Abstract
Cellular senescence, a state of permanent cell cycle arrest, represents a double-edged sword in biology—providing tumor-suppressive functions while contributing to tissue degeneration, chronic inflammation, and age-related diseases when senescent cells persist. A key driver of senescence is oxidative stress, primarily mediated by excessive reactive oxygen species that damage mitochondrial DNA, modulate redox-sensitive signaling pathways, and trigger the senescence-associated secretory phenotype. Emerging evidence highlights the pathogenic role of SASP in promoting local inflammation, immune evasion, and senescence propagation. This review explores the intricate interplay between redox imbalance and cellular senescence, emphasizing mitochondrial dysfunction, SASP dynamics, and their implications in aging and cancer. We discuss current senotherapeutic strategies—including senolytics, senomorphics, antioxidants, gene therapy, and immunotherapy—that aim to eliminate or modulate senescent cells to restore tissue homeostasis. Understanding the heterogeneity and context-specific behavior of senescent cells remains crucial for optimizing these therapies. Future research should focus on addressing key knowledge gaps, including the standardization of senescence biomarkers such as circulating miRNAs, refinement of predictive preclinical models, and development of composite clinical endpoints. These efforts are essential to translate mechanistic insights into effective senotherapeutic interventions and enable the safe integration of senescence-targeting strategies into routine clinical practice.
1. Introduction
Cellular senescence, a stable and irreversible form of cell cycle arrest, has emerged as a central biological process at the intersection of aging, chronic diseases, and cancer [1]. While initially recognized for its tumor-suppressive role by preventing the proliferation of damaged cells, senescence is now understood as a complex, context-dependent phenomenon that also drives tissue dysfunction, chronic inflammation, and age-related pathologies when senescent cells accumulate [2]. A pivotal factor in the induction and maintenance of senescence is oxidative stress, driven by an imbalance between reactive oxygen species (ROS) production and antioxidant defenses [3]. Excessive ROS not only cause direct macromolecular damage but also initiate signaling cascades that reinforce senescence and trigger the senescence-associated secretory phenotype (SASP), a pro-inflammatory state with systemic implications [3]. Recent studies emphasize the complex interaction between redox imbalance, mitochondrial dysfunction, deoxyribonucleic acid (DNA)damage response, and changes in cellular metabolism that help to maintain senescence. However, there is still debate about the double-edged role of senescence in both health and disease, as well as the most effective ways to target it therapeutically. In this comprehensive review, we explore the mechanistic links between oxidative stress and cellular senescence, summarize emerging senotherapeutic approaches—including senolytics, senomorphics, gene therapies, and redox modulators—and discuss the translational challenges in targeting senescence to improve healthspan and manage age-related diseases.
2. Definition and Biological Nature of Reactive Oxygen Species
The concept of oxidative stress was first explored in the mid-20th century and has gained increasing scientific interest since the 1970s [4]. It was originally described as an imbalance between oxidants and antioxidants, favoring the oxidants and leading to potential cellular damage [5]. Later, this concept was expanded to include disruption in redox signaling and regulation [6].
Reactive oxygen species are chemically reactive molecules that contain oxygen [7]. They include both radical forms—such as superoxide anion (O2−•) and hydroxyl radical (OH•)—and non-radical forms like hydrogen peroxide (H2O2) [7]. Some of these species are stable, while others are highly reactive and capable of causing significant cellular damage [7]. ROS can also interact with reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite (ONOO−), leading to similar harmful effects [8,9].
Among various ROS, those classified as highly reactive (hiROS), such as hydroxyl radicals and singlet oxygen, are especially damaging to proteins, lipids, and DNA [10]. Less reactive species (loROS), such as superoxide and hydrogen peroxide, are often involved in physiological processes like intracellular signaling [10].
This entire network of ROS, sometimes referred to as the ROS physiome, includes a wide variety of molecules with different reactivities [11]. While some ROS contribute to important cellular functions, excessive accumulation leads to oxidative stress and may initiate pathological processes [11].
2.1. Endogenous and Exogenous Sources of Reactive Oxygen and Nitrogen Species
Reactive oxygen species and reactive nitrogen species are continuously generated in biological systems, both under normal physiological conditions and in response to pathological stimuli [11,12]. The mitochondria are widely recognized as a major source of intracellular ROS, primarily through electron leakage during oxidative phosphorylation [13]. Inefficient electron transfer at respiratory complexes I and III leads to partial reduction of oxygen, forming superoxide anions and subsequently other ROS [14]. Given that adenosine triphosphate (ATP) synthesis is tightly coupled with oxygen consumption, mitochondrial metabolism inherently generates ROS as a byproduct of aerobic respiration [15].
In addition tomitochondrial respiration, ROS are also generated by several enzyme systems. These include nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX1–5), dual oxidases (DUOX1–2), xanthine oxidase, nitric oxide synthases, cytochrome P450 enzymes, and monoamine oxidases [16]. These enzymes are distributed across distinct cellular compartments such as the cytosol, plasma membrane, endoplasmic reticulum, and peroxisomes, each contributing to the oxidative burden in different contexts [17]. For example, peroxisomal β-oxidation of fatty acids and the activity of oxidoreductases also generate ROS, further amplifying redox signaling and oxidative damage [18].
Exogenous factors also play a substantial role in ROS/RNS generation [19]. Various external factors can trigger or increase the production of ROS. These include environmental pollutants, ionizing radiation, xenobiotics, certain drugs, such as chemotherapeutics, and specific dietary components [20,21,22]. Additionally, during immune responses, phagocytic cells such as neutrophils and macrophages produce a burst of ROS and RNS via NADPH oxidase and inducible nitric oxide synthase as part of host defense mechanisms [23,24].
2.2. Antioxidant Defense Networks and the Nrf2 Axis in Redox Homeostasis and Aging
The dynamic equilibrium between prooxidant and antioxidant forces governs cellular redox homeostasis and is vital for the preservation of physiological function [25]. Under basal conditions, this balance supports signaling fidelity, energy metabolism, and cellular integrity [26]. However, disruption of this equilibrium, typically in favor of oxidants, leads to oxidative and nitrosative stress, driving molecular damage and contributing to the onset of aging and degenerative diseases [26].
To mitigate oxidative insults, cells have evolved an intricate antioxidant defense system encompassing both enzymatic and non-enzymatic components [27]. These mechanisms function across three principal levels: preventing the formation of ROS, intercepting and neutralizing radicals, and repairing or removing oxidatively damaged biomolecules [28].
The frontline defense consists of enzymatic antioxidants, including superoxide dismutases (SODs), catalase (CAT), glutathione peroxidases (GPxs), glutathione reductase (GR), glutamyl transpeptidase (GGT), and thioredoxin reductases [29,30]. These enzymes neutralize ROS through catalysis, thereby preventing chain reactions that would otherwise propagate cellular injury [30]. Non-enzymatic antioxidants, such as reduced glutathione, ascorbate (vitamin C), tocopherol (vitamin E), uric acid, and carotenoids, act as radical scavengers by donating electrons, effectively terminating oxidative cascades without becoming reactive themselves [31,32].
Preventive antioxidants help to reduce the formation of ROS by binding transition metals like iron and copper. Plasma proteins such as transferrin, ferritin, ceruloplasmin, and albumin act in this way, limiting Fenton chemistry and preventing further ROS production [28,33]. A third layer of protection involves enzymatic repair systems that degrade oxidized lipids, proteins, and nucleic acids, alongside regeneration pathways that restore damaged macromolecules [28].
Central to redox regulation is the Kelch-like ECH-associated protein 1–nuclear factor erythroid 2–related factor 2 (Keap1–Nrf2) signaling pathway [34]. Under homeostatic conditions, Nrf2 is sequestered in the cytoplasm by Keap1, which targets it for ubiquitin-mediated degradation [34,35]. Upon oxidative or electrophilic challenge, conformational changes in Keap1 release Nrf2, allowing its nuclear translocation [36]. In the nucleus, Nrf2 forms a complex with small musculoaponeurotic fibrosarcoma (Maf) proteins. This complex binds to antioxidant response elements (AREs) and activates the transcription of several antioxidant genes. These include heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), superoxide dismutases (SODs), glutathione peroxidases (GPxs), ferritin, and thioredoxin [36,37].
This transcriptional network coordinates an adaptive response to redox perturbations and is pivotal for cytoprotection. However, Nrf2 activity declines with age, rendering cells more susceptible to oxidative stress and promoting senescence. Conversely, pharmacological activation of Nrf2 has been shown to delay senescence and extend cellular lifespan in preclinical models [35].
Exogenous antioxidants derived from the diet complement endogenous systems [38]. These include trace elements (e.g., selenium, zinc), vitamins C and E, carotenoids, and a broad spectrum of phytochemicals found in fruits, vegetables, coffee, tea, and whole grains [28]. Such compounds reduce lipid peroxidation, modulate signaling cascades, and support enzymatic antioxidant activity. In populations with impaired endogenous defenses, such as the elderly or individuals with chronic disease, dietary antioxidants may provide significant protection [28]. Elevated plasma levels of vitamin C and β-carotene have been correlated with improved cognitive performance and reduced oxidative stress in aging cohorts [39].
2.3. Circadian Regulation of Nrf2 Signaling: A Bidirectional Link Between the Molecular Clock and Redox Homeostasis
Emerging evidence indicates that the Nrf2/ARE pathway is under circadian regulation, integrating redox homeostasis with the body’s internal clock [40]. This link is of particular relevance in the context of aging and senescence, as both redox imbalance and circadian disruption are known hallmarks of aging tissues [41].
The molecular circadian clock is driven by two interlocking transcriptional–translational feedback loops (TTFLs) [42]. In the primary loop, the heterodimer composed of Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT-Like 1 (BMAL1) activates the transcription of core clock genes by binding to E-box elements within their promoter regions [42]. These target genes include Period homologs (PER1, PER2, and PER3) and Cryptochrome homologs (CRY1 and CRY2), which function as negative regulators by inhibiting the activity of the CLOCK/BMAL1 complex, thereby generating circadian oscillations [42].
Importantly, BMAL1 has been shown to bind directly to E-box motifs in the promoter region of the Nrf2 gene, thereby regulating the rhythmic expression of Nrf2 and its downstream antioxidant genes such as NAD(P)H quinone dehydrogenase 1 (NQO1), heme oxygenase 1 (HMOX1), and glutamate–cysteine ligase modifier subunit (GCLM) [43]. Cells show decreased antioxidant capacity and increased vulnerability to oxidative damage when Nrf2 expression reaches its lowest point during the circadian cycle [44].
BMAL1 thus plays a dual role as a regulator of both circadian rhythm and redox balance [45]. Studies in human lens epithelial cells have shown that aging is accompanied by a decline in BMAL1–CLOCK expression and a concomitant reduction in Nrf2-regulated antioxidant gene expression [46]. This is associated with increased levels of ROS. Overexpression of BMAL1 in these cells enhances the expression of Nrf2, NQO1, SOD, and peroxiredoxin 6 (PRDX6) at both the mRNA and protein levels, resulting in reduced oxidative stress [46].
BMAL1 can also activate nuclear factor Nrf2, which then interacts with other stress-response pathways. These include the nuclear factor kappa B (NF-κB) signaling pathway and the heat shock response (HSR). Together, they form a protective network that defends cells against ROS-induced apoptosis, inflammation, and tissue damage [40]. Loss of BMAL1 impairs this network: its deletion suppresses Nrf2 expression and antioxidant defense while simultaneously increasing the production of interleukin-1 beta (IL-1β), a proinflammatory cytokine [40].
Intriguingly, the relationship is bidirectional. Nrf2 can also influence circadian rhythm. In hepatocytes, activated Nrf2 has been shown to bind to enhancer regions of the Cryptochrome 2 (CRY2) gene, enhancing its expression and thereby modulating CLOCK/BMAL1-driven transcription. These findings suggest that Nrf2 and the circadian clock form an integrated feedback loop that synchronizes redox signaling with tissue-specific circadian timekeeping [40].
2.4. Redox Signaling: Dual Role of ROS in Cell Fate and Disease Progression
Beyond their damaging potential, ROS are now widely recognized as integral regulators of diverse cellular processes [47]. These include cell signaling, gene regulation, immune system activity, and control of cell survival, growth, and programmed cell death [47,48]. The concept of “redox biology” has emerged to describe this functional dimension of ROS, highlighting their role as signaling intermediates rather than mere byproducts of metabolism [47].
The effects of ROS on cells depend on their concentration and the specific cellular context [49]. Low-to-moderate levels modulate redox-sensitive signaling cascades, whereas excessive or prolonged exposure shifts ROS from regulatory agents to cytotoxic mediators [31]. Their effects are dictated by molecular species, subcellular localization, and interaction with target proteins [50]. In particular, redox-sensitive transcription factors and kinases, such as Nrf2, tumor protein p53 (p53), NF-κB, forkhead box O (FOXO), activator protein 1 (AP-1), and hypoxia-inducible factor 1-alpha (HIF-1α), serve as redox sensors, initiating adaptive or pathological responses depending on ROS dynamics [3].
In stem cell biology, ROS act as molecular switches that translate external signals such as nutrients, cytokines, and oxygen levels into internal decisions that control self-renewal, dormancy, or differentiation [51,52]. However, sustained or excessive ROS levels can drive stem cell exhaustion and compromise regenerative potential [51]. Reactive oxygen species produced by mitochondria also play a key role in “mitohormesis”. This is a process where low levels of oxidative stress trigger beneficial adaptive responses, helping to extend healthspan and delay the development of age-related diseases [53].
On the other hand, chronic oxidative or nitrosative stress resulting from persistent ROS accumulation or impaired detoxification disturbs redox homeostasis [31]. This leads to lipid peroxidation, protein oxidation, and DNA damage, particularly within mitochondrial and nuclear genomes [31]. Inadequate DNA repair mechanisms under oxidative stress conditions contribute to genomic instability and disease susceptibility [54].
High ROS levels are hallmarks of several chronic disorders, including diabetes, atherosclerosis, neurodegeneration, and inflammatory diseases [55]. In immune cells such as neutrophils and macrophages, ROS are crucial for microbial killing [56]. However, sustained activation contributes to tissue damage and chronic inflammation [31]. In the context of cancer, ROS exert paradoxical effects;they can both promote tumor initiation via DNA damage and mutations and support tumor progression by modulating oncogenic pathways, evading apoptosis, and sustaining proliferative signaling [57,58]. The interplay between ROS, p53, and NF-κB is particularly significant in shaping tumor cell fate and metastatic behavior [59]. Furthermore, oncogene-driven metabolic reprogramming can amplify ROS production, reinforcing malignant phenotypes [59].
An integrated summary of ROS types, sources, antioxidant systems, and their physiological and pathological roles is provided in Table 1.

Table 1.
Overview of reactive oxygen species: types, sources, antioxidant defenses, and biological roles.
3. Cellular Senescence: A Double-Edged Regulator of Aging and Disease
Cellular senescence represents a stable and irreversible state of cell cycle arrest initiated by a variety of intrinsic and extrinsic stressors, including telomere attrition, DNA damage, oxidative stress, and oncogene activation [60]. Senescence was first observed in cultured fibroblasts, where cells lost the ability to divide after a certain number of cycles. Today, it is recognized as a wider biological process involved in development, tissue repair, cancer prevention, and aging [2].
Senescent cells show several structural and molecular changes. These include an increase in cell size, a flattened shape, and expression of senescence-associated β-galactosidase (SA-β-gal). They also activate the DNA damage response (DDR), form senescence-associated heterochromatin foci (SAHF), and release inflammatory factors known as the senescence-associated secretory phenotype (SASP) [1]. Central regulators of the senescence program include cyclin-dependent kinase inhibitors such as p16INK4a and p21CIP1, which enforce proliferative arrest by inhibiting key cell cycle regulators [60].
