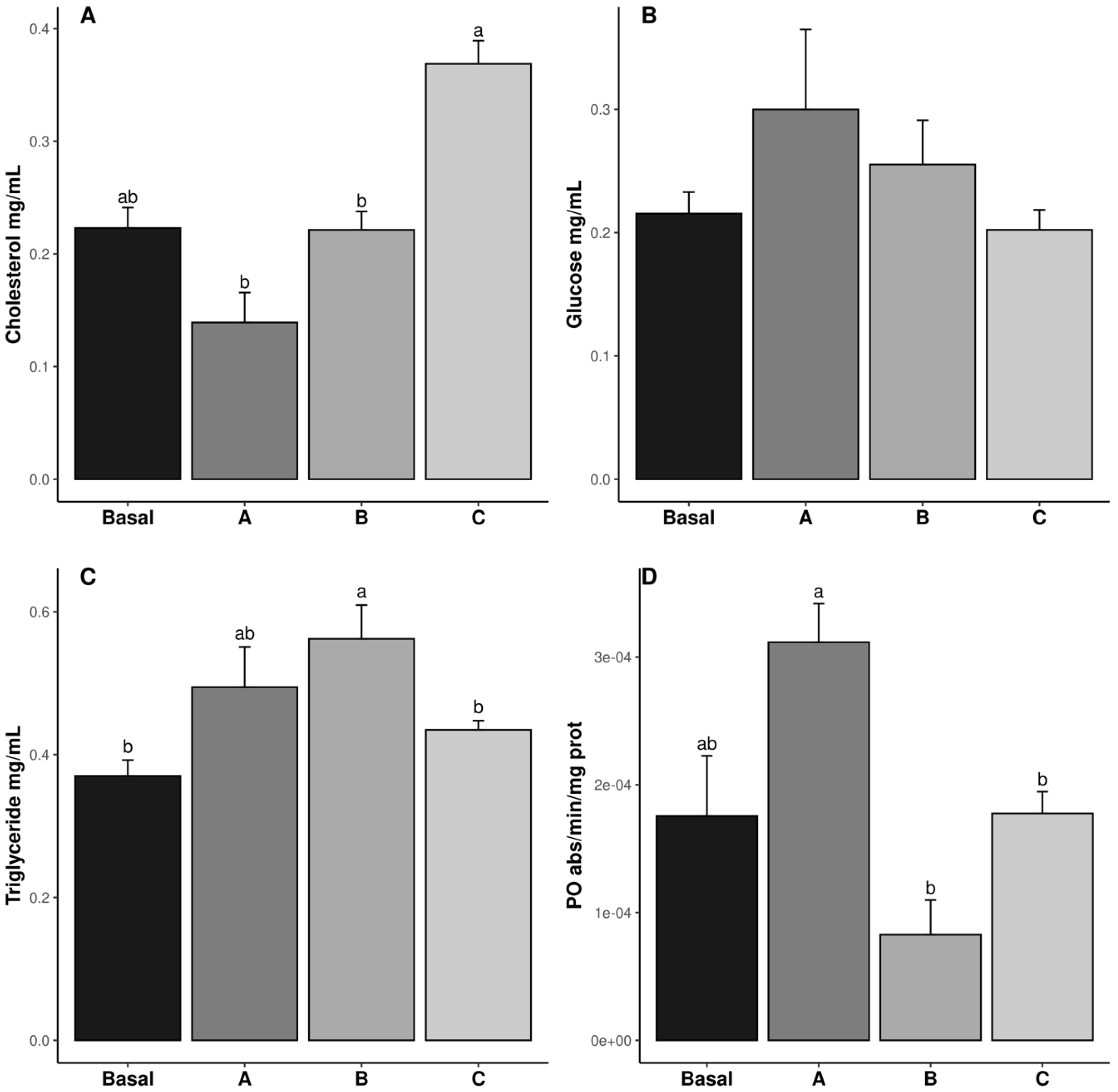
3.1. Hemolymph Biochemical Analysis
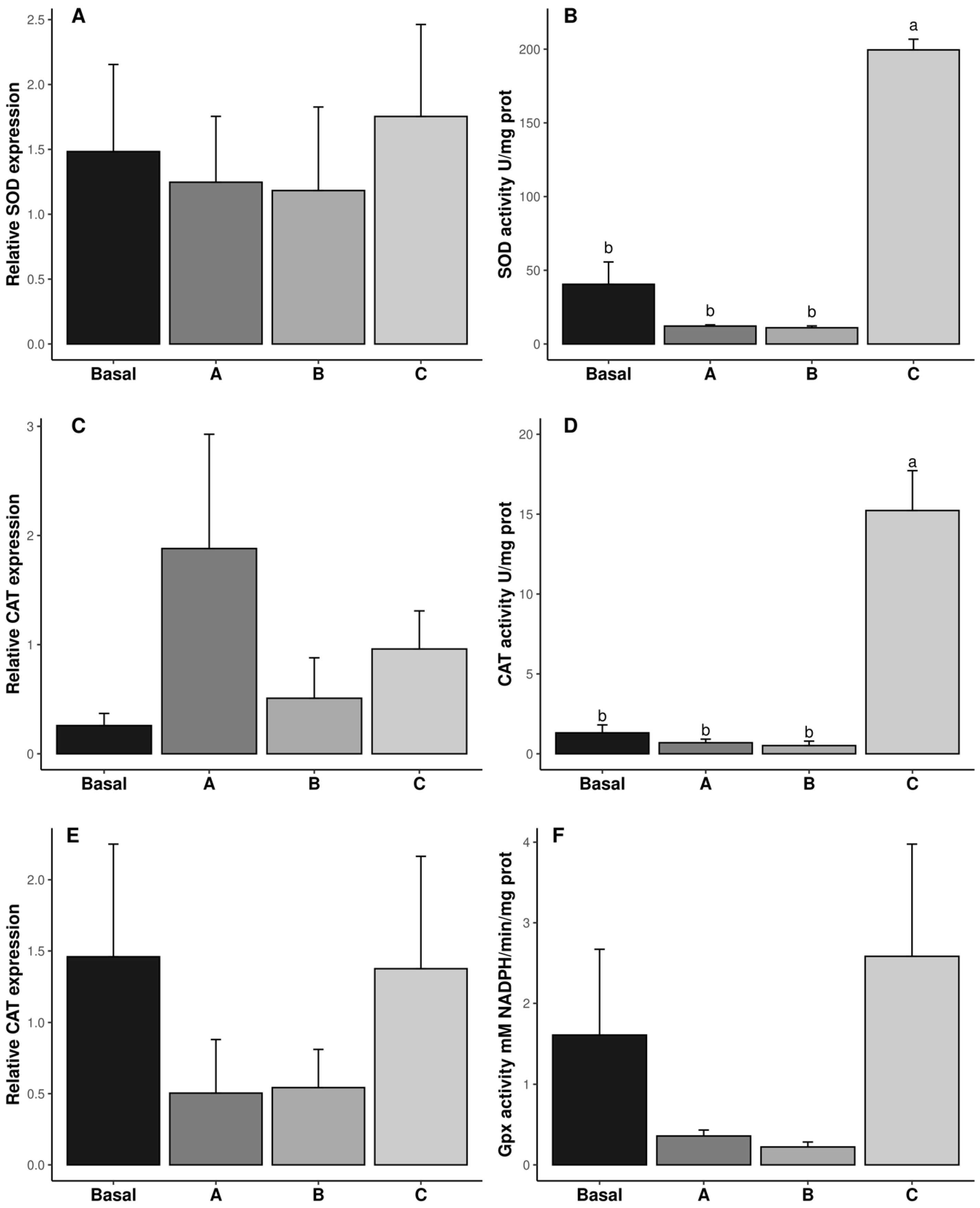
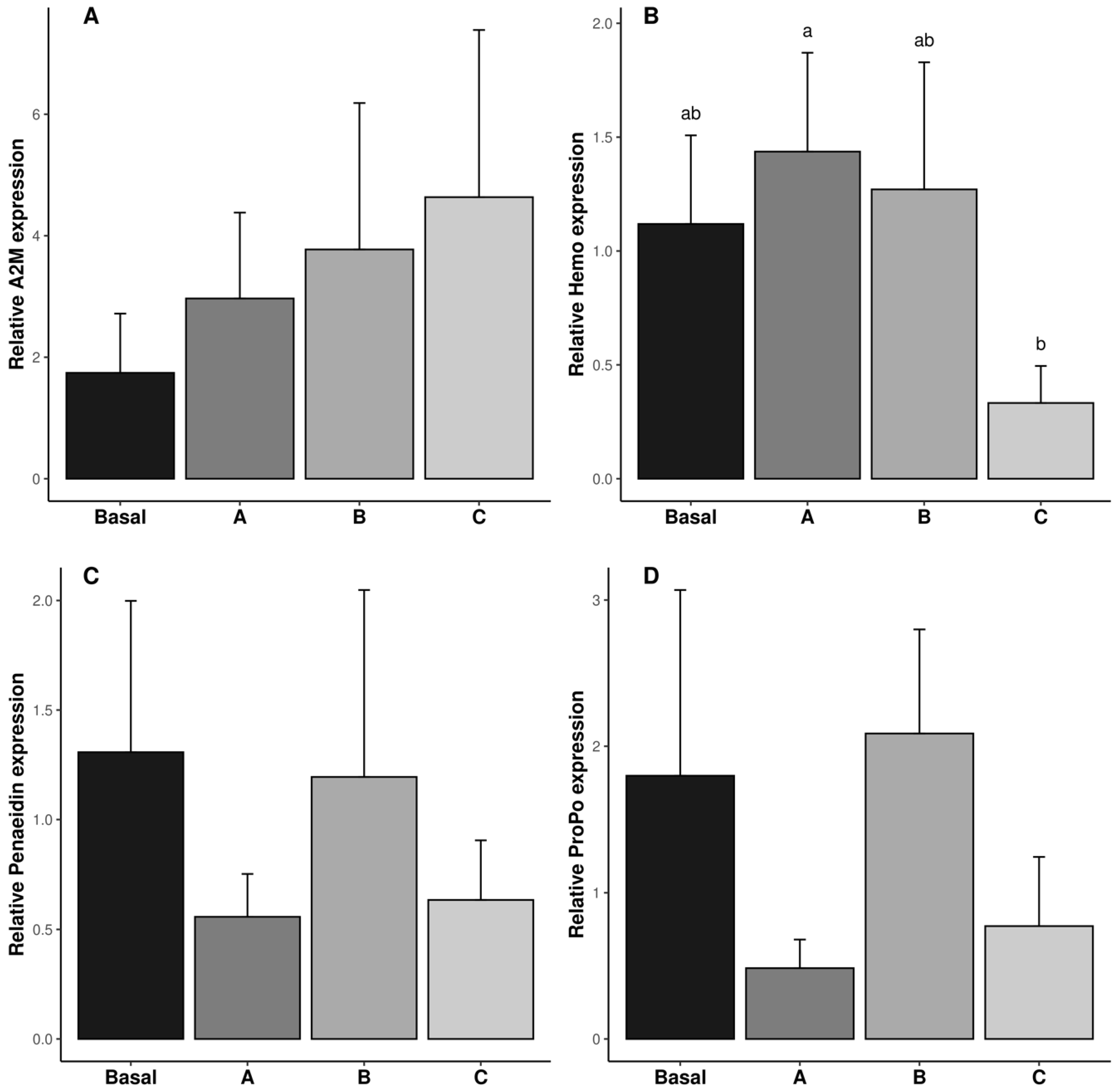
3.2. Biochemical Analysis and Relative Expression of Genes of Antioxidant Enzyme System in the Reproductive System
3.3. Spermatic Quantity and Quality
4. Discussion
4.1. Hemolymph Biochemical Analysis
4.2. Biochemical Analysis and Relative Expression of Genes in the Reproductive System
4.3. Relative Gene Expression of Immune-Related System
4.4. Spermatic Quantity and Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, A.; Thi, V.P.; To, H.; Maria, J.; Santigosa, E. Vitamin nutrition in shrimp aquaculture: A review focusing on the last decade. Aquaculture 2024, 578, 740004. [Google Scholar] [CrossRef]
- Lightner, D.V.; Hunter, B.; Magarelli, P.C., Jr.; Colvin, L.B. Ascorbic acid: Nutritional requirement and role in wound repair in penaeid shrimp. Proc. World Maric. Soc. 1979, 10, 513–528. [Google Scholar] [CrossRef]
- Strain, J.J.; Mulholland, C.W. Vitamin C and vitamin E—Synergistic interactions in vivo? Free Radic. Aging 1992, 62, 419–422. [Google Scholar]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Lee, K.-J. Dietary vitamin C reduces mortality of pacific white shrimp (Penaeus vannamei) post-larvae by Vibrio parahaemolyticus challenge. Fish Shellfish Immunol. 2024, 151, 109723. [Google Scholar] [CrossRef]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M. Effect of different levels of dietary vitamin C on growth performance, muscle composition, antioxidant and enzyme activity of Macrobrachium rosenbergii. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 477–486. [Google Scholar] [CrossRef]
- Asaikkutti, A.; Bhavan, P.S.; Vimala, K.; Karthik, M.; Cheruparambath, P. Effect of different levels dietary vitamin C on growth performance, muscle composition, antioxidant and enzyme activity of freshwater prawn, Macrobrachium malcolmsonii. Aquac. Rep. 2016, 3, 229–236. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Liau, S.-Y.; Huang, C.-T.; Nan, F.-H. Beta 1,3/1,6-glucan and vitamin C immunostimulant the non-specific immune response of white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 57, 269–277. [Google Scholar] [CrossRef]
