Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish
Abstract
1. Introduction
2. Material and Methods
2.1. Animals Breeding and Sample Collection
2.2. Antioxidant Enzyme Activity Assay
2.3. RNA Extraction, Library Preparation and Transcriptome Sequencing
2.4. Transcriptome Assembly and Differentially Expressed Genes Screening
2.5. Gut Microbiota Taxa Analysis
2.6. Histopathology Analysis
2.7. Immunohistochemistry Detection
2.8. Statistics Analysis
3. Results
3.1. Effects of Heat Stress on the Mortality of Wild Burbot
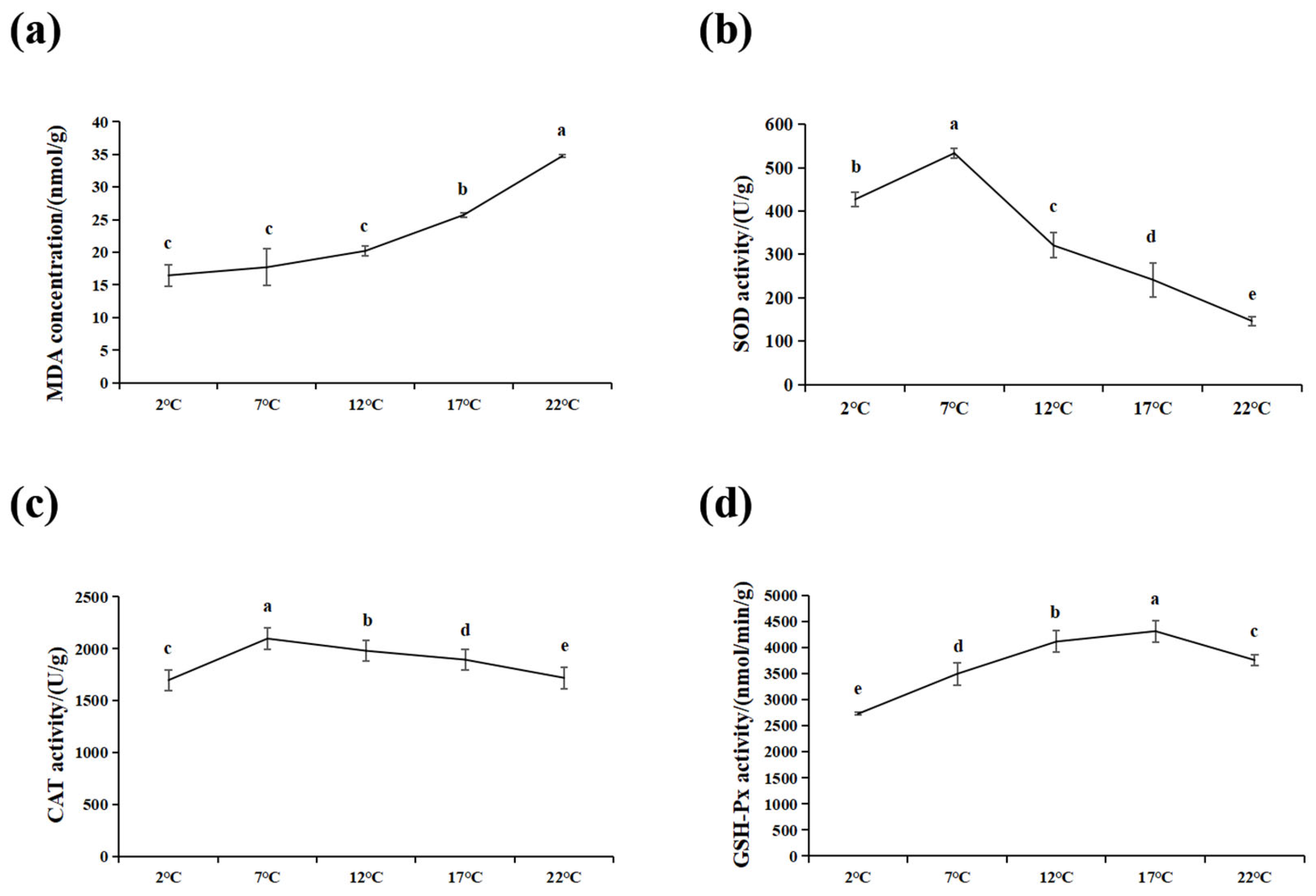
3.2. Effects of High-Temperature Stress on Oxidative Stress Parameters
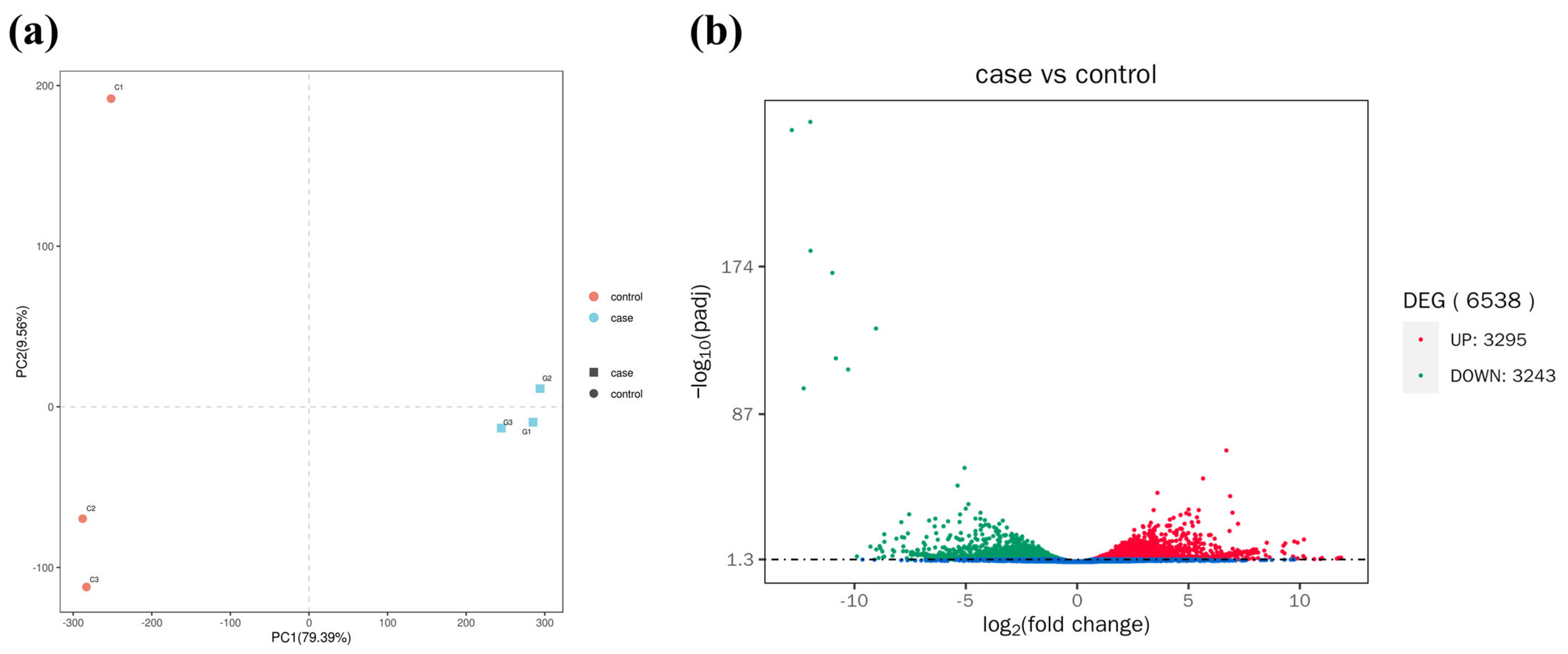
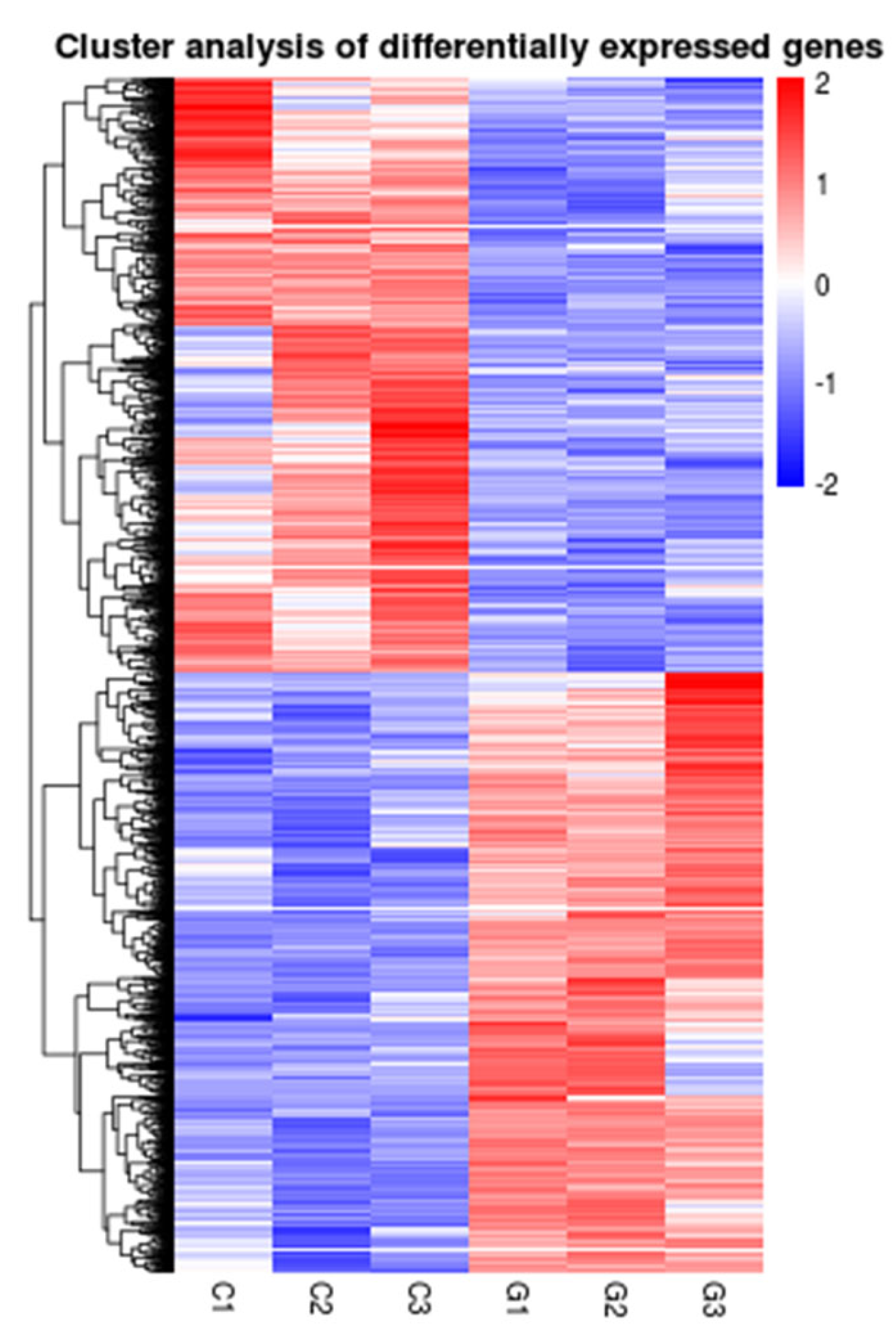
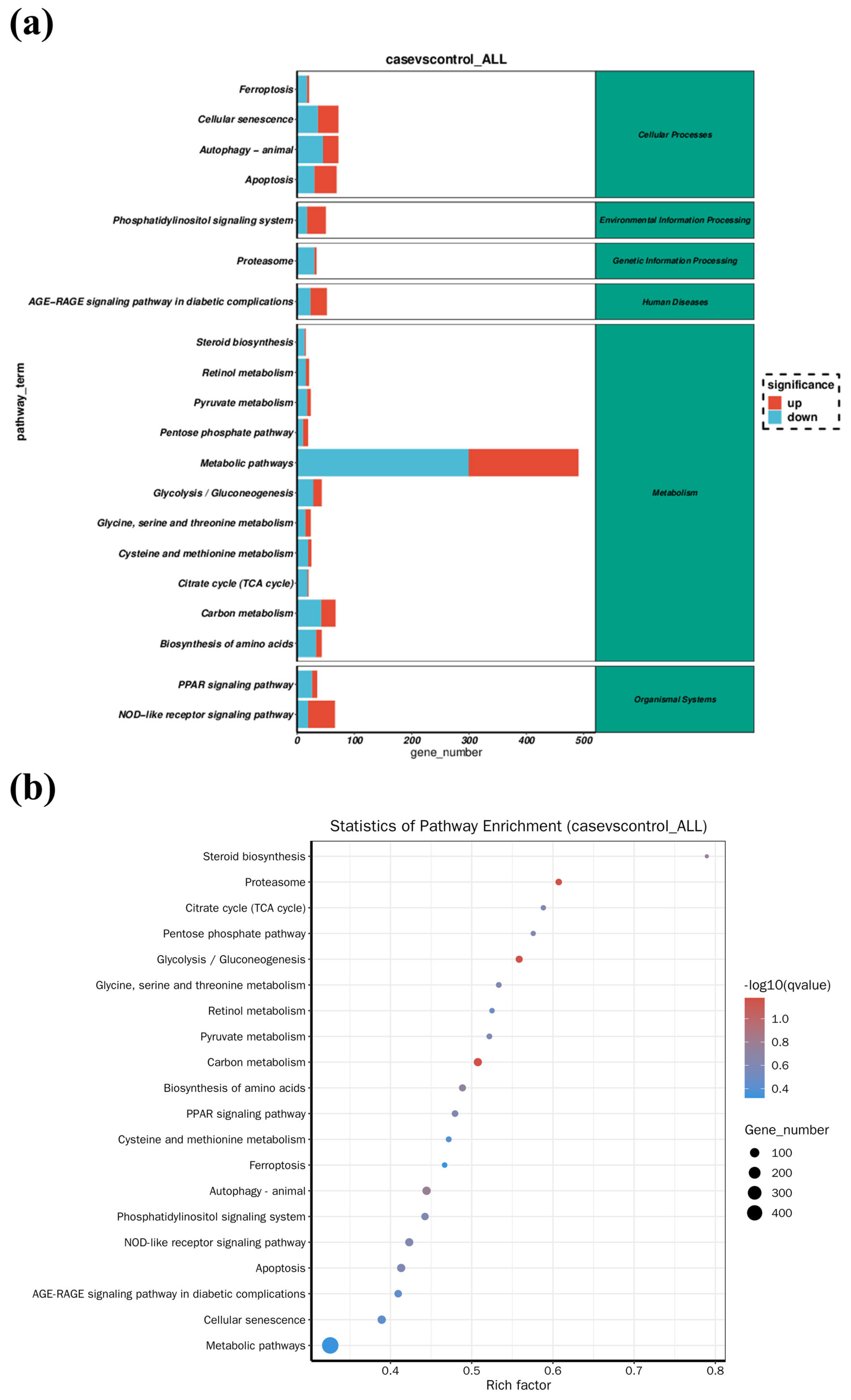
3.3. Changes in Gene Expression of Fish Liver
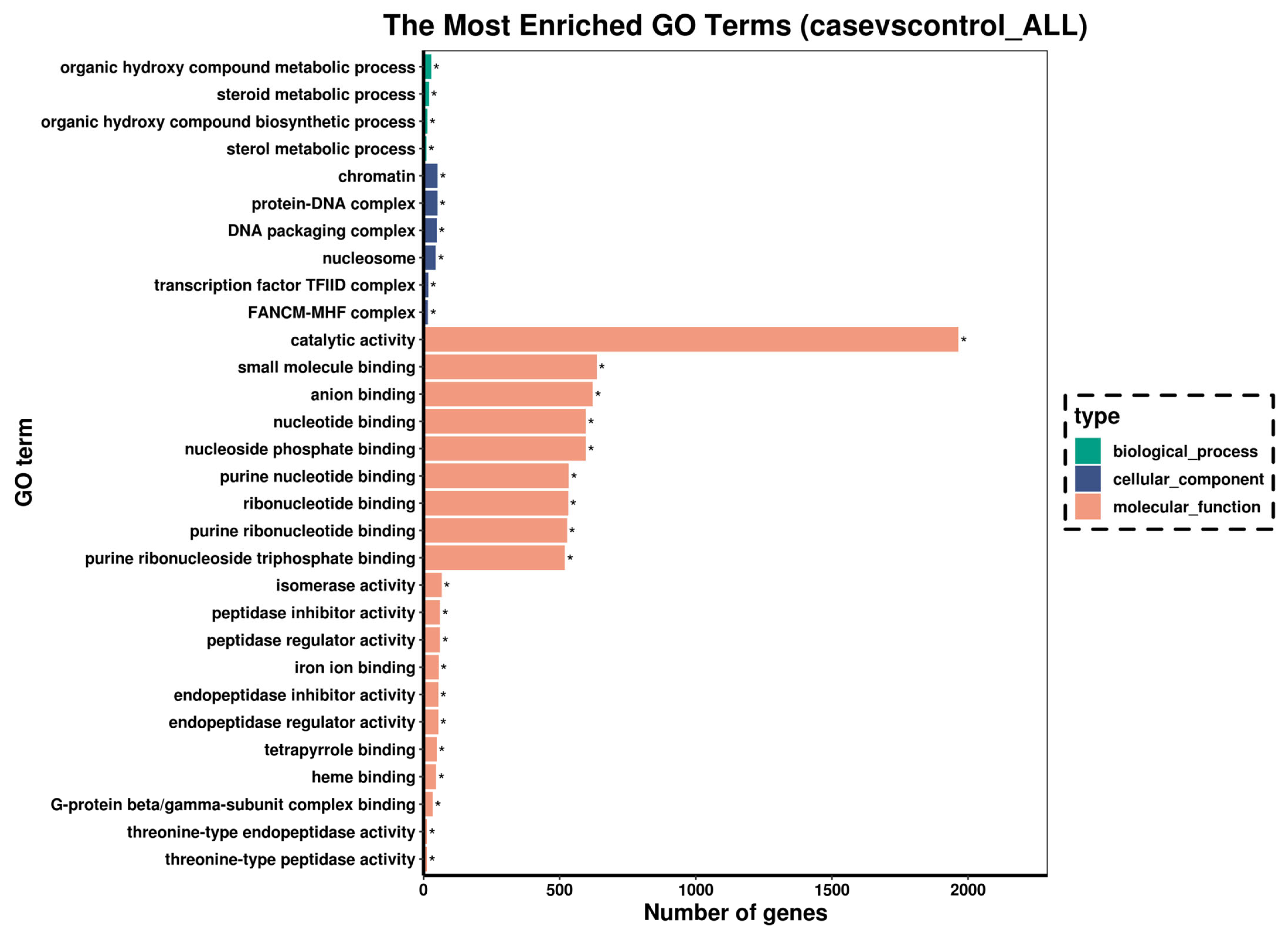
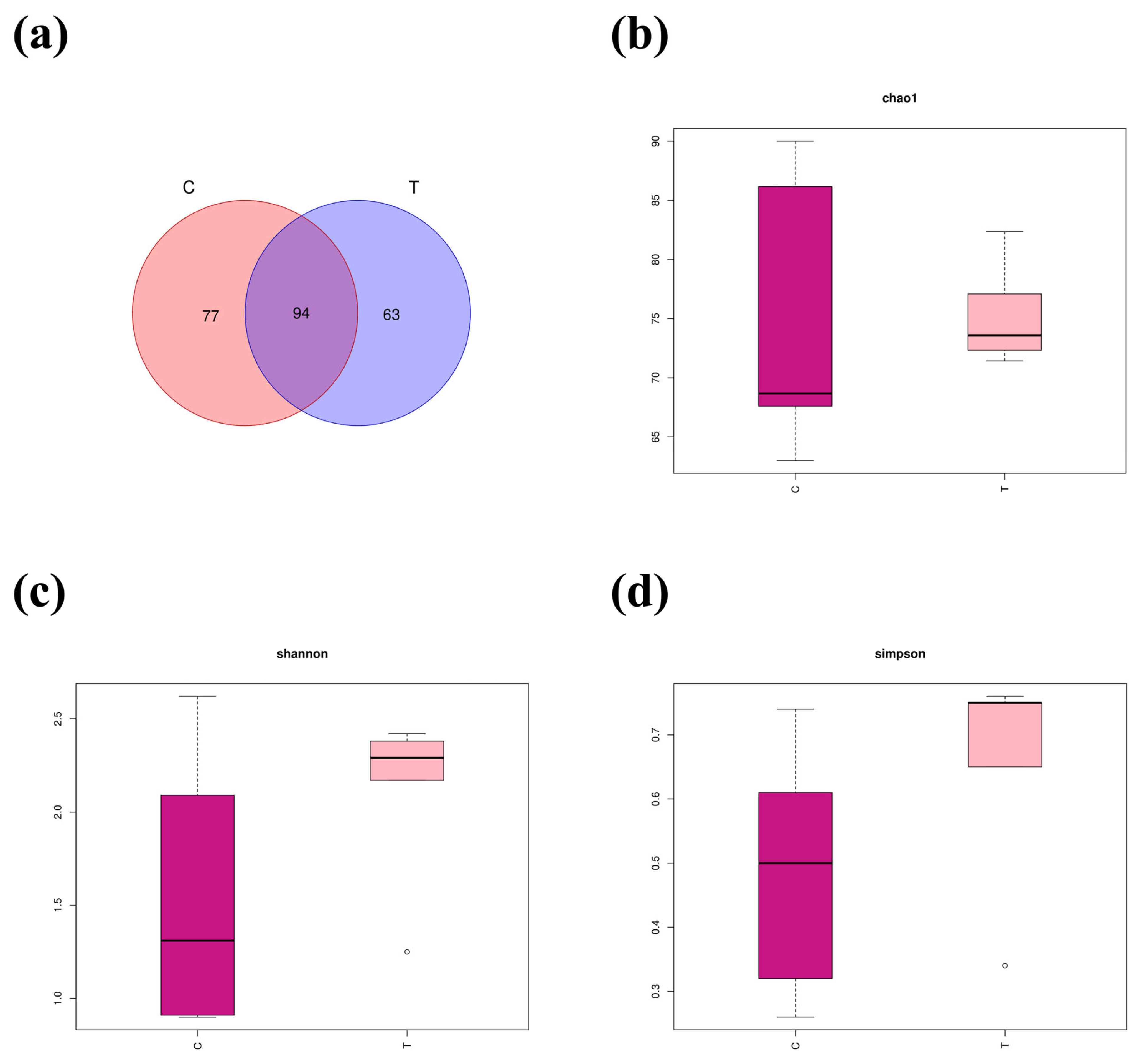
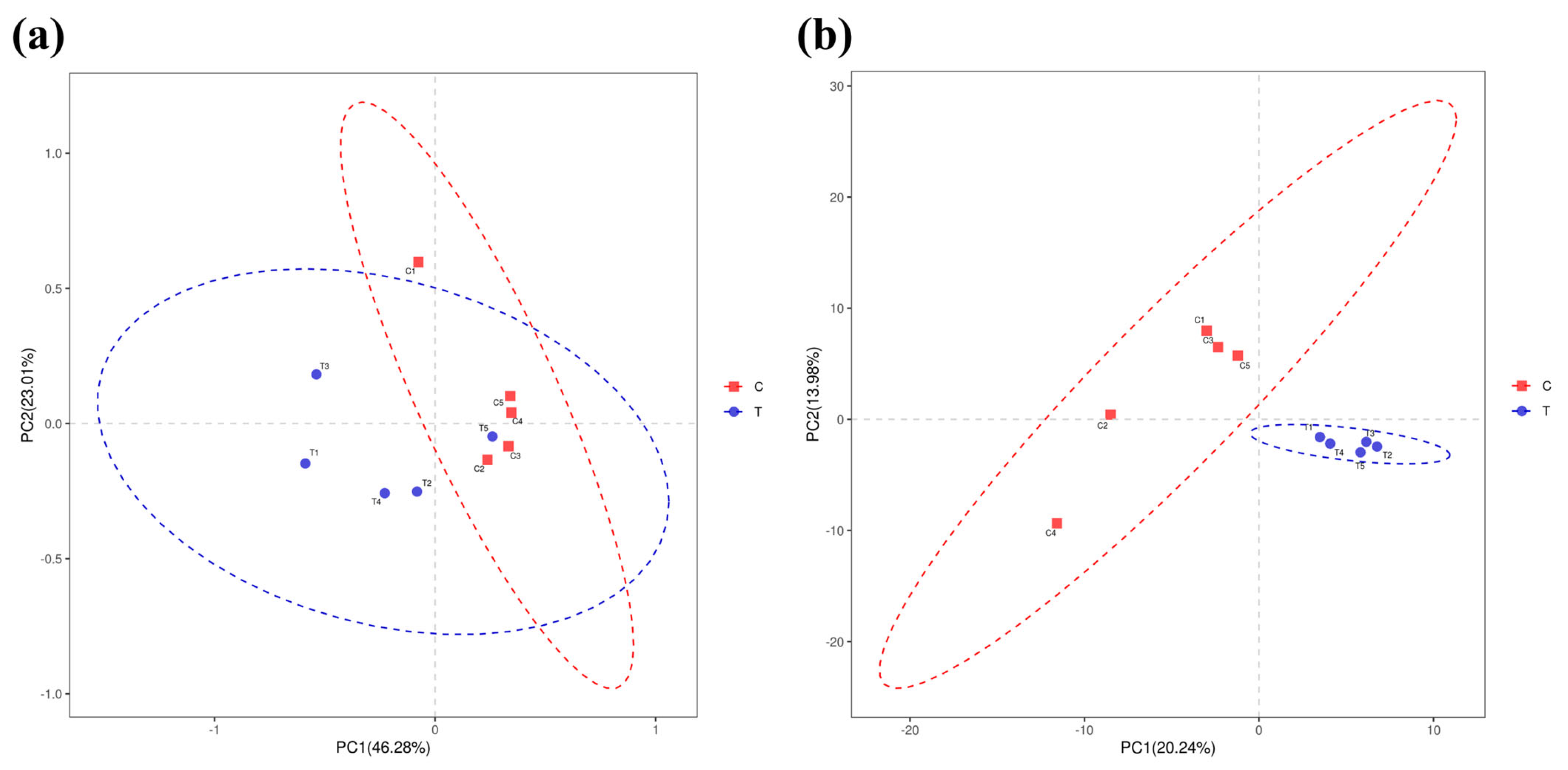
3.4. Intestinal Microbe Taxa Analysis
3.5. Correlation Analysis Between Intestinal Microbes and Liver Antioxidant, Immune, and Apoptosis Indexes
3.6. Hepatic Histology Observation
3.7. Effect of Heat Stress on FAS and Caspase3 Expression in Burbot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; VanTiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Ka, W.; Yuan, W.K.; Wang, J.L. The effect of acute heat stress on the innate immune function of rainbow trout based on the transcriptome. J. Therm. Biol. 2021, 96, 102834. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Ren, W.; Zheng, S.; Ren, Y. Histological, immune, and intestine microbiota responses of the intestine of rainbow trout (Oncorhynchus mykiss) to high temperature stress. Aquaculture 2024, 582, 740465. [Google Scholar] [CrossRef]