Crucially, new data indicates that circadian regulation affects both p16 and p21 [45]. Time-of-day-dependent control of cell cycle progression is facilitated by the nuclear RNA-binding protein NONO (non-POU domain-containing octamer-binding protein), which has been identified as a circadian output effector that directly and rhythmically activates p16INK4a transcription [61]. Similar to this, the circadian clock regulates the expression of p21CIP1/WAF1, and changes in core clock genes can change p21 levels, which can impact the fidelity and timing of cell cycle arrest [45].
Importantly, senescence can exert both beneficial and detrimental effects [62]. In its acute form, it is a well-regulated process that affects specific cell populations during tissue repair or after injury [2]. These senescent cells often promote their own removal by releasing signals through the SASP, which attracts immune cells and helps to restore tissue balance [2]. In contrast, chronic senescence arises from prolonged exposure to damaging stimuli and is marked by the persistence of senescent cells that escape immune clearance. These cells accumulate over time and contribute to the chronic inflammation and tissue dysfunction observed in aging and age-related diseases, including cancer, atherosclerosis, neurodegeneration, and renal pathologies [63].
3.1. Molecular Triggers of Cellular Senescence
Cellular senescence can be initiated by a variety of intrinsic and extrinsic stressors that converge on key signaling pathways to enforce a stable and irreversible cell cycle arrest [60]. These stimuli often reflect damage or dysfunction at the genomic, metabolic, or organelle level, serving as protective mechanisms to prevent propagation of potentially harmful cells [64]. However, the chronic accumulation of senescent cells contributes to tissue dysfunction and aging-related pathology [65]. The principal molecular triggers of senescence include telomere attrition, DNA damage, oxidative and metabolic stress, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and oncogenic signaling [2].
One of the earliest described forms of senescence, known as replicative senescence, arises from progressive telomere shortening [66]. Telomeres, composed of repetitive nucleotide sequences, protect chromosome ends from degradation [67]. Because of the limitations in DNA replication, known as the end-replication problem, telomeres become shorter with each cell division [68]. When telomeres become too short, the exposed DNA ends trigger a continuous DDR, which causes the cell to permanently stop dividing [66]. This checkpoint, triggered by telomere shortening, acts as a built-in timer that limits how many times a somatic cell can divide. It helps to protect the genome from instability [69,70].
Cells exposed to genotoxic agents—such as ultraviolet (UV) or ionizing radiation, oxidative byproducts of metabolism, or certain chemotherapeutic agents—develop DNA lesions that activate canonical DDR pathways [71]. This response is initiated by the sensing of DNA double-strand breaks (DSBs) primarily by the MRN complex (MRE11-RAD50-NBS1), which recruits and activates ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3-related) kinases [72].
ATM and ATR phosphorylate histone variant H2AX (forming γH2AX), which acts as a platform for the recruitment of DNA repair machinery and checkpoint signaling proteins such as CHK1 and CHK2 [73]. These kinases subsequently stabilize and activate the tumor suppressor p53, which induces the transcription of CDK inhibitor p21CIP1/WAF1. This inhibits CDK2 activity and prevents phosphorylation of the retinoblastoma protein (pRb), enforcing a G1/S cell cycle arrest [60,74].
Simultaneously, persistent stress or replicative signals upregulate p16INK4a, which inhibits CDK4/6 and reinforces the hypophosphorylated state of pRb [60]. The cooperation between the p53/p21 and p16/pRb pathways is essential for establishing and maintaining stable growth arrest characteristic of senescent cells [60]. Importantly, while p21 mediates the initial, reversible arrest in response to acute damage, p16 is associated with long-term, irreversible senescence, particularly in the context of telomere dysfunction and chronic oxidative stress [60]. These mechanisms together represent a protective, tumor-suppressive barrier that prevents propagation of cells with genomic instability.
Mitochondria are central regulators of energy metabolism and redox balance [75]. Dysfunction of the electron transport chain (ETC), especially at complexes I and III, leads to electron leakage and excessive ROS production [75]. These ROS can damage mitochondrial and nuclear components, inducing senescence through p53 and p21 activation [76]. Senescent cells triggered by mitochondrial impairment often shift from oxidative phosphorylation to glycolysis (Warburg-like metabolism), exhibit altered mitochondrial dynamics, and display persistent SASP expression [77]. The interplay between mitochondrial ROS and senescence establishes a self-perpetuating cycle of damage, metabolic reprogramming, and inflammatory signaling that contributes to tissue aging [62].
Metabolic stress, induced by conditions such as glucose overload or nutrient deprivation, contributes significantly to the induction and maintenance of cellular senescence [78]. Senescent cells experience major changes in their metabolism. They rely more on glycolysis, show reduced mitochondrial oxidative phosphorylation, and accumulate damaged mitochondria that produce high levels of ROS [79]. These metabolic changes contribute to redox imbalance and help to maintain the senescent state. They are regulated by nutrient and energy sensing pathways, particularly mTOR and AMPK signaling networks [62]. Chronic activation of mTOR signaling enhances anabolic metabolism, increases oxidative stress, and promotes the synthesis of SASP factors through the IL-1α–NF-κB axis [80]. In contrast, AMPK responds to energy stress by inhibiting mTOR activity and promoting autophagy and catabolic pathways, which help to mitigate oxidative burden and delay the onset of senescence [81]. The tumor suppressor protein p53 also contributes to the metabolic regulation of senescence by modulating mitochondrial function, glycolysis, and the expression of antioxidant enzymes. Experimental evidence from model organisms indicates that interventions targeting these metabolic pathways, such as caloric restriction or pharmacological mTOR inhibition, can restore metabolic homeostasis, suppress SASP expression, and extend both healthspan and lifespan [82].
The endoplasmic reticulum (ER) plays a central role in protein folding, post-translational modification, and quality control, ensuring proper proteostasis within the cell [83]. When the ER’s folding capacity is overwhelmed, due to excessive protein synthesis, oxidative stress, or perturbations in calcium homeostasis, misfolded or unfolded proteins accumulate within the ER lumen, triggering the unfolded protein response (UPR) [84]. At first, this signaling network serves a protective role. It helps to restore protein balance by stopping overall protein production, increasing the levels of molecular chaperones, and removing misfolded proteins through endoplasmic reticulum-associated degradation (ERAD) [84]. However, when ER stress is sustained or unresolved, the UPR transitions from a pro-survival to a pro-senescent or pro-apoptotic program. This switch is mediated by three primary UPR sensors: protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) [85,86,87]. PERK phosphorylates eIF2α, attenuating protein synthesis while selectively enhancing the translation of activating transcription factor 4 (ATF4), which can induce expression of the pro-senescent mediator p21CIP1 and the pro-apoptotic factor CHOP (C/EBP homologous protein) [86,87]. Collectively, these UPR arms intersect with canonical senescence pathways, particularly via p53/p21 axis activation, NF-κB signaling, and redox imbalance, promoting a stable growth arrest and contributing to SASP. Chronic ER stress and maladaptive UPR signaling have been increasingly implicated in the pathogenesis of age-related diseases and in the accumulation of senescent cells during tissue aging and degeneration [87].
Aberrant activation of oncogenes such as rat sarcoma viral oncogene homolog (RAS), rapidly accelerated fibrosarcoma (RAF), and mitogen-activated protein kinase kinase(MEK) can paradoxically induce senescence as an early tumor-suppressive mechanism [88]. This oncogene-induced senescence (OIS) is mediated through upregulation of p16 and alternate reading frame (ARF), which inhibit CDK activity and stabilize p53, respectively [89]. While initially protective, prolonged OIS can be detrimental, particularly through the chronic activity of SASP factors, which modulate the tumor microenvironment [90]. Persistent SASP signaling may promote epithelial–mesenchymal transition (EMT), angiogenesis, and inflammation, facilitating tumor progression in later stages [91]. Thus, OIS exemplifies the dual nature of senescence in cancer biology—as both a barrier and an enabler [74].
3.2. Gene Expression Dynamics in Cellular Senescence
Cellular senescence is a dynamic and multistep process characterized by progressive changes in gene expression that establish and maintain the senescent phenotype [60]. Transcriptomic analyses, particularly time-series studies of replicative senescence, have revealed distinct temporal phases marked by specific gene expression profiles [92]. In the early stages of senescence, genes linked to growth arrest and metabolic changes are mainly active. In later stages, there is a strong increase in genes that regulate inflammation, immune responses, and remodeling of the extracellular matrix. These changes are key features of SASP [92].
Both replicative senescence and stress-induced premature senescence (SIPS) share common transcriptional pathways, particularly those related to inflammation and immune surveillance [93]. The p53/p21 and p16INK4a/Rb signaling axes are central to the initiation and maintenance of growth arrest [94]. However, beyond these canonical pathways, epigenetic remodeling plays a critical role in stabilizing the senescent state [95]. Senescent cells show widespread loss of DNA methylation, along with increased methylation at specific sites. They also display changes in histone modifications that influence how tightly the DNA is packed and how accessible it is for gene expression [96]. For example, loss of the activating histone modification histone 3 lysine 4 trimethylation (H3K4me3) at promoters of cell cycle genes facilitates transcriptional silencing and reinforces proliferative arrest [97].
The tumor suppressor p53 emerges as a key modulator of senescence through its dual role in redox homeostasis and transcriptional regulation [76]. In response to moderate oxidative stress, p53 activates antioxidant defense genes—including manganese superoxide dismutase (MnSOD), Sestrins, and glutathione peroxidase 1—and metabolic regulators such as TP53-induced glycolysis and apoptosis regulator (TIGAR), glutaminase 2 (GLS2), and aldehyde dehydrogenase 4 family member A1 (ALDH4A1), which promote NADPH production, enhance mitochondrial function, and suppress glycolysis, thereby reducing ROS accumulation [76,98]. Conversely, under severe oxidative damage, p53 shifts its transcriptional program toward promoting apoptosis and senescence by upregulating prooxidant genes (e.g., NADPH oxidase and p53-induced genes 1–13 [PIG1–13]) and pro-apoptotic mediators such as Bcl-2-associated X protein (BAX) and p53 upregulated modulator of apoptosis (PUMA), while repressing antioxidant gene expression [76,98].
Beyond its canonical roles, p53 is intricately linked to the circadian clock, forming a bidirectional regulatory network. p53 can modulate the expression of Period 2 (PER2), a core component of the circadian machinery, thereby influencing circadian rhythmicity [99]. In turn, circadian clock proteins—such as PER2, BMAL1, and CRY1—can modulate the stability, localization, and activity of p53, affecting its transcriptional responses in cell cycle arrest, DNA repair, and apoptosis [100]. Notably, the circadian regulation of glutaminase 2, one of p53’s target genes, has been observed, suggesting time-of-day-dependent modulation of tumor metabolism and redox balance [100]. In addition, glutathione peroxidase activity has been shown to oscillate in a circadian manner, with peak levels reported around 2 a.m., indicating that enzymatic redox defense is temporally coordinated [101].
Mechanistic target of rapamycin (mTOR) further influences the bifurcation of cell fate downstream of p53 [102]. Maximal activation of p53 inhibits mTOR and favors reversible quiescence, while partial p53 activation permits continued mTOR signaling, pushing cells toward irreversible senescence [102]. These findings highlight the importance of signal intensity and duration in determining cell fate outcomes following stress.
3.3. Morphological and Functional Alterations in Senescent Cells
Senescent cells undergo major structural and functional changes that set them apart from cells that are actively dividing or in a resting state [62]. These alterations are not merely phenotypic markers but reflect underlying disruptions in cytoskeletal organization, organelle function, and intracellular signaling [103].
A hallmark of senescence is cellular enlargement and flattening, often accompanied by an irregular, spread morphology [2]. These features result from persistent growth arrest in G1 or G2 phases, during which biosynthetic activity continues, leading to cytoplasmic and nuclear expansion [66]. Enlarged nuclei, multinucleation, and nuclear envelope irregularities, including loss of lamin B1, are frequently observed [97,104,105]. Chromatin undergoes extensive remodeling, characterized by the formation of senescence-associated heterochromatin foci, redistribution of chromosomes, and global epigenetic reprogramming [96,97].
Senescent cells exhibit reorganization of the actin cytoskeleton, with increased formation of stress fibers and focal adhesions mediated by proteins such as ezrin and cofilin-1 [66,106,107,108]. These structural adaptations enhance cell spreading and adherence, possibly contributing to their anti-proliferative barrier function in early tumor suppression.
Several organelles also undergo significant remodeling. Lysosomes are increased in number and volume, reflected by elevated senescence-associated β-galactosidase (SA-β-gal) activity at pH 6.0—a widely used senescence biomarker [109,110]. However, despite their expansion, lysosomal function is impaired, leading to accumulation of nondegradable oxidized lipids, proteins, and lipofuscin [110,111]. Mitochondria in senescent cells are often swollen, fragmented, and dysfunctional, exhibiting increased ROS production, impaired mitophagy, and enhanced but ineffective biogenesis [2,112]. These features contribute to a feed-forward loop that exacerbates oxidative stress and reinforces the senescent state.
Endoplasmic reticulum expansion and stress are also prominent [2,87]. High secretory demand, particularly for SASP factors, strains ER capacity and impairs protein folding, leading to further ROS generation and unfolded protein accumulation [113]. Declines in chaperone expression and disulfide-bond formation capacity reduce proteostasis, contributing to ER dysfunction in aged and senescent cells [114].
Peroxisomes, crucial for ROS detoxification and lipid metabolism, also exhibit structural and functional decline in senescence [3,115]. Their impairment disrupts mitochondrial–peroxisomal crosstalk and worsens redox imbalance, promoting further cellular damage [2]. Restoration of peroxisomal function has been shown to delay senescence onset in experimental models [116].
Functionally, senescent cells are characterized by irreversible cell-cycle arrest, commonly mediated via the p53/p21Cip1 and p16Ink4a/pRb pathways [60]. While they do not proliferate, senescent cells remain metabolically active and secrete a complex mix of pro-inflammatory, pro-fibrotic, and tissue-remodeling factors known as the senescence-associated secretory phenotype (SASP) [60,117]. These include cytokines (e.g., interleukin-6 [IL-6], interleukin-8 [IL-8]), chemokines (e.g., C-C motif chemokine ligand 2 [CCL2]), growth factors (e.g., platelet-derived growth factor-AA [PDGF-AA], vascular endothelial growth factor [VEGF]), and proteases (e.g., matrix metalloproteinase 1 [MMP1], matrix metalloproteinase 3 [MMP3]) [60].
SASP factors exert pleiotropic effects [118]. They can promote tissue repair, embryonic development, and immune-mediated clearance of senescent or damaged cells [2]. Conversely, chronic SASP expression fosters low-grade systemic inflammation (“inflammaging”), tissue dysfunction, tumor promotion, and metabolic disorders [2]. SASP is regulated via both DDR-dependent mechanisms (via NF-κB and CCAAT/enhancer-binding protein beta [C/EBPβ]) and DDR-independent pathways, such as cyclic GMP-AMP synthase–stimulator of interferon genes (cGAS–STING)-mediated sensing of cytoplasmic DNA fragments derived from compromised nuclear integrity or infection-related stimuli [2,119,120]. An overview of the diverse effects mediated by SASP on tissue homeostasis and tumor progression is illustrated in Figure 1.