- Moe, Y.Y.; Koshio, S.; Teshima, I.; Ishikawa, M.; Matsunaga, Y.; Panganiban, A. Effect of vitamin C derivatives on the performance of larval kuruma shrimp, Marsupenaeus japonicus. Aquaculture 2004, 242, 501–512. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Hsu, T.S. Vitamin C requirement of grass shrimp, Penaeus monodon, as determined with L-ascorbyl-2-monophosphate. Aquaculture 1994, 122, 347–357. [Google Scholar] [CrossRef]
- Ravinder, S.A.; Chavez-Sanchez, M.C.; Martínez-Palacios, C.A.; Martínez-Rodriguez, I.E. Effect of supplementing ascorbic acid (L-ascorbyl-2-polyphosphate) in broodstock diet of the white shrimp Litopenaeus vannamei. J. World Aqua. Soc. 2000, 31, 137–144. [Google Scholar]
- Alava, V.R.; Kanazawa, A.; Teshima, S.; Koshio, S. Effect of phospholipids and n-3 highly unsaturated fatty acids on ovarian development of kuruma prawn. Nippon. Suisan Gakkaishi 1993, 59, 345–351. [Google Scholar] [CrossRef][Green Version]
- Alfaro Montoya, J. The reproductive conditions of male shrimps, genus Penaeus, sub-genus Litopenaeus (open thelyca penaeoid shrimps): A review. Aquaculture 2010, 300, 1–9. [Google Scholar] [CrossRef]
- Ortiz-Guillén, S.; Magaña-Gallegos, E.; Arévalo, M.; Cuzon, G.; Gaxiola, G. Partial substitution of frozen-fresh food by an experimental diet in Litopenaeus vannamei (Boone, 1931) (Decapoda: Dendrobranchiata: Penaeidae) broodstock. Anim. Reprod. Sci. 2022, 242, 107001. [Google Scholar] [CrossRef]
- Chimsung, N. Maturation diets for black tiger shrimp (Penaeus monodon) broodstock: A review. Songklanakarin J. Sci. Technol. 2014, 36, 265–273. [Google Scholar]
- Montalvo, G.; Morones, R.; Barral, X.; Arenas, M.; Barreto, A.; Escalante, K.; Cuzon, G.; Gaxiola, G. Metabolic, antooxidant and inmune resposnse of broodstock males of Penaeus brasiliensis, Latreille, 1817 fed diets supplemented by vitamin C. J. Crustac. Biol. 2022, 42, ruac056. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Tyagi, A.; Benjakul, S. Shrimp Culture Technology Farming, Health Management and Quality Assurance; Springer: Singapore, 2025; p. 458. [Google Scholar]