- Pérez-Casanova, J.C.; Afonso, L.O.B.; Johnson, S.C.; Currie, S.; Gamperl, A.K. The stress and metabolic responses of juvenile Atlantic cod Gadus morhua L. to an acute thermal challenge. J. Fish Biol. 2008, 72, 899–916. [Google Scholar] [CrossRef]
- Sharifian, M.; English, C.J.; Whitehead, D.L.; Barnes, A.C. Changes in mucosal epithelia of marine Atlantic salmon (Salmo salar) under chronic heat stress. Aquaculture 2025, 598, 742064. [Google Scholar] [CrossRef]
- Harrison, P.M.; Gutowsky, L.F.; Martins, E.G.; Patterson, D.A.; Cooke, S.J.; Power, M. Temporal plasticity in thermal-habitat selection of burbot Lota lota a diel-migrating winter-specialist. J. Fish Biol. 2016, 88, 2111–2129. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Sasinowski, W.; Targońska, K.; Mamcarz, A. The Influence of Temperature on Successful Reproductions of Burbot, Lota Lota (L.) Under Hatchery Conditions. Pol. J. Nat. Sci. 2010, 25, 93–105. [Google Scholar] [CrossRef]
- Ashton, N.K.; Jensen, N.R.; Ross, T.J.; Young, S.P.; Hardy, R.S.; Cain, K.D. Temperature and Maternal Age Effects on Burbot Reproduction. North Am. J. Fish. Manag. 2019, 39, 1192–1206. [Google Scholar] [CrossRef]
- Paragamian, V.L.; Wakkinen, V.D. Seasonal movement of burbot in relation to temperature and discharge in the Kootenai River, Idaho, USA and British Columbia, Canada. Am. Fish. Soc. Symp. 2008, 59, 55. [Google Scholar]