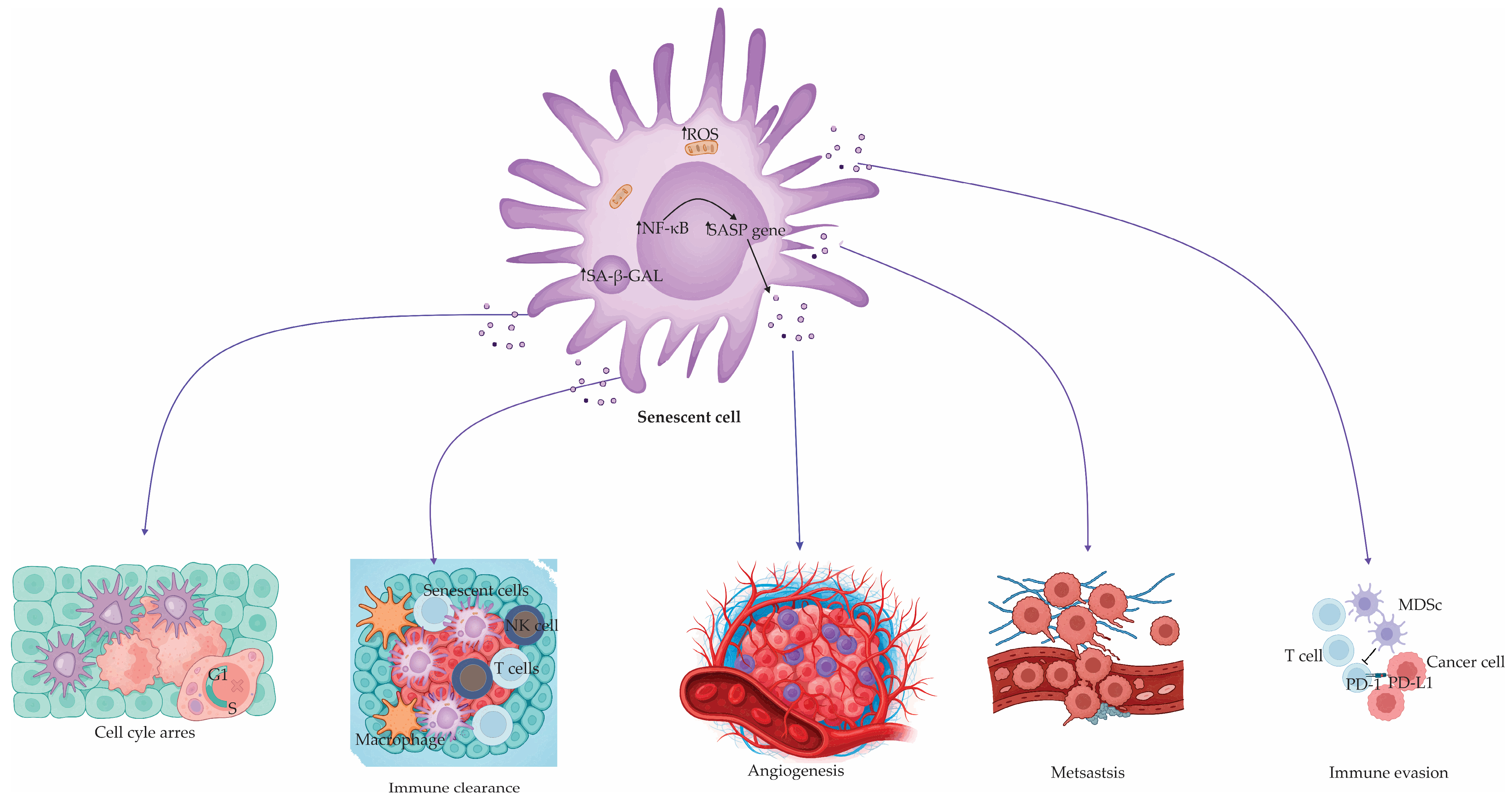
Figure 1.
Senescent cell and SASP-mediated effects on tissue and tumor biology. Senescent cells, despite their proliferative arrest, remain metabolically active and secrete an array of bioactive factors collectively termed the senescence-associated secretory phenotype (SASP). SASP factors are transcriptionally regulated predominantly by NF-κB and contribute to a broad spectrum of physiological and pathological outcomes. The figure illustrates how SASP factors influence the following: (1) induction and maintenance of cell cycle arrest; (2) recruitment of immune cells (e.g., NK cells, T cells, macrophages), facilitating immune clearance of tumor cells; (3) promotion of angiogenesis, supporting tumor vascularization; (4) enhancement of metastatic dissemination of tumor cells; (5) immune evasion via mechanisms such as PD-1/PD-L1 axis activation and recruitment of myeloid-derived suppressor cells (MDSCs). This pleiotropic activity underscores the dualistic nature of senescence in health and disease, balancing regenerative processes with the risk of chronic inflammation and malignancy progression.
Organ-specific senescent phenotypes also vary. For instance, senescent mesenchymal stem cells (MSCs) adopt a flattened, polygonal morphology, while senescent microglia become hypertrophic with shortened processes [121]. In aging kidneys, tubular epithelial cells display characteristic senescent markers alongside decreased proliferative capacity [122]. These morphological and functional features reflect the localized responses to stress and damage in different tissues. A summary of the molecular triggers, cellular changes, and clinical implications of senescence is provided in Table 2.

Table 2.
Molecular triggers, features, and clinical implications of cellular senescence.
4. Oxidative Stress as a Central Driver and Sustainer of Cellular Senescence
Oxidative stress represents a pivotal biological force that not only initiates but also perpetuates cellular senescence [123]. The excessive accumulation of ROS disrupts redox homeostasis and induces macromolecular damage, particularly within DNA and mitochondria, thereby activating stress-responsive signaling pathways that enforce cell cycle arrest and senescence [31,124]. Among the earliest and most critical consequences of oxidative stress is the activation of DDR, often via ROS-induced lesions at telomeric sites [125,126,127]. This leads to the stabilization of p53 and the transcriptional upregulation of cyclin-dependent kinase inhibitors such as p21CIP1 and p16INK4a, culminating in durable growth arrest and the formation of SAHF [128,129,130].
Mitochondrial dysfunction is a critical driver and hallmark of cellular senescence [131]. Damage to mitochondrial DNA (mtDNA), which lacks protective histones and has limited repair capacity, compromises the integrity of respiratory chain complexes and impairs oxidative phosphorylation. This dysfunction results in inefficient electron transfer and increased electron leakage, particularly at complexes I and III, leading to the excessive generation of ROS [132,133]. The accumulation of ROS further damages mitochondrial and nuclear components, establishing a self-perpetuating loop of oxidative stress that stabilizes the senescent phenotype [132,133].
Alongside mitochondrial dysfunction, impaired peroxisomal function exacerbates redox imbalance. Peroxisomes play a vital role in detoxifying ROS, especially hydrogen peroxide, through catalase activity. Their insufficiency in senescent cells contributes to sustained oxidative damage and the reinforcement of redox-sensitive signaling pathways involved in growth arrest and SASP induction [134].
In response to mitochondrial distress, senescent cells activate compensatory pathways aimed at restoring energy homeostasis. One such response involves mitochondrial biogenesis driven by activation of the ataxia-telangiectasia mutated (ATM) kinase, which, through downstream effectors including protein kinase B (Akt) and mechanistic target of rapamycin complex 1 (mTORC1), promotes transcriptional coactivators such as Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [135,136]. This axis attempts to increase mitochondrial mass and function, but in the context of senescence, the biogenesis process is often dysfunctional or insufficient. Concurrently, a metabolic shift toward aerobic glycolysis (Warburg-like effect) occurs, favoring ATP production independent of the impaired electron transport chain [135,136].
Despite these adaptive responses, senescent cells remain in a state of chronic energetic stress, characterized by altered NAD+/NADH ratios, sustained AMPK activation, and elevated ROS levels. Namely, ROS also activate metabolic checkpoints such as AMPK, which senses altered AMP/ATP and NAD+/NADH ratios [137]. AMPK engagement contributes to senescence by stabilizing p21 and p16 mRNA transcripts through inhibition of the RNA-binding protein human antigen R (HuR) [138,139]. This stabilization promotes the activation of p53 and Rb pathways, reinforcing the senescent arrest program [139].
Importantly, oxidative stress-induced senescence is not a passive consequence but a tightly regulated process supported by self-sustaining signaling loops [111]. One example of this feedback loop includes several key molecules: cyclin-dependent kinase inhibitor 1A (CDKN1A or p21), growth arrest and DNA damage-inducible alpha (GADD45A), mitogen-activated protein kinase 14 (MAPK14), growth factor receptor-bound protein 2 (GRB2), and transforming growth factor-beta (TGF-β). In this pathway, ROS help to sustain DDR signaling and promote continued expression of p21, reinforcing the senescent state [140,141]. This leads to a stable senescent state that is difficult to reverse [140]. Moreover, ROS actively participate in shaping SASP, a pro-inflammatory secretome that exacerbates tissue dysfunction, propagates paracrine senescence, and promotes chronic inflammation [3].
Compelling experimental data further reveal the reciprocal crosstalk between mitochondrial and telomeric ROS [142]. ROS localized to mitochondria can induce telomere shortening and genomic instability, while telomere-specific oxidative damage reciprocally impairs mitochondrial function, compounding cellular stress [143,144]. This bidirectional signaling underlines the systemic nature of redox imbalance in senescence.
In sum, oxidative stress is not merely a correlate but a causative and sustaining force in cellular senescence. Through its roles in DNA damage, mitochondrial impairment, and pro-senescent signaling, ROS are deeply embedded in the molecular circuitry of aging and age-related disease. Therapeutic strategies aimed at restoring redox homeostasis hold promise for mitigating senescence and its pathological consequences.
4.1. DNA Damage Response, Oxidative Stress, and the Reinforcement of Senescence
The DNA damage response (DDR) is a fundamental signaling cascade that not only initiates but also sustains cellular senescence, acting at the intersection of nuclear genome integrity, oxidative stress, and mitochondrial function [140,145]. In response to persistent genotoxic stress, such as double-strand DNA breaks induced by ROS, radiation, or replication stress, DDR is activated through key sensors like ATM and ATR, leading to the phosphorylation of downstream effectors such as CHK1, CHK2, and p53 [130]. This cascade triggers the transcriptional upregulation of cell cycle inhibitors, including p21CIP1 and p16INK4a, establishing growth arrest [130].
However, DDR signaling extends beyond nuclear checkpoint control. Sustained DDR activity promotes mitochondrial remodeling and functional decline, leading to increased production of ROS [140]. Mechanistically, DDR activation engages a signaling axis involving growth arrest and DNA damage-inducible protein 45 (GADD45), which activates p38 mitogen-activated protein kinase (p38 MAPK). This, in turn, signals through GRB2 and TGF-β pathways, resulting in transcriptional and metabolic reprogramming that enhances mitochondrial ROS production [140].
The elevated ROS levels not only damage mitochondrial and cytoplasmic components but also further compromise nuclear DNA integrity [7]. This establishes a self-amplifying feedback loop in which ROS perpetuate DNA lesions, thereby sustaining DDR signaling and maintaining DNA damage foci, including 53BP1- and γH2AX-marked chromatin domains. These persistent DDR foci function as epigenetic scaffolds that stabilize the senescent phenotype by continuously enforcing checkpoint signaling and facilitating the secretion of pro-inflammatory SASP components [140].
Importantly, pharmacological inhibition of ROS leads to restoration of mitochondrial membrane potential, while combined suppression of ROS and mTOR signaling can further mitigate senescence-associated mitochondrial defects and DNA double-strand breaks [146,147,148]. These findings underscore the role of mitochondrial ROS as active contributors to DDR persistence and senescence maintenance.
The downstream signaling cascade of DDR—including ATM activation and subsequent Akt/mTORC1 phosphorylation—serves not only to regulate DNA repair but also to promote mitochondrial biogenesis via peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC-1β) [149]. This adaptive response, though initially compensatory, paradoxically supports the senescent phenotype by reinforcing mitochondrial-derived ROS production and metabolic burden [149].
4.2. Oxidative Stress and Its Modulatory Role in Senescence-Associated Signaling Pathways
Oxidative stress plays a crucial role in orchestrating the molecular signaling that underpins cellular senescence [150]. One of the hallmark features of senescent cells is irreversible cell cycle arrest, which is accompanied by a broad transcriptional suppression of genes involved in mitotic progression, DNA replication, and chromosomal segregation [60]. Reactive oxygen species, generated under oxidative stress, profoundly affect the expression and activity of these gene networks, thereby reinforcing the senescence phenotype [151].
Oxidative stress is a strong trigger of cellular senescence. It mainly acts by causing lasting DNA damage and disturbing the balance of the cellular redox system. Among the principal mediators of this response are the p53–p21 and p16INK4a–Rb tumor suppressor pathways, which function as key transcriptional and cell cycle checkpoints sensitive to redox perturbations [152,153]. Oxidative ROS trigger DNA lesions that activate DDR. This cascade begins with the activation of ATM or ATM and ATR kinases, which phosphorylate checkpoint kinases CHK2 and CHK1, respectively [154]. These kinases, in turn, stabilize p53 and promote its transcriptional activity, including the upregulation of p21CIP1, a CDK inhibitor that disrupts CDK2 and CDK1 activity, thereby blocking G1/S and G2/M cell cycle transitions [155]. Simultaneously, ROS can upregulate p16INK4a, either directly via redox-sensitive transcriptional programs or indirectly through p38 MAPK activation downstream of DDR. p16INK4a inhibits CDK4 and CDK6, preventing the phosphorylation of pRb and maintaining Rb in its active, hypophosphorylated state. This results in the transcriptional silencing of E2F target genes, which are required for S-phase entry, thus enforcing durable growth arrest [151]. Together, these pathways suppress the activity of multiple cyclin–CDK complexes, including those involving cyclins D, E, and A, thereby locking the cell in a senescent state [151]. The interplay between oxidative stress, DNA damage response, and the p53–p21 and p16–Rb pathways in driving cellular senescence is schematically represented in Figure 2.
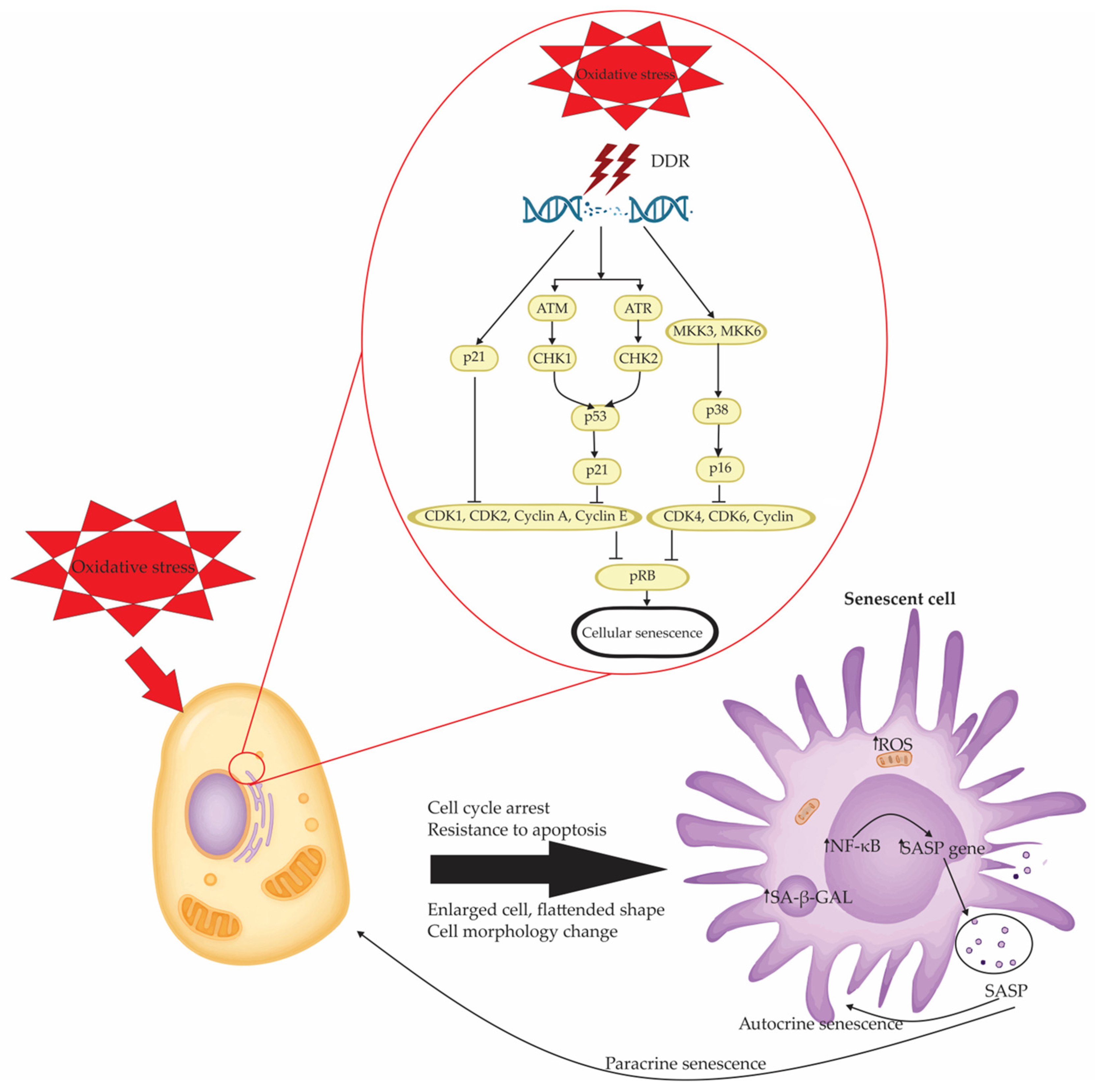
Figure 2.