- Diamond, S.; Powell, A.; Shields, R.J.; Rowley, A.F. Is spermatophore melanization in captive shrimp (Litopenaeus vannamei) a result of an auto-immune response? Aquaculture 2008, 285, 14–18. [Google Scholar] [CrossRef]
- Braga, A.; Lopes, D.; Magalhães, V.; Klosterhoff, M.C.; Romano, L.A.; Poersch, L.H.; Wasielesky, W. Hemocytic melanization in shrimp spermatophores. Aquaculture 2018, 486, 64–67. [Google Scholar] [CrossRef]
- Sánchez, A.; Pascual, C.; Sánchez, A.; Vargas-Albores, F.; Le Moullac, G.; Rosas, C. Hemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: The effect of acclimation. Aquaculture 2001, 198, 13–28. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Carpenter, C.E. Food content determination. In Food Analysis (Food Science and Text Series); Suzanne-Nielsen, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 117–119. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of the Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Zeng, W.; Martinuzzi, F.; MacGregor, A. Development and application of a novel UV method for the analysis of ascorbic acid. J. Pharm. Biomed. Anal. 2005, 5, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hernández-López, J. Diseño de Técnicas para la Cuantificación de Moléculas Plasmáticas de Camarón. Ph.D. Thesis, Centro de Investigación en Alimentación y Desarrollo, A.C., Hermosillo, Sonora, Mexico, 2001. [Google Scholar]
- Marie, B.; Genard, B.; Rees, J.-F.; Zal, F. Effect of ambient oxygen concentration on activities of enzymatic antioxidant defenses and aerobic metabolism in the hydrothermal vent worm, Paralvinella grasslei. Mar. Biol. 2006, 150, 273–284. [Google Scholar] [CrossRef]
- Flohé, L.; Ötting, F. Superoxide dismutase assays. Method. Enzymol. 1985, 105, 93–105. [Google Scholar]