- Neufeld, M.D.; Davis, C.A.; Cain, K.D.; Jensen, N.R.; Ireland, S.C.; Lewandowski, C. Evaluation of methods for the collection and fertilization of burbot eggs from a wild stock for conservation aquaculture operations. J. Appl. Ichthyol. 2011, 27, 9–15. [Google Scholar] [CrossRef]
- Pandakov, P.G.; Teofilova, T.M.; Kodzhabashev, N.D. Status of the Burbot (Lota lota L.) in the Lower Danube (Bulgaria)–a species threatened by climate change. ZooKeys 2020, 910, 143. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, G.H.; Kuo, R.L.; Shih, S.R. Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect. 2017, 19, 570579. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquacult. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Steiner, K.; Laroche, O.; Walker, S.P.; Symonds, J.E. Effects of water temperature on the gut microbiome and physiology of Chinook salmon (Oncorhynchus tshawytscha) reared in a freshwater recirculating system. Aquaculture 2022, 560, 738529. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Rebekka, A.H.; Guido, G.; Gökhan, S.H.; Michael, T.; Ali, C. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Brena, B.M.; Pirez, M.; de Molina, M.C.R.; Luquet, C.M. Oxidative effects and toxin bioaccumulation after dietary microcystin intoxication in the hepatopancreas of the crab Neohelice (Chasmagnathus) granulata. Ecotoxicol. Environ. Saf. 2015, 120, 136–141. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef]