Mechanistic pathways linking oxidative stress to cellular senescence and SASP activation. Oxidative stress induces cellular senescence primarily through DNA damage response (DDR) activation, leading to a cascade of molecular events that enforce cell cycle arrest. The central DDR machinery involves the ATM/checkpoint kinase 2 (CHK2)/p53/p21 axis and the Rad3-related (ATR)/checkpoint kinase 1 (CHK1)/mitogen-activated protein kinase kinase 3 (MKK3)–p38/p16 pathway. These converging routes inhibit cyclin-dependent kinases (CDK1, CDK2, CDK4, CDK6) and cyclins (A, E, D), ultimately suppressing phosphorylation of the retinoblastoma protein (pRB) and halting cell cycle progression. This checkpoint blockade ensures a stable senescent phenotype characterized by resistance to apoptosis, cellular enlargement, morphological changes, and flattened cell shape. Additionally, senescent cells exhibit increased senescence-associated β-galactosidase (SA-β-GAL) activity and heightened mitochondrial ROS (↑ROS) production, which further amplifies redox imbalance. NF-κB activation drives the transcription of SASP genes, culminating in the secretion of diverse inflammatory and tissue-remodeling factors. These SASP components perpetuate senescence in an autocrine manner and propagate paracrine senescence in surrounding cells, thereby contributing to tissue dysfunction, chronic inflammation, and tumorigenic processes.
A notable redox-sensitive effector in this context is thrombospondin-1 (TSP1), a matricellular glycoprotein that exerts multiple effects on cellular homeostasis and is upregulated in senescent cells [156]. TSP1 expression is triggered by DNA damage, inflammatory cytokines like TGF β1, and increased oxidative stress, which places it downstream of important signals that initiate senescence such that itacts as a feedback amplifier [157,158,159]. Once secreted, TSP1 binds to its receptor CD47 (also known as integrin-associated protein), triggering the activation of NADPH oxidase 1 (Nox1) [156]. This leads to increased ROS production, which amplifies nuclear p53 signaling, upregulates p21CIP1/WAF1, and enforces cell cycle arrest. This TSP1–CD47–Nox1 axis thereby strengthens the DNA damage response and sustains the senescence phenotype.
Importantly, this axis also engages in crosstalk with other redox-regulatory systems. For example, TSP1-induced CD47 activation inhibits nitric oxide signaling, a crucial antioxidant and vasoprotective pathway, further shifting the redox balance in favor of oxidative stress [160]. This contributes not only to intracellular DNA damage and senescence propagation but also to extracellular microenvironment remodeling, including impaired angiogenesis and vascular dysfunction, particularly relevant in aging tissues [160]. Overall, TSP1 acts as both a downstream effector and an upstream amplifier of oxidative stress and p53-driven senescence, forming a feed-forward loop that reinforces redox imbalance and permanent growth arrest.
Furthermore, exposure to environmental stressors, such as polystyrene nanoplastics (PS-NPs), has been shown to provoke oxidative stress via NADPH oxidase 2 (Nox2) activation [161]. This culminates in enhanced activation of both the p53–p21 and Rb–p16 pathways, contributing to premature senescence in spermatogenic cells [162].
At the transcriptional level, oxidative stress alters the expression of genes involved in vesicular trafficking, cell adhesion, and membrane receptor signaling, reflecting the adaptive remodeling of senescent cells [151]. Additionally, senescent cells often exhibit increased expression of anti-apoptotic B-cell lymphoma 2 (BCL-2) family proteins (e.g., BCL-2, B-cell lymphoma-extra large [BCL-XL], BCL-w), while pro-apoptotic mediators such as caspase-3 are downregulated, supporting their long-term survival despite accumulating damage [151].
4.3. Mitochondrial Damage and Oxidative Stress in Senescence Induction
Mitochondria are central hubs in regulating redox homeostasis, and their dysfunction is intimately linked to the onset and progression of cellular senescence [163]. One of the key mechanisms involves damage to mtDNA, which is highly susceptible to oxidative insults due to its close proximity to the electron transport chain and limited repair capacity [163]. Elevated reactive oxygen species, either intrinsically generated or induced by external stressors, can directly damage mtDNA or exacerbate mitochondrial dysfunction via alterations in telomerase reverse transcriptase (TERT) activity and modulation of tumor suppressor pathways such as p53 and Ras [164].
Persistent mitochondrial dysfunction is a hallmark of senescent cells and plays a central role in reinforcing the senescence program. Damaged mitochondria constantly produce high levels of ROS. This creates a self-sustaining cycle where oxidative stress further harms the mitochondria, leading to ongoing DNA damage and activation of redox-sensitive signaling pathways [163]. This sustained ROS production not only drives initial senescence induction but also actively contributes to the maintenance of the senescent phenotype.
One critical mechanism by which ROS mediate long-term growth arrest involves the activation of the GADD45–p38 mitogen-activated protein kinase (p38 MAPK)–growth factor receptor-bound protein 2 (GRB2)–transforming growth factor beta receptor 2 (TGFBR2)–TGF-β axis [140]. The GADD45 family, activated downstream of persistent DDR and oxidative stress, promotes phosphorylation of p38 MAPK, a key regulator of stress-responsive gene expression [165]. Activated p38 MAPK interacts with GRB2 and enhances TGF-β signaling through increased expression and activation of TGFBR2 [166].
TGF-β signaling, in this context, exerts strong anti-proliferative effects by inducing cyclin-dependent kinase inhibitors (such as p15INK4b and p21CIP1) and promoting chromatin remodeling at E2F target gene promoters, thereby stabilizing Rb-mediated cell cycle arrest [166]. Importantly, this signaling cascade is not merely a transient checkpoint but constitutes a positive-feedback mechanism that ensures the irreversibility of senescence once established. The convergence of ROS production, DDR persistence, and TGF-β–mediated signaling creates a robust network that sustains the senescent state, rendering it resistant to mitogenic cues and apoptosis [3,140].
Thus, mitochondrial damage and the associated oxidative stress are not merely byproducts of cellular aging but active participants in the molecular orchestration of senescence, with implications for aging-related pathologies and therapeutic targeting.
4.4. Crosstalk Between Oxidative Stress, SASP, and the Nrf2–mTOR Axis
Oxidative stress is a key upstream signal that modulates both the onset and persistence of SASP [2,90]. Reactive oxygen species not only initiate DNA damage responses but also amplify SASP gene expression by activating redox-sensitive transcription factors such as NF-κB and C/EBPβ [2,167,168]. These transcriptional regulators drive the production of proinflammatory mediators like TNF-α, IL-1β, and IFN-γ, which can further propagate oxidative stress in neighboring cells, creating a self-reinforcing loop that sustains tissue-level senescence and inflammation [169,170].
Within this oxidative context, the Nrf2 transcription factor plays a dual role [171]. Under oxidative pressure, Nrf2 translocates into the nucleus and induces a specific arm of SASP, designated the Nrf2-induced secretory phenotype (NISP) [172]. This transcriptional program selectively promotes immune surveillance by recruiting CCR2-positive monocytes, facilitating the clearance of damaged or senescent cells [173]. Thus, Nrf2 acts as a protective tumor suppressor by curbing the long-term persistence of potentially harmful senescent cells [174].
Recent studies support the notion that Nrf2 directly and indirectly regulates mTOR activity, positioning the Nrf2–mTOR axis as a central redox–senescence regulatory hub [175]. At the transcriptional level, in vitro and organismal models have shown that Nrf2 activation can drive mTOR gene expression by binding to its promoter region, thereby promoting mTORC1 signaling even under basal conditions [176]. Nrf2 also enhances mTOR signaling through upregulation of RagD, a small GTPase essential for lysosomal targeting and activation of mTORC1 in nutrient-sensing pathways [177]. These mechanisms suggest that the oxidative stress-responsive Nrf2 participates in metabolic and growth regulatory feedback loops by tuning mTOR activity via transcriptional and post-translational modulators.
Conversely, mTOR stabilizes Nrf2 by inhibiting its degradation through suppression of the β-TrCP E3 ubiquitin ligase pathway [177]. Under normal conditions, β-TrCP recognizes phosphorylated Neh6 domain motifs on Nrf2 (e.g., via GSK-3β), leading to Cullin-1-mediated Nrf2 degradation independent of Keap1 [178]. mTOR activation suppresses β-TrCP expression/activity, thereby enhancing Nrf2 nuclear accumulation and antioxidant gene transcription, as demonstrated in pathological models (e.g., diabetic nephropathy) [17,179,180,181].
Finally, the feedback architecture of this axis is particularly relevant in aging and senescence. Persistent mTORC1 hyperactivity, common in aging tissues, can maintain SASP, while concurrently reinforcing Nrf2 stabilization [78]. Depending on the cellular context, Nrf2 may drive either pro-survival antioxidant responses or immune-mediated clearance via SASP regulation [182]. Together, this reciprocal regulation integrates redox stress sensing (via Nrf2) with metabolic and growth signaling (via mTOR), reinforcing senescence through feed-forward loops and offering a coherent mechanistic framework for targeting this axis in age-related disease and cancer.
4.5. MicroRNAs as Modulators and Biomarkers of Oxidative Stress-Driven Senescence
MicroRNAs (miRNAs) have emerged as crucial post-transcriptional regulators of gene expression that participate in both the onset and maintenance of cellular senescence, particularly in the context of oxidative stress [183,184,185]. A variety of miRNAs, including miR-21, miR-22, miR-34a, miR-146a, and members of the miR-17-92 and miR-200 families, are differentially expressed in senescent cells and aged tissues [185]. Importantly, oxidative stress can both influence miRNA biogenesis and modulate the expression profiles of specific miRNAs, thereby contributing to senescence induction [185]. Cellular oxidative stress can impair the miRNA biogenesis machinery, notably through ROS-mediated inactivation of protein tyrosine phosphatase 1B (PTP1B), which leads to phosphorylation and functional inhibition of Argonaute 2 (AGO2). This disruption compromises miRNA loading into the RNA-induced silencing complex (RISC) and represents a critical upstream regulatory checkpoint in the initiation of senescence, as demonstrated in RAS-induced premature senescence models. In this context, ROS serve as both inducers of DNA damage and as upstream modulators of miRNA silencing efficiency, coupling oxidative stress directly to the failure of post-transcriptional regulation that enforces cell cycle arrest and senescence propagation [186].
At the same time, specific miRNAs act as downstream amplifiers and fine-tuners of oxidative stress-induced senescence programs. Reactive oxygen species directly upregulate miR 210 and miR 494, which impair mitochondrial function, inhibit autophagy, and exacerbate DNA damage via downregulation of key repair proteins such as the MRN complex, thereby reinforcing senescence hallmarks [3,187].
Additionally, families of miRNAs—including miR-21, miR-34a, miR-146a, and members of the miR-17-92 and miR-200 clusters—are transcriptionally altered in response to oxidative stress and contribute to feedback loops that regulate redox homeostasis, inflammatory signaling, and cell cycle exit in aging cells and tissues [188]. Beyond their intracellular regulatory roles, miRNAs are secreted into the circulation, where they are stabilized within extracellular vesicles or bound to carrier proteins, enabling them to act as intercellular signaling molecules and accessible biomarkers [189]. Numerous studies have demonstrated that circulating miRNAs—such as miR-126, miR-93, miR-21, and miR-146a—are altered in aging and age-related diseases [190,191]. Notably, miR-21, which is elevated in age-associated pathologies and various cancers, is found at lower levels in centenarians, indicating a possible role in healthy aging and longevity prediction [192].
A comprehensive overview of oxidative stress-driven mechanisms of senescence and their clinical implications is presented in Table 3.

Table 3.
Oxidative stress-driven mechanisms of cellular senescence and associated clinical implications.
5. Senotherapy: Targeting Senescent Cells for Healthspan Extension
Senotherapy encompasses a group of therapeutic strategies specifically designed to counteract the deleterious effects of cellular senescence by either eliminating senescent cells (senolytics) or modulating their secretory profile (senomorphics) [193]. As senescent cells accumulate in tissues with age or in response to stress, they exert adverse effects primarily through the secretion of pro-inflammatory and matrix-degrading factors collectively known as SASP [118]. Persistent SASP signaling not only promotes chronic inflammation but also drives the senescence of neighboring cells, thereby amplifying tissue dysfunction in a paracrine manner [2].
Senescent cells have been implicated in the pathogenesis of a wide spectrum of age-associated disorders, including neurodegenerative diseases, cardiovascular dysfunction, diabetes, osteoporosis, and chronic renal and hepatic diseases [194]. Their accumulation at sites of pathology is increasingly recognized as a contributor to disease progression and organ failure [195]. Consequently, the targeted elimination of these cells has emerged as a promising strategy to restore tissue homeostasis and delay age-related decline [195].
Given the role of oxidative stress in reinforcing the senescent state and driving SASP production, future senotherapy efforts may benefit from combined regimens that also modulate redox balance, offering synergistic benefits in controlling the detrimental consequences of cellular senescence.
5.1. Senolytic Strategies: Selective Clearance of Senescent Cells to Restore Tissue Integrity
The therapeutic targeting of senescent cells (SNCs) through senolytics has emerged as a promising strategy to counteract chronic inflammation, tissue dysfunction, and age-associated diseases [196]. Unlike conventional therapies, senolytics act by selectively inducing apoptosis in senescent cells that evade immune clearance through activation of pro-survival pathways [196,197]. These interventions aim not only to reduce the burden of dysfunctional cells but also to mitigate the proinflammatory milieu driven by SASP [193].
Initial breakthroughs came with the identification of dasatinib and quercetin (D+Q) as effective senolytic agents [198]. This combination targets tyrosine kinases and phosphoinositide 3-kinase (PI3K)-dependent survival signaling and has shown efficacy in both preclinical models and early-phase clinical trials for conditions such as idiopathic pulmonary fibrosis and cancer [199,200,201,202]. Intermittent dosing of D+Q has been shown to improve physical function and reduce senescence markers in treated patients [193,200]. Moreover, in ovarian cancer models, D+Q mitigated chemotherapy-induced senescence in adipose stromal cells, thereby inhibiting metastatic dissemination [201].