- Góth, L. A simple method for determination of serum catalase activity and revision of references range. Clin. Chim. Acta 1991, 196, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Madhusoodanan, U.K.; Sharanabasappa, M.; Sandip-Ghosh, J.J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clin. Chim. Acta 2003, 337, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Aguilera-Rivera, D.; Prieto-Davó, A.; Rodriguez-Fuentes, G.; Escalante-Herrera, K.; Hernpandez-López, J.; Chávez-Sanchéz, C.; Rodriguez-Canul, R.; Gaxiola, G. Immune response of the pacific white shrimp Litopenaeus vannamei previously rared in biofloc and after an infection assay with Vibrio harveyi. J. World Aqua. Soc. 2018, 50, 119–136. [Google Scholar] [CrossRef]
- Montalvo, G.; Campos, S.; Arenas, M.; Barreto, A.; Escalante, K.; Cuzon, G.; Gaxiola, G. Immune gene expression and antioxidant response to vitamin E enriched diets for males Litopenaeus vannamei breeder (Boone, 1931). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 268, 111187. [Google Scholar] [CrossRef]
- Leung-Trujillo, J.R.; Lawrence, A.L. Observation on the decline in sperm quality of Penaeus setiferus under laboratory conditions. Aquaculture 1987, 65, 363–370. [Google Scholar] [CrossRef]
- A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 12 September 2024).
- Emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 10 September 2024).
- nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 10 September 2024).
- He, H.; Lawrence, A.L. Vitamin E requirement of Penaeus vannamei. Aquaculture 1993, 118, 245–255. [Google Scholar] [CrossRef]
- Premkumar, K.; Bowlus, C.L. Ascorbic acid does not increase the oxidative stress induced by dietary iron in C3H mice. J. Nutr. 2004, 134, 435–438. [Google Scholar] [CrossRef]
- Sakuma, N.; Yoshikawa, M.; Hibino, T.; Sato, T.; Kamiya, Y.; Ohte, N.; Tamai, N.; Kunimatsu, M.; Kimura, G.; Inoue, M. Ascorbic acid protects against peroxidative modification of low-density lipoprotein, maintaining its recognition by LDL receptors. J. Nutr. Sci. Vitaminol. 2001, 47, 28–31. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: A meta-analysis of 13 randomized controlled trials. J. Chiropr. Med. 2008, 7, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Feller, A.G.; Rudman, D. Role of carnitine in human nutrition. J. Nutr. 1988, 118, 541–547. [Google Scholar] [CrossRef]