- Feng, S.; Zeng, Y.; Cai, Z.; Wu, J.; Zhou, J. Polystyrene microplastics alter the intestinal microbiota function and the hepatic metabolism status in marine medaka (Oryzias melastigma). Sci. Total Environ. 2020, 759, 143558. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol a exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in cd-1 mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, L.; Duan, Y.; Gao, Y.; Luo, Y. Gut microbiota exaggerates triclosan-induced liver injury via gut-liver axis. J. Hazard. Mater. 2021, 421, 126707. [Google Scholar] [CrossRef] [PubMed]
- Ukeda, H.; Kawana, D.; Maeda, S.; Sawamura, M. Spectrophotometric Assay for Superoxide Dismutase Based on the Reduction of Highly Water-soluble Tetrazolium Salts by Xanthine-Xanthine Oxidase. Biosci. Biotechnol. Biochem. 1999, 63, 485–488. [Google Scholar] [CrossRef] [PubMed]
- De Groot, H.; Auferkamp, O.; Bramey, T.; de Groot, K.; Kirsch, M.; Korth, H.G.; Petrat, F.; Sustmann, R. Non-oxygen-forming pathways of hydrogen peroxide degradation by bovine liver catalase at low hydrogen peroxide fluxes. Free. Radic. Res. 2006, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Gunzler, W.A. Assay of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, Y.F.; Wang, G.C.; Chen, Y.N.; Guo, J.Q.; Pan, C.L.; Liu, E.G.; Ling, Q.F. Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotox. Environ. Saf. 2019, 181, 130–137. [Google Scholar] [CrossRef]