The BCL-2 protein family represents a key target in senescence due to their upregulation in SNCs [203]. Navitoclax (ABT-263), a potent inhibitor of BCL-2, BCL-XL, and BCL-W, promotes apoptosis in senescent cells, particularly in chemotherapy-induced models of resistant cancers such as pancreatic and breast tumors [200,204,205]. However, its clinical translation is limited by hematologic toxicity, particularly thrombocytopenia. Similar limitations apply to its structural analog, ABT-737 [206]. To circumvent these drawbacks, ongoing research is exploring tissue-specific delivery vectors, intermittent regimens, and novel inhibitors with enhanced selectivity.
Flavonoids such as quercetin, fisetin, apigenin, and resveratrol act as senolytic compounds by interfering with ROS signaling, SASP induction, and the cell cycle arrest pathway. Quercetin reduces ROS and inflammatory cytokines, upregulates antioxidant responses via Nrf2, and downregulates markers of senescence such as p16 and p53 while promoting SOD and CAT expression; it also modulates microRNA-155–SIRT1–NF-κB signaling to inhibit SASP-driven inflammation [207,208,209,210]. Fisetin similarly diminishes oxidative stress, promotes senolytic clearance, mimics caloric restriction, and extends lifespan in vivo—though its effects depend on timing relative to oxidative challenge [208,211,212].
Apigenin, a flavone, directly suppresses SASP through inhibition of IL-1α–IRAK1/4, p38-MAPK, and NF-κB signaling in senescent fibroblasts, reducing secretion of pro-inflammatory cytokines without necessarily inducing senescent-cell death [210,213]. Other compounds under evaluation include cardiac glycosides (digoxin, ouabain), glutaminase inhibitors, and mTOR inhibitors like rapamycin, which indirectly suppress SASP signaling via modulation of NF-κB translation [214,215,216].
Mechanistically distinct approaches, such as the forkhead box O4–D-retro-inverso (FOXO4-DRI) peptide, disrupt the FOXO4–p53 interaction, freeing p53 to trigger senescence-specific apoptosis [62,217]. Likewise, heat shock protein 90 (HSP90) inhibitors destabilize chaperone networks that sustain senescent cell viability [218].
Despite these advances, several challenges hinder clinical translation. Senescent cells are heterogeneous across tissues and disease contexts, displaying variable dependency on pro-survival pathways [219]. As such, a senolytic agent effective in one setting may be ineffective—or deleterious—in another. Furthermore, repeated or widespread senescent cell clearance may impair regenerative capacity or provoke compensatory cell damage [2]. Kowald and colleagues have emphasized that while senolytics remove harmful cells, their inappropriate use may accelerate tissue dysfunction [220].
5.2. Senomorphics: Modulating the Harmful Phenotype of Senescent Cells
Senomorphics represent an alternative and increasingly valued strategy in the therapeutic modulation of cellular senescence [221]. Rather than eliminating senescent cells, senomorphic agents aim to suppress or reshape the senescence-associated secretory phenotype, a key driver of chronic inflammation and tissue degeneration in aging [193]. By preserving the structural integrity of SNCs while mitigating their deleterious paracrine signaling, senomorphics offer a refined approach for managing age-related pathologies, especially in tissues where transient senescence plays protective or regenerative roles [90,193,222].
SASP consists of a dynamic and complex mix of pro-inflammatory cytokines (e.g., IL-6, IL-8, IL-1β), chemokines, matrix metalloproteinases, growth factors, and bioactive lipids [90]. It is governed by interconnected signaling axes, including NF-κB, mTOR, Janus kinase/signal transducer and activator of transcription (JAK/STAT), and p38 MAPK [223,224]. Persistent SASP activity promotes local inflammation, stem cell dysfunction, and propagation of senescence to surrounding cells, thereby contributing to conditions such as cancer, osteoarthritis, and neurodegeneration [90,169]. Importantly, the composition and function of SASP evolve temporally: early SASP components can mediate tissue repair and immune evasion, while chronic SASP tends to drive inflammatory deterioration [90].
A variety of agents have been shown to suppress SASP activity. Rapamycin and its analogs (rapalogs), which inhibit mTOR signaling, reduce SASP output by blocking IL-1α translation, a key SASP initiator [193]. Similarly, JAK inhibitors such as ruxolitinib and baricitinib effectively blunt IL-6/IL-8-driven SASP propagation and restore tissue homeostasis in both preclinical and clinical settings [225,226]. Metformin, beyond its antidiabetic action, activates AMPK and downregulates mTOR, thereby exerting senomorphic effects and reducing low-grade inflammation linked to aging and metabolic disease [227]. Other phytochemicals, including apigenin, kaempferol, and rutin, suppress SASP expression via interference with ATM–TRAF6–HIF1α signaling or inhibition of MAPK-dependent transcription [228,229,230].
In addition to synthetic and plant-derived compounds, newer targets for senomorphic intervention are emerging [231]. Inhibitors of nicotinamide phosphoribosyltransferase (NAMPT), such as FK866, interfere with nicotinamide adenine dinucleotide (NAD+) biosynthesis and suppress therapy-induced senescent cancer stem-like cells [232]. Certain compounds can reduce SASP without causing cell death. These include inhibitors of p38 MAPK, bromodomain and extraterminal domain (BET) proteins, and O-linked N-acetylglucosamine glycosylation (O-GlcNAcylation) [233,234]. Although some glucocorticoids show senomorphic activity via NF-κB inhibition, their systemic immunosuppressive effects limit long-term utility [193].
Importantly, the context-dependent roles of SASP require a nuanced therapeutic approach. While suppressing chronic SASP is desirable, indiscriminate inhibition may interfere with its beneficial roles in wound healing and tumor surveillance [235]. Thus, temporally and spatially regulated senomorphic therapies are under development, often guided by tissue-specific delivery systems and AI-driven compound optimization [193]. Precision in targeting SASP regulators is essential to selectively suppress deleterious factors while preserving reparative or immune-supportive elements [193].
In clinical translation, senomorphics may be used alone or in combination with senolytics [193]. Sequential regimens—such as SASP suppression prior to senescent cell clearance, or post-clearance suppression to prevent SASP propagation—are under active investigation [193,236]. Moreover, the absence of non-invasive biomarkers to assess the SASP burden in vivo remains a major challenge for stratifying patients and tracking therapeutic response.
5.3. Redox Modulation in Senescence Management
Oxidative stress plays a central role in the initiation and propagation of cellular senescence [111]. The duality of reactive oxygen species—as both signaling molecules and sources of cellular damage—has positioned redox modulation as a key therapeutic avenue in senescence-targeted interventions [12]. Depending on the context, redox-based therapies can either amplify ROS to induce senescence or cell death in tumor cells, or attenuate ROS to prevent stress-induced senescence and chronic inflammation in aging tissues [237,238].
One compound of interest is resveratrol, a polyphenolic antioxidant found in grapes and berries, which exhibits dose-dependent dual activity [239]. At low concentrations, it suppresses inflammation-induced senescence, particularly in nucleus pulposus cells, while at higher doses, it paradoxically induces oxidative stress and DNA damage, promoting senescence or apoptosis in cancer cells via mitochondrial disruption and stress-activated kinases [239,240,241]. Clinical findings further support the redox-dependent actions of resveratrol; in combination with copper, it acts as a prooxidant that reduces chemotherapy-associated toxicity in gastric cancer patients [242].
Ginsenoside Rh2, derived from ginseng, has been shown to modulate redox signaling by attenuating NF-κB/IL-8-driven SASP in doxorubicin-resistant breast cancer models, thereby inhibiting cell proliferation and restoring chemosensitivity [243]. Similarly, apigenin, a dietary flavonoid, exerts its senescence-inducing effects via sustained oxidative signaling through p21 activation, independent of canonical p53 or p16-Rb pathways, suggesting a promising role in redox-based sensitization of colorectal cancer cells to therapy [244].
A wide spectrum of natural compounds—such as phloretin, genistein, sulforaphane, quercetin, silybin, and curcumin analogs—have demonstrated potential to modulate oxidative stress and induce senescence selectively in cancerous cells [244]. Several of these agents have advanced into preclinical validation, highlighting their promise as redox-active senotherapeutics with both antioxidant and prooxidant capacities [244].
In parallel, antioxidant strategies are being explored to mitigate the detrimental effects of senescence in non-cancerous tissues [245,246]. Enzymatic mimetics of superoxide dismutase and glutathione peroxidase, as well as Nrf2 activators like sulforaphane, have shown efficacy in restoring redox homeostasis and limiting oxidative DNA damage [247,248,249]. Inhibitors of NADPH oxidase (e.g., diphenyleneiodonium) directly reduce ROS production, dampening SASP and its associated inflammatory cascade [250].
Collectively, redox modulation represents a versatile platform in senotherapy—whether by enhancing ROS to selectively eliminate damaged or malignant cells or by curbing oxidative stress to preserve tissue integrity and suppress chronic inflammation. The therapeutic outcome hinges on precise control of ROS levels, cell context, and the integration of redox pathways with key senescence effectors such as p21, p53, and NF-κB.
5.4. Gene Therapy Approaches in Senotherapeutics
Gene therapy has recently gained momentum as a precision strategy to combat cellular senescence by directly targeting the genetic regulators of aging and stress responses [251]. This approach harnesses advanced molecular tools to either restore function in cells undergoing premature senescence or eliminate senescent cells that contribute to tissue dysfunction and chronic disease [251].
Interventions aimed at modulating the expression of key cell cycle regulators, such as CDK2 and the retinoblastoma (RB) tumor suppressor, have demonstrated the potential to delay senescence onset and maintain proliferative competence in aging cells [252]. By correcting or compensating for disruptions in these pathways, gene therapy may preserve genomic integrity and cellular plasticity, particularly in high-turnover tissues susceptible to degenerative changes [253].
An alternative application, known as suicide gene therapy, is being explored to selectively ablate senescent cells through the activation of endogenous apoptotic mechanisms [62]. In this context, senescence-specific promoters drive the expression of cytotoxic genes only in cells exhibiting a senescent phenotype, thus enabling their precise removal while sparing surrounding healthy tissue. This targeted approach offers a means to reduce senescent cell burden without the systemic effects associated with conventional senolytic drugs [62].
By integrating gene repair and targeted ablation strategies, gene therapy provides a versatile and highly customizable platform for senescence management. Its therapeutic potential is particularly relevant for age-related pathologies, such as cataracts, neurodegeneration, and fibrotic disorders, where the accumulation of senescent cells disrupts tissue architecture and function. As delivery systems and vector safety continue to improve, gene therapy is poised to become a cornerstone of next-generation senotherapeutics aimed at extending healthspan and delaying the molecular hallmarks of aging.
5.5. Harnessing the Immune System for Senescent Cell Clearance
Recent advancements have revealed the immune system’s essential role in identifying and eliminating senescent cells, opening the door to immunotherapeutic strategies aimed at promoting healthy aging and enhancing cancer treatment [253]. Senescent cells often display altered expression of surface proteins, making them susceptible to immune surveillance mechanisms that can be therapeutically exploited [254].
One promising approach involves engineering chimeric antigen receptor (CAR) T cells to specifically recognize and destroy senescent cells expressing surface molecules such as urokinase-type plasminogen activator receptor (uPAR) [255]. Preclinical models have demonstrated that uPAR-targeted CAR-T cells efficiently eliminate senescent tumor cells induced by MEK and CDK4/6 inhibition, thereby suppressing tumor progression in lung cancer models [255].
Natural killer (NK) cells also participate in senescent cell clearance through recognition of upregulated ligands for activating receptors like natural killer group 2 member D (NKG2D) [256]. Enhancing NKG2D-mediated signaling may potentiate NK cell cytotoxicity against senescent targets and contribute to improved immune surveillance in aging tissues and malignancies [256,257].
Checkpoint blockade therapies, widely used in oncology, are now being considered in the context of senescence [258]. Senescent cells often overexpress programmed death-ligand 1 (PD-L1), contributing to immune evasion and chronic inflammation [259]. Thus, immune checkpoint inhibitors targeting PD-L1 could selectively restore immune-mediated clearance of senescent cells, particularly in aged or tumor-bearing hosts [260,261].
Furthermore, manipulation of the tumor microenvironment to improve immunogenicity of senescent cells is a developing area of interest. Epigenetic repression of pro-inflammatory SASP genes—such as those encoding CCL2 and CXCL9/10—by enhancer of zeste homolog 2 (EZH2) has been shown to dampen immune cell infiltration in pancreatic cancer models [262]. Pharmacologic inhibition of EZH2 reactivates SASP gene expression, enhances chemokine-mediated recruitment of NK and T cells, and facilitates the immune-mediated elimination of therapy-induced senescent (TIS) cells [262].
Collectively, these findings support the development of immune-based senotherapeutics that selectively augment the immune system’s ability to target senescent cells. Such strategies hold promise not only for cancer therapy but also for mitigating the deleterious effects of senescent cell accumulation in age-related diseases. A comprehensive overview of senotherapeutic strategies, their mechanisms, and clinical implications is summarized in Table 4.

Table 4.
Senotherapeutic strategies, mechanisms of action, and clinical implications.
6. Conclusions
Cellular senescence stands at the crossroads of oxidative stress, mitochondrial dysfunction, and chronic inflammation. Excessive ROS damage organelles and DNA, triggering a persistent DNA damage response (DDR) and reinforcing SASP, which can shift senescence from a protective arrest to a driver of tissue degeneration. This transition is regulated by the nuclear factor Nrf2 and mechanistic target of rapamycin (mTOR) axis, which integrates metabolic signals with antioxidant defenses to determine whether senescence remains beneficial or becomesharmful through a process known as inflammaging. Current research is shifting from general antioxidant therapies to precision senotherapeutics. These include redox modulators combined with senolytics or senomorphics, senescence-targeted gene editing, promoter-driven suicide constructs, and engineered CAR-T or NK cells targeting uPAR or NKG2D. The goal is to eliminate or reprogram senescent cells while preserving tissue regeneration. However, three key challenges remain: addressing the variability of senescent cells across tissues and diseases, managing the dual roles of SASP, and developing non-invasive, ROS-sensitive biomarkers—such as circulating microRNA panels or imaging agents—to guide treatment selection, monitor response, and adjust dosing.
7. Future Directions
Despite major strides in elucidating the molecular underpinnings of oxidative stress-induced senescence and its therapeutic targeting, several critical gaps must be addressed to ensure successful clinical translation.
One promising area lies in the identification and validation of non-invasive biomarkers of senescence. Circulating microRNAs (miRNAs) have emerged as potential diagnostic and prognostic tools due to their stability in bodily fluids and their capacity to reflect intracellular signaling states. Specifically, miR-21 and miR-146a are consistently dysregulated in aging and senescence-associated diseases. miR-21, which modulates the p53 pathway, TGF-β signaling, and oxidative stress responses, is upregulated in various cancers and cardiovascular pathologies, whereas reduced levels have been associated with healthy aging. Similarly, miR-146a plays a pivotal role in dampening inflammatory signaling via the NF-κB axis, and its altered expression is linked to immune senescence and inflammaging. Both miRNAs show potential as dynamic biomarkers for monitoring senescence burden and therapeutic response. However, their implementation in clinical settings requires standardization of detection protocols, robust cohort-based validation, and longitudinal studies to establish temporal specificity.