- Fernandez, M.L. Guinea pigs as models for cholesterol and lipoprotein metabolism. J. Nutri. 2001, 131, 10–20. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Marieshwari, B.N.; Bhuvaragavan, S.; Sruthi, K.; Mullainadhan, P.; Janarthanan, S. Insect phenoloxidase and its diverse roles: Melanogenesis and beyond. J. Comp. Physiol. B 2023, 193, 1–23. [Google Scholar] [CrossRef]
- Amparyup, P.; Charoensapsri, W.; Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013, 34, 990–1001. [Google Scholar] [CrossRef]
- Perveen, S.; Yang, L.; Xie, X.; Han, X.; Gao, Q.; Wang, J.; Wang, C.; Yin, F. Vitamin C elicits the activation of immunological responses in swimming crab (Portunus trituberculatus) hemocytes against Mesanophrys sp. Aquaculture 2022, 547, 737447. Aquaculture 2022, 547, 737447. [Google Scholar] [CrossRef]
- Hong, Y.A.; Park, C.W. Catalytic antioxidants in the kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Brand, I. Kinetics of glutathione peroxidase. Biochim. Biophys. Acta (BBA)–Enzymol. 1969, 191, 541–549. [Google Scholar] [CrossRef]
- Castillo-Velarde, E.R. Vitamin C in health and disease. Rev. Fac. Med. Hum. 2019, 19, 95–100. [Google Scholar]
- Lee, M.-H.; Shiauf, S.-Y. Dietary vitamin C and its derivatives affect immune responses in grass shrimp, Penaeus monodon. Fish Shellfish Immunol. 2022, 12, 119–129. [Google Scholar] [CrossRef]
- Ogura, Y. Catalase activity at high concentration of hydrogen peroxide. Arch. Biochem. Biophy. 1955, 57, 288–300. [Google Scholar] [CrossRef]
- Barral-Pintos, X.; Arévalo, M.; Escalante, K.; Arenas, M.; Morones, R.; Velázquez, E.; Gaxiola, G. Sperm quality of Litopenaeus vannamei fed fresh or experimental food in two culture systems. J. World Aqua. Soc. 2024, 55, e13025. [Google Scholar] [CrossRef]