- Ma, F.; Ma, B.H.; Zhang, B.X.; He, Y.D.; Wang, Y. Disturbance of oxidation/antioxidant status and histopathological damage in tsinling lenok trout under acute thermal stress. Trop. Anim. Health Prod. 2023, 55, 287. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Jia, Y.d.; Jing, Q.Q.; Zhai, J.M.; Guan, C.T.; Huang, B. Alternations in oxidative stress, apoptosis, and innate-immune gene expression at mRNA levels in subadult tiger puffer (Takifugu rubripes) under two different rearing systems. Fish Shellfish Immunol. 2019, 92, 756–764. [Google Scholar] [CrossRef]
- Hasnain, S.S.; Shuter, B.J.; Minns, C.K.; Fraser, D. Phylogeny influences the relationships linking key ecological thermal metrics for North American freshwater fish species. Can. J. Fish. Aquat. Sci. 2013, 70, 964–972. [Google Scholar] [CrossRef]
- Pörtner, H.O. Climate-dependent evolution of Antarctic ectotherms: An integrative analysis. Deep. Sea Res. 2006, 53, 1071–1104. [Google Scholar] [CrossRef]
- Pörtner, H.; Peck, M. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total. Environ. 2022, 806, 151252. [Google Scholar] [CrossRef] [PubMed]
- Ashaf-Ud-Doulah, M.; Mamun, A.; Rahman, M.L.; Islam, S.M.M.; Jannat, R.; Hossain, M.A.R.; Shahjahan, M. High temperature acclimation alters upper thermal limits and growth performance of Indian major carp, rohu, Labeo rohita (Hamilton, 1822). J. Therm. Biol. 2020, 93, 102738. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, L.L.; Yuan, L.; Liu, M.Z.; Du, Y.H.; Yang, L.H.; Ren, B.; Guo, D.S. Integration of physiological, transcriptomic and metabolomic reveals molecular mechanism of Paraisaria dubia response to Zn2+ stress. J. Fungi. 2023, 9, 693. [Google Scholar] [CrossRef]