Additionally, clinical and translational validation of senotherapeutic strategies remains an unmet need. While several senolytic and senomorphic compounds have shown promise in preclinical and early-phase clinical trials, the heterogeneity of senescent phenotypes across tissues and diseases demands the development of predictive preclinical models that more faithfully recapitulate human senescence. Three-dimensional organoids, patient-derived xenografts, and humanized mouse models offer opportunities for more relevant drug testing and biomarker discovery.
Equally critical is the standardization of clinical endpoints. Current trials often rely on surrogate markers, such as a reduction in SASP components or improvements in physical function, which may not capture the full spectrum of therapeutic effects. Future studies should incorporate composite outcomes encompassing functional, molecular, and quality-of-life indicators, along with validated biomarkers such as circulating miRNAs and imaging modalities capable of tracking senescent cell burden in vivo.
Moreover, advances in systems biology, artificial intelligence, and single-cell omics are poised to revolutionize the senescence field. These tools will enable the deconvolution of senescence heterogeneity, identification of senescence subtypes, and prediction of individual responses to senotherapeutics.
In conclusion, while targeting senescence offers transformative potential for age-related and degenerative diseases, its success will hinge upon integrative efforts that bridge mechanistic insight, biomarker development, and rigorous translational validation.
Author Contributions
Conceptualization, B.S., M.D.S., B.S.S., S.L.; methodology, B.S., I.J., N.Z., B.M.; investigation, V.K., A.A.A., S.E., D.J., S.J.; validation, I.R., N.M., B.M.; writing—original draft preparation, B.S., M.D.S., B.S.S., J.N., I.M.B.; writing—review and editing, I.J., M.M.K., B.M.; visualization, B.S., D.J., N.Z., S.J.; supervision, A.A.A., S.E., S.L., B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
As this is a review article, no original research data were generated or analyzed. The manuscript provides a comprehensive synthesis and critical appraisal of previously published studies, all of which are appropriately cited. Consequently, a data availability statement is not applicable.
Acknowledgments
The authors sincerely thank the Faculty of Medical Sciences, University of Kragujevac, for their continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AGO2 | Argonaute 2 |
| ALDH4A1 | aldehyde dehydrogenase 4 family member A1 |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein 1 |
| AREs | antioxidant response elements |
| ARF | alternate reading frame |
| ATR | Rad3-related |
| ATM | ataxia-telangiectasia mutated |
| ATF6 | activating transcription factor 6 |
| Akt | protein kinase B |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| BCL-XL | B-cell lymphoma-extra large |
| BET | bromodomain and extraterminal domain |
| CAR | chimeric antigen receptor |
| CAT | catalase |
| CCL2 | C-C motif chemokine ligand 2 |
| CD47 | cluster of differentiation 47 |
| CDK | cyclin-dependent kinase |
| CDKN1A | cyclin-dependent kinase inhibitor 1A |
| cGAS–STING | GMP-AMP synthase–stimulator of interferon genes |
| CHK2 | checkpoint kinase 2 |
| CHOP | C/EBP homologous protein |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| D | dasatinib |
| DDR | DNA damage response |
| DNA | deoxyribonucleic acid |
| DRI | D-retro-inverso |
| EMT | epithelial–mesenchymal transition |
| ER | endoplasmic reticulum |
| ETC | electron transport chain |
| EZH2 | enhancer of zeste homolog 2 |
| FOXO | forkhead box O |
| FOXO4 | forkhead box O4 |
| GADD45A | growth arrest and DNA damage-inducible alpha |
| GGT | glutamyl transpeptidase |
| GLS2 | glutaminase 2 |
| GR | glutathione reductase |
| GRB2 | growth factor receptor-bound protein 2 |
| H3K4me3 | histone 3 lysine 4 trimethylation |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HO-1 | heme oxygenase-1 |
| HSP90 | heat shock protein 90 |
| HuR | human antigen R |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| IRE1 | inositol-requiring enzyme 1 |
| JAK | Janus kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| loROS | less reactive ROS |
| Maf | musculoaponeurotic fibrosarcoma |
| MAPK14 | mitogen-activated protein kinase 14 |
| MEK | mitogen-activated protein kinase kinase |
| miRNAs | microRNAs |
| MKK3 | mitogen-activated protein kinase kinase 3 |
| MMP1 | matrix metalloproteinase 1 |
| MMP3 | matrix metalloproteinase 3 |
| MnSOD | manganese superoxide dismutase |
| mTOR | mechanistic target of rapamycin |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| MSC | mesenchymal stem cells |
| mtDNA | mitochondrial DNA |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NAD+ | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NISP | Nrf2-induced secretory phenotype |
| NK | natural killer |
| NKG2D | natural killer group 2 member D |
| Nox1 | NADPH oxidase 1 |
| Nox2 | NADPH oxidase 2 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| O-GlcNAcylation | O-linked N-acetylglucosamine glycosylation |
| OIS | oncogene-induced senescence |
| PDGF-AA | platelet-derived growth factor-AA |
| PD-L1 | programmed death-ligand 1 |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| PGC-1β | peroxisome proliferator-activated receptor gamma coactivator 1-beta |
| PI3K | phosphoinositide 3-kinase |
| PIG1–13 | p53-induced genes 1–13 |
| p53 | tumor protein p53 |
| pRB | retinoblastoma protein |
| PS-NPs | polystyrene nanoplastics |
| PUMA | p53 upregulated modulator of apoptosis |
| Q | quercetin |
| RAF | rapidly accelerated fibrosarcoma |
| RAS | rat sarcoma viral oncogene homolog |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA-β-gal | senescence-associated β-galactosidase |
| SAHF | senescence-associated heterochromatin foci |
| SASP | senescence-associated secretory phenotype |
| SIPS | stress-induced premature senescence |
| SNCs | senescent cells |
| SODs | superoxide dismutases |
| STAT | signal transducer and activator of transcription |
| TERT | telomerase reverse transcriptase |
| TGF-β | transforming growth factor-beta |
| TIGAR | TP53-induced glycolysis and apoptosis regulator |
| TIS | therapy-induced senescent |
| TNF-α | tumor necrosis factor-alpha |
| TSP1 | thrombospondin-1 |
| uPAR | urokinase-type plasminogen activator receptor |
| UPR | unfolded protein response |
| UV | ultraviolet |
References
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. Excli J. 2021, 20, 956–967. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Loreto Palacio, P.; Godoy, J.R.; Aktas, O.; Hanschmann, E.M. Changing Perspectives from Oxidative Stress to Redox Signaling-Extracellular Redox Control in Translational Medicine. Antioxidants 2022, 11, 1181. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Pooja, G.; Shweta, S.; Patel, P. Oxidative stress and free radicals in disease pathogenesis: A review. Discov. Med. 2025, 2, 104. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; HA, A.L.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Masuda, D.; Nakanishi, I.; Ohkubo, K.; Ito, H.; Matsumoto, K.-I.; Ichikawa, H.; Chatatikun, M.; Klangbud, W.K.; Kotepui, M.; Imai, M.; et al. Mitochondria Play Essential Roles in Intracellular Protection against Oxidative Stress—Which Molecules among the ROS Generated in the Mitochondria Can Escape the Mitochondria and Contribute to Signal Activation in Cytosol? Biomolecules 2024, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Bhatti, G.K.; Gupta, A.; Pahwa, P.; Khullar, N.; Singh, S.; Navik, U.; Kumar, S.; Mastana, S.S.; Reddy, A.P.; Reddy, P.H.; et al. Targeting mitochondrial bioenergetics as a promising therapeutic strategy in metabolic and neurodegenerative diseases. Biomed. J. 2022, 45, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic Regulation of Redox Balance in Cancer. Cancers 2019, 11, 955. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Wang, X.; Jiang, C.; Zhao, Y.; Lu, M.; Zhang, J. Peroxisome-Mediated Reactive Oxygen Species Signals Modulate Programmed Cell Death in Plants. Int. J. Mol. Sci. 2022, 23, 10087. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress-The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Paithankar, J.G.; Stopper, H.; Pandey, A.; Sharma, A. Environmental Chemical-Induced Reactive Oxygen Species Generation and Immunotoxicity: A Comprehensive Review. Antioxid. Redox Signal. 2024, 40, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Qiu, H.; Kuolee, R.; Harris, G.; Chen, W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect. Immun. 2009, 77, 1015–1021. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospíšil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef] [PubMed]
- Huete-Acevedo, J.; Mas-Bargues, C.; Arnal-Forné, M.; Atencia-Rabadán, S.; Sanz-Ros, J.; Borrás, C. Role of Redox Homeostasis in the Communication Between Brain and Liver Through Extracellular Vesicles. Antioxidants 2024, 13, 1493. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Moyo, M.; Van Staden, J. Natural antioxidants: Fascinating or mythical biomolecules? Molecules 2010, 15, 6905–6930. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Ngo, V.; Karunatilleke, N.C.; Brickenden, A.; Choy, W.-Y.; Duennwald, M.L. Oxidative Stress-Induced Misfolding and Inclusion Formation of Nrf2 and Keap1. Antioxidants 2022, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 system and inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef]
- Panova, I.G.; Tatikolov, A.S. Endogenous and Exogenous Antioxidants as Agents Preventing the Negative Effects of Contrast Media (Contrast-Induced Nephropathy). Pharmaceuticals 2023, 16, 1077. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Bevinakoppamath, S.; Ramachandra, S.C.; Yadav, A.K.; Basavaraj, V.; Vishwanath, P.; Prashant, A. Understanding the Emerging Link Between Circadian Rhythm, Nrf2 Pathway, and Breast Cancer to Overcome Drug Resistance. Front. Pharmacol. 2021, 12, 719631. [Google Scholar] [CrossRef]
- Lananna, B.V.; Musiek, E.S. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Sun, Q.; Zeng, C.; Du, L.; Dong, C. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp. Ther. Med. 2021, 21, 190. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, Y.; Tao, R.; Panayi, A.C.; Mi, B.; Liu, G. Emerging Insight Into the Role of Circadian Clock Gene BMAL1 in Cellular Senescence. Front. Endocrinol. 2022, 13, 915139. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Lindsay, R.T.; Rhodes, C.J. Reactive Oxygen Species (ROS) in Metabolic Disease—Don’t Shoot the Metabolic Messenger. Int. J. Mol. Sci. 2025, 26, 2622. [Google Scholar] [CrossRef] [PubMed]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kuk, M.U.; So, M.K.; Song, E.S.; Lee, H.; Ahn, S.K.; Kwon, H.W.; Park, J.T.; Park, S.C. Targeting Mitochondrial Oxidative Stress as a Strategy to Treat Aging and Age-Related Diseases. Antioxidants 2023, 12, 934. [Google Scholar] [CrossRef]
- Van Houten, B.; Santa-Gonzalez, G.A.; Camargo, M. DNA repair after oxidative stress: Current challenges. Curr. Opin. Toxicol. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Martinvalet, D.; Walch, M. Editorial: The Role of Reactive Oxygen Species in Protective Immunity. Front. Immunol. 2021, 12, 832946. [Google Scholar] [CrossRef]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.-S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, S.; Papanikolaou, N.A. Reactive Oxygen Species Mechanisms that Regulate Protein–Protein Interactions in Cancer. Int. J. Mol. Sci. 2024, 25, 9255. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, D.; Traini, V.; Silvestris, I.; Fabbiano, G.; Passamonti, F.; Bolli, N.; Taiana, E. The pleiotropic nature of NONO, a master regulator of essential biological pathways in cancers. Cancer Gene Ther. 2024, 31, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, H.; Wu, H. Cellular Senescence in Health, Disease, and Lens Aging. Pharmaceuticals 2025, 18, 244. [Google Scholar] [CrossRef] [PubMed]
- Demirci, D.; Dayanc, B.; Mazi, F.A.; Senturk, S. The Jekyll and Hyde of Cellular Senescence in Cancer. Cells 2021, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Yeo, H.L.; Wong, S.W.; Zhao, Y. Cellular Senescence: Mechanisms and Therapeutic Potential. Biomedicines 2021, 9, 1769. [Google Scholar] [CrossRef]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Aksenova, A.Y.; Mirkin, S.M. At the Beginning of the End and in the Middle of the Beginning: Structure and Maintenance of Telomeric DNA Repeats and Interstitial Telomeric Sequences. Genes 2019, 10, 118. [Google Scholar] [CrossRef]
- Bonnell, E.; Pasquier, E.; Wellinger, R.J. Telomere Replication: Solving Multiple End Replication Problems. Front. Cell Dev. Biol. 2021, 9, 668171. [Google Scholar] [CrossRef]
- Saretzki, G. Role of Telomeres and Telomerase in Cancer and Aging. Int. J. Mol. Sci. 2023, 24, 9932. [Google Scholar] [CrossRef]
- Shin, J.S.; Hong, A.; Solomon, M.J.; Lee, C.S. The role of telomeres and telomerase in the pathology of human cancer and aging. Pathology 2006, 38, 103–113. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Y.; Li, C.; Xue, Y.; Ba, X. Targeting the DNA Damage Response for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15907. [Google Scholar] [CrossRef]
- Tatsukawa, K.; Sakamoto, R.; Kawasoe, Y.; Kubota, Y.; Tsurimoto, T.; Takahashi, T.S.; Ohashi, E. Resection of DNA double-strand breaks activates Mre11-Rad50-Nbs1- and Rad9-Hus1-Rad1-dependent mechanisms that redundantly promote ATR checkpoint activation and end processing in Xenopus egg extracts. Nucleic Acids Res. 2024, 52, 3146–3163. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A key player in DNA damage response and a promising target for cancer therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Huang, Y.Q.; Hu, J.J.; Liang, J.; He, C.Y.; Wan, W.D.; Jiang, C.H.; Wu, H.; Li, N. Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants. Aging Dis. 2024, 16, 1483–1512. [Google Scholar] [CrossRef]
- Choudhury, F.K. Mitochondrial Redox Metabolism: The Epicenter of Metabolism during Cancer Progression. Antioxidants 2021, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, Y. p53, oxidative stress, and aging. Antioxid. Redox Signal. 2011, 15, 1669–1678. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, Y.; Kim, M.S.; Kang, C. Metabolic remodeling in cancer and senescence and its therapeutic implications. Trends Endocrinol. Metab. 2024, 35, 732–744. [Google Scholar] [CrossRef]
- Russo, L.; Babboni, S.; Andreassi, M.G.; Daher, J.; Canale, P.; Del Turco, S.; Basta, G. Treating Metabolic Dysregulation and Senescence by Caloric Restriction: Killing Two Birds with One Stone? Antioxidants 2025, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.Y.; Lee, H.; Song, E.S.; Kuk, M.U.; Joo, J.; Oh, S.; Kwon, H.W.; Park, J.T.; Park, S.C. Targeting Mitochondrial Metabolism as a Strategy to Treat Senescence. Cells 2021, 10, 3003. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar] [CrossRef]