| Gene | Sequence | Size pb | GenBank Accession No. | |
|---|---|---|---|---|
| Forward | Reverse | |||
| β-actin | 5′-TGTGTGACGACGAAGTAGCC-3′ | 5′-TGGTCGTGAAGGTGTAACCA-3′ | 142 | AF300705 |
| SOD | 5′-AGCTTACATCTCCATCCTGG-3′ | 5′-ATCTGAGGACTGACTGTGC-3′ | 189 | DQ298207 |
| CAT | 5′-ACTCCCATTGCTGTTCGT-3′ | 5′-ATCCCAATTTCCTTCTTCTG-3′ | 195 | JX162772 |
| GPx | 5′-AGTCGATGTCAACGGGTCAAC-3′ | 5′-GCTGAACCTCTTAAACGGCTG-3′ | 180 | AY973252 |
| a2M | 5′-GTTTCCATCACCGCCTCA-3′ | 5′-ACCTTATCCTGCGGTGCCA-3′ | 227 | EF182745 |
| ProFo | 5′-ACCGTACAAGGAAGAGGAAC-3′ | 5′-TCTCGCAGGTCGTTGTTGAT-3′ | 222 | AY723296 |
| Hemo | 5′-GTCTTAGTGGTTCTTGGGCTTGTC-3′ | 5′-GGTCTCCGTCCTGAATGTCTCC-3′ | 124 | KJ151291 |
| Penaeidin | 5′-CTGGTCTTCTTGCCTCCTT-3′ | 5′-ATATCCCTTTCCCACGTGAC-3′ | 121 | AF390139 |
| Treatment | Mean | SE |
|---|---|---|
| Basal | 0.058 | 0.00585684 |
| A | 0.04 | 0.01921331 |
| B | 0.03 | 0.01964186 |
| C | 0.047 | 0.03985511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo, G.; Caballeros, S.; Escalante, K.; Barreto, A.; Arenas, M.; Gaxiola, G. Effect of Vitamin C on the Antioxidant and Immune Response of Male White Shrimp (Penaeus vannamei) Broodstock. Antioxidants 2025, 14, 988. https://doi.org/10.3390/antiox14080988
Montalvo G, Caballeros S, Escalante K, Barreto A, Arenas M, Gaxiola G. Effect of Vitamin C on the Antioxidant and Immune Response of Male White Shrimp (Penaeus vannamei) Broodstock. Antioxidants. 2025; 14(8):988. https://doi.org/10.3390/antiox14080988
Chicago/Turabian StyleMontalvo, Grecia, Sarabí Caballeros, Karla Escalante, Alvaro Barreto, Martín Arenas, and Gabriela Gaxiola. 2025. "Effect of Vitamin C on the Antioxidant and Immune Response of Male White Shrimp (Penaeus vannamei) Broodstock" Antioxidants 14, no. 8: 988. https://doi.org/10.3390/antiox14080988
APA StyleMontalvo, G., Caballeros, S., Escalante, K., Barreto, A., Arenas, M., & Gaxiola, G. (2025). Effect of Vitamin C on the Antioxidant and Immune Response of Male White Shrimp (Penaeus vannamei) Broodstock. Antioxidants, 14(8), 988. https://doi.org/10.3390/antiox14080988