- Zheng, X.W.; Liu, X.L.; Zhang, L.L.; Wang, Z.M.; Yuan, Y.; Li, J.; Li, Y.Y.; Huang, H.H.; Cao, X.; Fan, Z.Q. Toxicity mechanism of Nylon microplastics on Microcystis aeruginosa through three pathways: Photosynthesis, oxidative stress and energy metabolism. J. Hazard. Mater. 2022, 426, 128094. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Li, X.; Wang, Q.; Li, J.; Li, W. Fatty acid binding protein regulate antimicrobial function via Toll signaling in Chinese mitten crab. Fish Shellfish Immun. 2017, 63, 9–17. [Google Scholar] [CrossRef]
- Luo, S.; Cai, L.; Liu, Y.; Wang, W. Functional analysis of a dietary recombinant Fatty acid binding protein 10 (FABP10) on the Epinephelus coioides in response to acute low temperature challenge. Fish Shellfish Immun. 2014, 36, 475–484. [Google Scholar] [CrossRef]
- Morash, A.J.; Le Moine, C.M.R.; McClelland, G.B. Genome duplication events have led to a diversification in the CPT I gene family in fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R579–R589. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Lai, J.; Liu, Y.; Song, M.; Li, F.; Gong, Q. Integrated biochemical, transcriptomic and metabolomic analyses provide insight into heat stress response in Yangtze sturgeon (Acipenser dabryanus). Ecotoxicol. Environ. Saf. 2023, 249, 114366. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.J.; Clark, B.E.; Van Eenennaam, J.P.; Schreier, A.D.; Todgham, A.E. The effects of warm temperature acclimation on constitutive stress, immunity, and metabolism in white sturgeon (Acipenser transmontanus) of different ploidies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 224, 23–34. [Google Scholar] [CrossRef]
- Sobhkhez, M.; Skjesol, A.; Thomassen, E.; Tollersrud, L.G.; Iliev, D.B.; Sun, B.; Jørgensen, J.B. Structural and functional characterization of salmon STAT1, STAT2 and IRF9 homologs sheds light on interferon signaling in teleosts. FEBS Open Bio. 2014, 4, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Kessler, D.S.; Veals, S.A.; Levy, D.E.; Darnell, J.E., Jr. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc. Nati. Acad. Sci. USA 1990, 87, 8555–8559. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Lyu, L.K.; Wen, H.S.; Li, Y.; Li, J.F.; Zhao, J.; Zhang, S.M.; Song, M.; Wang, X.J. Deep transcriptomic analysis of black rockfish (Sebastes schlegelii) provides new insights on responses to acute temperature stress. Sci. Rep. 2018, 8, 9113. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Luo, X.C.; Dan, X.M.; Huang, X.Z.; Qiao, W.; Zhong, Z.P.; Li, A.X. Orange-spotted grouper (Epinephelus coioides) TLR2, MyD88 and IL-1β involved in anti-Cryptocaryon irritans response. Fish Shellfish Immunol. 