- Araki, K.; Nagata, K. Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 2011, 3, a007526. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Martin, N.; Bernard, D. Cellular senescence links mitochondria-ER contacts and aging. Commun. Biol. 2021, 4, 1323. [Google Scholar] [CrossRef]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A Review in the Theme: Cellular Mechanisms of Endoplasmic Reticulum Stress Signaling in Health and Disease. Am. J. Physiol.-Cell Physiol. 2015, 308, C415–C425. [Google Scholar] [CrossRef] [PubMed]
- Toropov, A.L.; Deryabin, P.I.; Shatrova, A.N.; Borodkina, A.V. Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells. Int. J. Mol. Sci. 2023, 24, 14089. [Google Scholar] [CrossRef]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-induced senescence: A double edged sword in cancer. Acta Pharmacol. Sin. 2018, 39, 1553–1558. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Abdullah Mohamed, H.; T.A., H.; Elsayed Ahmed, R.; et al. SASP Modulation for Cellular Rejuvenation and Tissue Homeostasis: Therapeutic Strategies and Molecular Insights. Cells 2025, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Byun, H.O.; Jee, B.A.; Cho, H.; Seo, Y.H.; Kim, Y.S.; Park, M.H.; Chung, H.Y.; Woo, H.G.; Yoon, G. Implications of time-series gene expression profiles of replicative senescence. Aging Cell 2013, 12, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ohtani, N.; Hara, E. Irreversibility of cellular senescence: Dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Div. 2007, 2, 10. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The role of the dynamic epigenetic landscape in senescence: Orchestrating SASP expression. Npj Aging 2024, 10, 48. [Google Scholar] [CrossRef]
- Criscione, S.W.; Teo, Y.V.; Neretti, N. The Chromatin Landscape of Cellular Senescence. Trends Genet. 2016, 32, 751–761. [Google Scholar] [CrossRef]
- Duarte, L.F.; Young, A.R.; Wang, Z.; Wu, H.A.; Panda, T.; Kou, Y.; Kapoor, A.; Hasson, D.; Mills, N.R.; Ma’ayan, A.; et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 2014, 5, 5210. [Google Scholar] [CrossRef]
- Budanov, A.V. The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell. Biochem. 2014, 85, 337–358. [Google Scholar] [CrossRef]
- Ali, R.; Zhen, Y.; Zanna, X.; Lin, J.; Zhang, C.; Ma, J.; Zhong, Y.; Husien, H.M.; Saleh, A.A.; Wang, M. Impact of Circadian Clock PER2 Gene Overexpression on Rumen Epithelial Cell Dynamics and VFA Transport Protein Expression. Int. J. Mol. Sci. 2024, 25, 12428. [Google Scholar] [CrossRef]
- Munteanu, C.; Turti, S.; Achim, L.; Muresan, R.; Souca, M.; Prifti, E.; Mârza, S.M.; Papuc, I. The Relationship between Circadian Rhythm and Cancer Disease. Int. J. Mol. Sci. 2024, 25, 5846. [Google Scholar] [CrossRef]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef]
- Leontieva, O.V.; Demidenko, Z.N.; Gudkov, A.V.; Blagosklonny, M.V. Elimination of proliferating cells unmasks the shift from senescence to quiescence caused by rapamycin. PLoS ONE 2011, 6, e26126. [Google Scholar] [CrossRef]
- Machado-Oliveira, G.; Ramos, C.; Marques, A.R.A.; Vieira, O.V. Cell Senescence, Multiple Organelle Dysfunction and Atherosclerosis. Cells 2020, 9, 2146. [Google Scholar] [CrossRef]
- Heckenbach, I.; Mkrtchyan, G.V.; Ezra, M.B.; Bakula, D.; Madsen, J.S.; Nielsen, M.H.; Oró, D.; Osborne, B.; Covarrubias, A.J.; Idda, M.L.; et al. Nuclear morphology is a deep learning biomarker of cellular senescence. Nat. Aging 2022, 2, 742–755. [Google Scholar] [CrossRef]
- Matias, I.; Diniz, L.P.; Damico, I.V.; Araujo, A.P.B.; Neves, L.D.S.; Vargas, G.; Leite, R.E.P.; Suemoto, C.K.; Nitrini, R.; Jacob-Filho, W.; et al. Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus. Aging Cell 2022, 21, e13521. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, J.; Surina, S.; Hengstschläger, M.; Lomakin, A.J. Form follows function: Nuclear morphology as a quantifiable predictor of cellular senescence. Aging Cell 2023, 22, e14012. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chang, C.Y.; Lin, B.Z.; Wu, Y.L.; Wu, M.H.; Lin, L.T.; Huang, W.C.; Holz, J.D.; Sheu, T.J.; Lee, J.S.; et al. Up-regulation of cofilin-1 in cell senescence associates with morphological change and p27(kip1)-mediated growth delay. Aging Cell 2021, 20, e13288. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Hinds, P.W. Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer Res. 2006, 66, 2708–2715. [Google Scholar] [CrossRef][Green Version]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. Lysosomes in senescence and aging. EMBO Rep. 2023, 24, e57265. [Google Scholar] [CrossRef]
- Nousis, L.; Kanavaros, P.; Barbouti, A. Oxidative Stress-Induced Cellular Senescence: Is Labile Iron the Connecting Link? Antioxidants 2023, 12, 1250. [Google Scholar] [CrossRef]
- Camacho-Encina, M.; Booth, L.K.; Redgrave, R.E.; Folaranmi, O.; Spyridopoulos, I.; Richardson, G.D. Cellular Senescence, Mitochondrial Dysfunction, and Their Link to Cardiovascular Disease. Cells 2024, 13, 353. [Google Scholar] [CrossRef]
- Giroud, J.; Combémorel, E.; Pourtier, A.; Abbadie, C.; Pluquet, O. Unraveling the functional and molecular interplay between cellular senescence and the unfolded protein response. Am. J. Physiol.-Cell Physiol. 2025, 328, C1764–C1782. [Google Scholar] [CrossRef]
- Sabath, N.; Levy-Adam, F.; Younis, A.; Rozales, K.; Meller, A.; Hadar, S.; Soueid-Baumgarten, S.; Shalgi, R. Cellular proteostasis decline in human senescence. Proc. Natl. Acad. Sci. USA 2020, 117, 31902–31913. [Google Scholar] [CrossRef]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Kim, J.; Bai, H. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging. Antioxidants 2022, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, Y.; Jia, Y.; Zhou, Q.; Wang, Q.; Jiang, X. Cellular senescence: From homeostasis to pathological implications and therapeutic strategies. Front. Immunol. 2025, 16, 1534263. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Salotti, J.; Johnson, P.F. Regulation of senescence and the SASP by the transcription factor C/EBPβ. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef]
- Takahashi, A.; Loo, T.M.; Okada, R.; Kamachi, F.; Watanabe, Y.; Wakita, M.; Watanabe, S.; Kawamoto, S.; Miyata, K.; Barber, G.N.; et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 2018, 9, 1249. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Yi, X.; Dou, Q.; Yang, X.; He, Y.; Chen, J.; Chen, K. Cellular senescence of renal tubular epithelial cells in acute kidney injury. Cell Death Discov. 2024, 10, 62. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress and cell senescence as drivers of ageing: Chicken and egg. Ageing Res. Rev. 2024, 102, 102558. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Dong, R.; Wang, J.; Guan, R.; Sun, J.; Jin, P.; Shen, J. Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer. Antioxidants 2025, 14, 104. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, E.; Leone, S.; Sgura, A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W.; Adams, P.D. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol. Cell Biol. 2007, 27, 2343–2358. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Graifer, D.; Stefanovie, B.; Krejci, L. Mitochondrial dysfunction in DDR-related cancer predisposition syndromes. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2016, 1865, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, O.; Van Veldhoven, P.P.; Brees, C.; Ho, Y.S.; Terlecky, S.R.; Fransen, M. Intraperoxisomal redox balance in mammalian cells: Oxidative stress and interorganellar cross-talk. Mol. Biol. Cell 2011, 22, 1440–1451. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, X.; Yan, C.; Sun, S.; Chen, Z.; Lin, F. Mitochondrial dysfunction and aging: Multidimensional mechanisms and therapeutic strategies. Biogerontology 2025, 26, 142. [Google Scholar] [CrossRef]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef]
- Hinchy, E.C.; Gruszczyk, A.V.; Willows, R.; Navaratnam, N.; Hall, A.R.; Bates, G.; Bright, T.P.; Krieg, T.; Carling, D.; Murphy, M.P. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 2018, 293, 17208–17217. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Kuwano, Y.; Kim, H.H.; Gorospe, M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: Implications for cellular senescence. Biol. Chem. 2008, 389, 243–255. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Cui, X.; Wang, J.; Liu, S.; Li, H.; Yang, J.; Zhang, C.; Zhang, W. Smooth muscle-specific HuR knockout induces defective autophagy and atherosclerosis. Cell Death Dis. 2021, 12, 385. [Google Scholar] [CrossRef]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef]
- Passos, J.F.; von Zglinicki, T.; Kirkwood, T.B. Mitochondria and ageing: Winning and losing in the numbers game. Bioessays 2007, 29, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Assalve, G.; Lunetti, P.; Rocca, M.S.; Cosci, I.; Di Nisio, A.; Ferlin, A.; Zara, V.; Ferramosca, A. Exploring the Link Between Telomeres and Mitochondria: Mechanisms and Implications in Different Cell Types. Int. J. Mol. Sci. 2025, 26, 993. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef]
- Shreeya, T.; Ansari, M.S.; Kumar, P.; Saifi, M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Senescence: A DNA damage response and its role in aging and Neurodegenerative Diseases. Front. Aging 2023, 4, 1292053. [Google Scholar] [CrossRef]
- Andreux, P.A.; Houtkooper, R.H.; Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013, 12, 465–483. [Google Scholar] [CrossRef]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef]
- Dalle Pezze, P.; Nelson, G.; Otten, E.G.; Korolchuk, V.I.; Kirkwood, T.B.; von Zglinicki, T.; Shanley, D.P. Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput. Biol. 2014, 10, e1003728. [Google Scholar] [CrossRef]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. Embo J. 2016, 35, 724–742. [Google Scholar] [CrossRef]
- Faraonio, R. Oxidative Stress and Cell Senescence Process. Antioxidants 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Feng, X.-D.; Huang, C.-S.; Li, J.; Wang, H.; Wang, X.-M.; Li, L.; Ma, L.-Q. Oxidative stress and cellular senescence: Roles in tumor progression and therapeutic opportunities. MedComm—Oncol. 2024, 3, e70007. [Google Scholar] [CrossRef]
- Lawless, C.; Jurk, D.; Gillespie, C.S.; Shanley, D.; Saretzki, G.; von Zglinicki, T.; Passos, J.F. A stochastic step model of replicative senescence explains ROS production rate in ageing cell populations. PLoS ONE 2012, 7, e32117. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Miro-Mur, F.; Alijotas-Reig, J.; Tchkonia, T.; Schwartz, S.; Kirkland, J.L.; Oshima, J. Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex. Aging 2023, 15, 4012–4034. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Wang, S.; Mao, G. DNA damage response(DDR): A link between cellular senescence and human cytomegalovirus. Virol. J. 2023, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Amani, J.; Gorjizadeh, N.; Younesi, S.; Najafi, M.; Ashrafi, A.M.; Irian, S.; Gorjizadeh, N.; Azizian, K. Cyclin-dependent kinase inhibitors (CDKIs) and the DNA damage response: The link between signaling pathways and cancer. DNA Repair 2021, 102, 103103. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Rogers, N.M.; Ghimire, K. Thrombospondin-1 CD47 Signalling: From Mechanisms to Medicine. Int. J. Mol. Sci. 2021, 22, 4062. [Google Scholar] [CrossRef]
- Ghantous, L.; Volman, Y.; Hefez, R.; Wald, O.; Stern, E.; Friehmann, T.; Chajut, A.; Bremer, E.; Elhalel, M.D.; Rachmilewitz, J. The DNA damage response pathway regulates the expression of the immune checkpoint CD47. Commun. Biol. 2023, 6, 245. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Chen, J.; Xu, Y. Thrombospondin-1: A Key Protein That Induces Fibrosis in Diabetic Complications. J. Diabetes Res. 2020, 2020, 8043135. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tian, L.; Li, L.; Chen, Z.; Ding, C.; Tian, J.; Song, D.; Yao, S.; Ren, W. Thrombospondin-1-mediated crosstalk between autophagy and oxidative stress orchestrates repair of blast lung injury. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1870, 167026. [Google Scholar] [CrossRef]
- Singla, B.; Aithbathula, R.V.; Pervaiz, N.; Kathuria, I.; Swanson, M.; Ekuban, F.A.; Ahn, W.; Park, F.; Gyamfi, M.; Cherian-Shaw, M.; et al. CD47 Activation by Thrombospondin-1 in Lymphatic Endothelial Cells Suppresses Lymphangiogenesis and Promotes Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1234–1250. [Google Scholar] [CrossRef]
- Bu, W.; Cui, Y.; Jin, Y.; Wang, X.; Jiang, M.; Huang, R.; Egbobe, J.O.; Zhao, X.; Tang, J. Unmasking the Invisible Threat: Biological Impacts and Mechanisms of Polystyrene Nanoplastics on Cells. Toxics 2024, 12, 908. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Lu, C.; Cheng, Y.; Jiang, X.; Yang, B.; Li, Y.; Chen, Q.; Ao, L.; Cao, J.; et al. Polystyrene nanoplastics exposure triggers spermatogenic cell senescence via the Sirt1/ROS axis. Ecotoxicol. Environ. Saf. 2024, 279, 116461. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Humayun, A.; Fornace, A.J., Jr. GADD45 in Stress Signaling, Cell Cycle Control, and Apoptosis. Adv. Exp. Med. Biol. 2022, 1360, 1–22. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Ding, S.; Zhang, M. Cross-talk of inflammation and cellular senescence: A new insight into the occurrence and progression of osteoarthritis. Bone Res. 2024, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.; Jara-Gutiérrez, C.; Paz-Araos, M.; Vázquez, M.C.; Díaz, P.; Murgas, P. The Relationship between Reactive Oxygen Species and the cGAS/STING Signaling Pathway in the Inflammaging Process. Int. J. Mol. Sci. 2022, 23, 15182. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Baird, L.; Taguchi, K.; Zhang, A.; Takahashi, Y.; Suzuki, T.; Kensler, T.W.; Yamamoto, M. A NRF2-induced secretory phenotype activates immune surveillance to remove irreparably damaged cells. Redox Biol. 2023, 66, 102845. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, Q.; Lu, F.; Lei, J.; Zhou, Y.; Liu, Y.; Zhu, N.; Yu, Y.; Ning, Z.; She, T.; et al. Nrf2 signaling pathway: Current status and potential therapeutic targetable role in human cancers. Front. Oncol. 2023, 13, 1184079. [Google Scholar] [CrossRef]
- Zoungrana, L.I.; Krause-Hauch, M.; Wang, H.; Fatmi, M.K.; Bates, L.; Li, Z.; Kulkarni, P.; Ren, D.; Li, J. The Interaction of mTOR and Nrf2 in Neurogenesis and Its Implication in Neurodegenerative Diseases. Cells 2022, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Bendavit, G.; Aboulkassim, T.; Hilmi, K.; Shah, S.; Batist, G. Nrf2 Transcription Factor Can Directly Regulate mTOR: LINKING CYTOPROTECTIVE GENE EXPRESSION TO A MAJOR METABOLIC REGULATOR THAT GENERATES REDOX ACTIVITY. J. Biol. Chem. 2016, 291, 25476–25488. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Panieri, E.; Telkoparan-Akillilar, P.; Suzen, S.; Saso, L. The NRF2/KEAP1 Axis in the Regulation of Tumor Metabolism: Mechanisms and Therapeutic Perspectives. Biomolecules 2020, 10, 791. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/β-TrcP/Nrf2 pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, S.; Sohn, H.Y.; Koh, Y.H.; Jo, C. TFEB activates Nrf2 by repressing its E3 ubiquitin ligase DCAF11 and promoting phosphorylation of p62. Sci. Rep. 2019, 9, 14354. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef]
- Smith-Vikos, T.; Slack, F.J. MicroRNAs and their roles in aging. J. Cell Sci. 2012, 125, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ankasha, S.J.; Shafiee, M.N.; Wahab, N.A.; Ali, R.A.R.; Mokhtar, N.M. Post-transcriptional regulation of microRNAs in cancer: From prediction to validation. Oncol. Rev. 2018, 12, 344. [Google Scholar] [CrossRef]
- Bu, H.; Wedel, S.; Cavinato, M.; Jansen-Dürr, P. MicroRNA Regulation of Oxidative Stress-Induced Cellular Senescence. Oxid. Med. Cell Longev. 2017, 2017, 2398696. [Google Scholar] [CrossRef]
- Williams, J.; Smith, F.; Kumar, S.; Vijayan, M.; Reddy, P.H. Are microRNAs true sensors of ageing and cellular senescence? Ageing Res. Rev. 2017, 35, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Catanesi, M.; d’Angelo, M.; Tupone, M.G.; Benedetti, E.; Giordano, A.; Castelli, V.; Cimini, A. MicroRNAs Dysregulation and Mitochondrial Dysfunction in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5986. [Google Scholar] [CrossRef]
- Saha, S. Role of microRNA in Oxidative Stress. Stresses 2024, 4, 269–281. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular transport of microRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
- Jung, H.J.; Suh, Y. Circulating miRNAs in ageing and ageing-related diseases. J. Genet. Genom. 2014, 41, 465–472. [Google Scholar] [CrossRef]
- Mharrach, I.; Tadlaoui, K.A.; Aqerrout, M.; Laraqui, A.; Ameur, A.; El Ghazzaly, A.; Ennibi, K.; Ennaji, M.M. Diagnostic value of miR-21 and miR-221 as potential biomarkers for early diagnosis of prostate cancer. Mol. Clin. Oncol. 2025, 22, 40. [Google Scholar] [CrossRef]
- Saliev, T.; Singh, P.B. Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules 2025, 15, 860. [Google Scholar] [CrossRef]
- Kaur, J.; Farr, J.N. Cellular senescence in age-related disorders. Transl. Res. 2020, 226, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lam, E.W.; Tchkonia, T.; Kirkland, J.L.; Sun, Y. Senescent Cells: Emerging Targets for Human Aging and Age-Related Diseases. Trends Biochem. Sci. 2020, 45, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Al-Naggar, I.M.A.; Kuchel, G.A.; Xu, M. Senolytics: Targeting senescent cells for age-associated diseases. Curr. Mol. Biol. Rep. 2020, 6, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lelarge, V.; Capelle, R.; Oger, F.; Mathieu, T.; Le Calvé, B. Senolytics: From pharmacological inhibitors to immunotherapies, a promising future for patients’ treatment. NPJ Aging 2024, 10, 12. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Rad, A.N.; Grillari, J. Current senolytics: Mode of action, efficacy and limitations, and their future. Mech. Ageing Dev. 2024, 217, 111888. [Google Scholar] [CrossRef]
- Imawari, Y.; Nakanishi, M. Senescence and senolysis in cancer: The latest findings. Cancer Sci. 2024, 115, 2107–2116. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, B.; Lu, W.; Cheng, Y.; Zhu, J.; Ai, G.; Zhang, X.; Liu, X.; Cheng, Z. Senolytic drugs dasatinib and quercetin combined with Carboplatin or Olaparib reduced the peritoneal and adipose tissue metastasis of ovarian cancer. Biomed. Pharmacother. 2024, 174, 116474. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Shou, X.; Liao, W.; Jin, C.; Chen, C.; Zhang, C.; Gao, W.; Zhang, J.; Ge, W.; et al. Senolytic Therapy Enabled by Senescent Cell-Sensitive Biomimetic Melanin Nano-Senolytics. Adv. Healthc. Mater. 2024, 13, e2401085. [Google Scholar] [CrossRef]
- Basu, A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol. Ther. 2022, 230, 107943. [Google Scholar] [CrossRef]
- Nor Hisam, N.S.; Ugusman, A.; Rajab, N.F.; Ahmad, M.F.; Fenech, M.; Liew, S.L.; Mohamad Anuar, N.N. Combination Therapy of Navitoclax with Chemotherapeutic Agents in Solid Tumors and Blood Cancer: A Review of Current Evidence. Pharmaceutics 2021, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, Y.; Huang, H.C.; Mitchison, T.J. Navitoclax (ABT-263) accelerates apoptosis during drug-induced mitotic arrest by antagonizing Bcl-xL. Cancer Res. 2011, 71, 4518–4526. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Warnier, M.; Leers, C.; Vernier, M.; Goehrig, D.; Médard, J.J.; Vindrieux, D.; Ziegler, D.V.; Bernard, D. Targeting chemoresistant senescent pancreatic cancer cells improves conventional treatment efficacy. Mol. Biomed. 2023, 4, 4. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, Y.; Liuyang, Z.; Li, H.; Jiang, Z.; Tao, J.; Liu, H.; Xie, A.; Feng, Y.; Dong, X.; et al. Quercetin Prevents Radiation-Induced Oral Mucositis by Upregulating BMI-1. Oxid. Med. Cell Longev. 2021, 2021, 2231680. [Google Scholar] [CrossRef]
- Della Vedova, L.; Baron, G.; Morazzoni, P.; Aldini, G.; Gado, F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals 2025, 18, 138. [Google Scholar] [CrossRef]
- Li, S.; Cao, H.; Shen, D.; Jia, Q.; Chen, C.; Xing, S.L. Quercetin protects against ox-LDL-induced injury via regulation of ABCAl, LXR-α and PCSK9 in RAW264.7 macrophages. Mol. Med. Rep. 2018, 18, 799–806. [Google Scholar] [CrossRef]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef]
- Tavenier, J.; Nehlin, J.O.; Houlind, M.B.; Rasmussen, L.J.; Tchkonia, T.; Kirkland, J.L.; Andersen, O.; Rasmussen, L.J.H. Fisetin as a senotherapeutic agent: Evidence and perspectives for age-related diseases. Mech. Ageing Dev. 2024, 222, 111995. [Google Scholar] [CrossRef]
- Watkins, B.A.; Mitchell, A.E.; Shin, A.C.; Dehghani, F.; Shen, C.-L. Dietary flavonoid actions on senescence, aging, and applications for health. J. Nutr. Biochem. 2025, 139, 109862. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Soriani, O.; Bernard, D. Cardiac Glycosides as Senolytic Compounds. Trends Mol. Med. 2020, 26, 243–245. [Google Scholar] [CrossRef]
- Kim, J.; Brunetti, B.; Kumar, A.; Mangla, A.; Honda, K.; Yoshida, A. Inhibition of glutaminase elicits senolysis in therapy-induced senescent melanoma cells. Cell Death Dis. 2024, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Rapamycin promotes endothelial-mesenchymal transition during stress-induced premature senescence through the activation of autophagy. Cell Commun. Signal 2020, 18, 43. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Zhang, M.; Xia, K.; Feng, X.; Li, Y.; Liang, X.; et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging 2020, 12, 1272–1284. [Google Scholar] [CrossRef]
- Qin, M.; Ren, J.; Chen, X.; Zhou, W.; Zhang, S.; Zhang, W.; Shi, M.; Zhang, M.; Liu, H.; Ma, Y.; et al. CD19-targeted HSP90 inhibitor nanoparticle combined with TKIs reduces tumor burden and enhances T-cell immunity in murine B-cell malignancies. Theranostics 2025, 15, 3589–3609. [Google Scholar] [CrossRef]
- Kirschner, K.; Rattanavirotkul, N.; Quince, M.F.; Chandra, T. Functional heterogeneity in senescence. Biochem. Soc. Trans. 2020, 48, 765–773. [Google Scholar] [CrossRef]
- Kowald, A.; Kirkwood, T.B.L. Senolytics and the compression of late-life mortality. Exp. Gerontol. 2021, 155, 111588. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting Cellular Senescence in Aging and Age-Related Diseases: Challenges, Considerations, and the Emerging Role of Senolytic and Senomorphic Therapies. Aging Dis. 2024, 15, 2554–2594. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): A complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 2021, 15, 3242–3255. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of the p38 MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014, 39, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Griveau, A.; Wiel, C.; Ziegler, D.V.; Bergo, M.O.; Bernard, D. The JAK1/2 inhibitor ruxolitinib delays premature aging phenotypes. Aging Cell 2020, 19, e13122. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, L.; Feng, J.; Song, H.; Zhang, S.; Yang, L.; Liu, Y.; Wang, T. Low-Dose Baricitinib Plus Narrow-Band Ultraviolet B for the Treatment of Progressive Non-Segmental Vitiligo: A Prospective, Controlled, Open-Label Study. Pigment. Cell Melanoma Res. 2025, 38, e13209. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, T.; Zhang, H.; Fan, X.; Zhang, Q.; Chen, H. Kaempferol enhances intestinal repair and inhibits the hyperproliferation of aging intestinal stem cells in Drosophila. Front. Cell Dev. Biol. 2024, 12, 1491740. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Hu, S.; Song, Y.; Jin, W.; Zou, J.; Zhang, Y.; Guo, J.; An, P.; Luo, J.; et al. Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells. Targets 2025, 3, 23. [Google Scholar] [CrossRef]
- Nacarelli, T.; Fukumoto, T.; Zundell, J.A.; Fatkhutdinov, N.; Jean, S.; Cadungog, M.G.; Borowsky, M.E.; Zhang, R. NAMPT Inhibition Suppresses Cancer Stem-like Cells Associated with Therapy-Induced Senescence in Ovarian Cancer. Cancer Res. 2020, 80, 890–900. [Google Scholar] [CrossRef]
- Wakita, M.; Takahashi, A.; Sano, O.; Loo, T.M.; Imai, Y.; Narukawa, M.; Iwata, H.; Matsudaira, T.; Kawamoto, S.; Ohtani, N.; et al. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat. Commun. 2020, 11, 1935. [Google Scholar] [CrossRef]
- Loison, I.; Pioger, A.; Paget, S.; Metatla, I.; Vincent, A.; Abbadie, C.; Dehennaut, V. O-GlcNAcylation inhibition redirects the response of colon cancer cells to chemotherapy from senescence to apoptosis. Cell Death Dis. 2024, 15, 762. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes. Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, Y.; He, X.; Gong, Y.; Huang, J.; Wu, P. Harnessing the interaction between redox signaling and senescence to restrain tumor drug resistance. Front. Cell Dev. Biol. 2025, 13, 1639772. [Google Scholar] [CrossRef]
- Sutkowy, P.; Czeleń, P. Redox Balance in Cancer in the Context of Tumor Prevention and Treatment. Biomedicines 2025, 13, 1149. [Google Scholar] [CrossRef]
- Ji, S.; Zheng, Z.; Liu, S.; Ren, G.; Gao, J.; Zhang, Y.; Li, G. Resveratrol promotes oxidative stress to drive DLC1 mediated cellular senescence in cancer cells. Exp. Cell Res. 2018, 370, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wang, X.; Zheng, Z.; Ren, G.; Zhu, H.; Qiao, M.; Li, G. Resveratrol drives cancer cell senescence via enhancing p38MAPK and DLC1 expressions. Food Funct. 2022, 13, 3283–3293. [Google Scholar] [CrossRef]
- Ma, F.; Ma, Y.; Liu, K.; Gao, J.; Li, S.; Sun, X.; Li, G. Resveratrol induces DNA damage-mediated cancer cell senescence through the DLC1-DYRK1A-EGFR axis. Food Funct. 2023, 14, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, V.; Ramaswamy, A.; Bhargava, P.; Srinivas, S.; Mandavkar, S.; Chaugule, D.; Peelay, Z.; Baheti, A.; Tandel, H.; Jadhav, V.K.; et al. A pro-oxidant combination of resveratrol and copper reduces chemotherapy-related non-haematological toxicities in advanced gastric cancer: Results of a prospective open label phase II single-arm study (RESCU III study). Med. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef]
- Hou, J.; Yun, Y.; Xue, J.; Jeon, B.; Kim, S. Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytother. Res. 2020, 34, 1659–1669. [Google Scholar] [CrossRef]
- Mamun, A.A.; Sufian, M.A.; Uddin, M.S.; Sumsuzzman, D.M.; Jeandet, P.; Islam, M.S.; Zhang, H.J.; Kong, A.N.; Sarwar, M.S. Exploring the role of senescence inducers and senotherapeutics as targets for anticancer natural products. Eur. J. Pharmacol. 2022, 928, 174991. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Neganova, M. Antioxidant Senotherapy by Natural Compounds: A Beneficial Partner in Cancer Treatment. Antioxidants 2025, 14, 199. [Google Scholar] [CrossRef]
- Ebrahimirad, F.; Mirmahdizade, S.E.; Mahmoodieh, B.; Najafi, S.; Banihashemian, S.M.; Nikakhtar, S.; Mobaraki, H.; Sadeghi, A.; Kossari, N.; SadatRafiei, S.K.; et al. Antioxidant strategies against cellular senescence: Unveiling the power of synthetic versus natural antioxidants in a systematic review. Front. Aging 2025, 6, 1543360. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Ścianowski, J. New glutathione peroxidase mimetics-Insights into antioxidant and cytotoxic activity. Bioorg Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef]
- Hariton, F.; Xue, M.; Rabbani, N.; Fowler, M.; Thornalley, P.J. Sulforaphane Delays Fibroblast Senescence by Curbing Cellular Glucose Uptake, Increased Glycolysis, and Oxidative Damage. Oxid. Med. Cell Longev. 2018, 2018, 5642148. [Google Scholar] [CrossRef]
- Liao, K.M.; Chen, C.J.; Luo, W.J.; Hsu, C.W.; Yu, S.L.; Yang, P.C.; Su, K.Y. Senomorphic effect of diphenyleneiodonium through AMPK/MFF/DRP1 mediated mitochondrial fission. Biomed. Pharmacother. 2023, 162, 114616. [Google Scholar] [CrossRef]
- Yu, J.; Li, T.; Zhu, J. Gene Therapy Strategies Targeting Aging-Related Diseases. Aging Dis. 2023, 14, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M. Gene therapy for p16-overexpressing cells. Aging 2018, 10, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef]
- Prata, L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Yang, D.; Sun, B.; Li, S.; Wei, W.; Liu, X.; Cui, X.; Zhang, X.; Liu, N.; Yan, L.; Deng, Y.; et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 2023, 15, eadd1951. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; He, Y.; Chen, S.; Yang, W.; Chen, X.; Feng, S.; Xiong, L.; Peng, Y.; Shao, Z. PGC1α-Inducing Senomorphic Nanotherapeutics Functionalized with NKG2D-Overexpressing Cell Membranes for Intervertebral Disc Degeneration. Adv. Sci. 2024, 11, e2400749. [Google Scholar] [CrossRef]
- Jain, S.S.; Burton Sojo, G.; Sun, H.; Friedland, B.N.; McNamara, M.E.; Schmidt, M.O.; Wellstein, A. The Role of Aging and Senescence in Immune Checkpoint Inhibitor Response and Toxicity. Int. J. Mol. Sci. 2024, 25, 7013. [Google Scholar] [CrossRef]
- Salminen, A. The role of the immunosuppressive PD-1/PD-L1 checkpoint pathway in the aging process and age-related diseases. J. Mol. Med. 2024, 102, 733–750. [Google Scholar] [CrossRef]
- Majewska, J.; Agrawal, A.; Mayo, A.; Roitman, L.; Chatterjee, R.; Sekeresova Kralova, J.; Landsberger, T.; Katzenelenbogen, Y.; Meir-Salame, T.; Hagai, E.; et al. p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells. Nat. Cell Biol. 2024, 26, 1336–1345. [Google Scholar] [CrossRef]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Murphy, K.C.; DeMarco, K.D.; Gopalan, S.; Liu, H.; Parikh, C.N.; Lopez-Diaz, Y.; Faulkner, M.; Li, J.; Morris, J.P.t.; et al. EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance. Nat. Cancer 2023, 4, 872–892. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).