2011, 30, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.D.; Hu, W.; Fulenchek, S.; Yamazaki, T.; Kurosaki, T.; Bazan, J.F.; Pasare, C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Nati. Acad. Sci. USA 2011, 109, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.W.; Kang, H.; Kong, J.R.; Xie, R.C.; Liu, Y.; Wang, W.N.; Xie, F.X.; Wang, C.; Sun, Z.M. Molecular cloning, characterization and expression analysis of (B-cell lymphoma-2) Bcl-2 in the orangespotted grouper (Epinephelus coioides). Dev. Comp. Immunol. 2017, 76, 150–162. [Google Scholar] [CrossRef]
- Nakano, K.; Iwanaga, M.; Utsunomiya, A.; Uchimaru, K.; Watanabe, T. Functional analysis of aberrantly spliced caspase 8 variants in adult T-cell leukemia cells. Mol. Cancer Res. 2019, 17, 2522–2536. [Google Scholar] [CrossRef]
- Botrus, G.; Miller, R.M.; Uson Junior, P.L.S.; Kannan, G.; Han, H.; Von Hoff, D.D. Increasing stress to induce apoptosis in pancreatic cancer via the unfolded protein response (UPR). Int. J. Mol. Sci. 2022, 24, 577. [Google Scholar] [CrossRef]
- Soriano, E.L.; Ramírez, D.T.; Araujo, D.R.; Gómez-Gil, B.; Castro, L.I.; Sánchez, C.G. Effect of temperature and dietary lipid proportion on gut microbiota in yellowtail kingfish Seriola lalandi juveniles. Aquaculture 2018, 497, 269–277. [Google Scholar] [CrossRef]
- Bestion, E.; Jacob, S.; Zinger, L.; Di Gesu, L.; Richard, M.; White, J.; Cote, J. Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat. Ecol. Evol. 2017, 1, 0161. [Google Scholar] [CrossRef]
- Terova, G.; Díaz, N.; Rimoldi, S.; Ceccotti, C.; Gliozheni, E.; Piferrer, F. Effects of sodium butyrate treatment on histone modifications and the expression of genes related to epigenetic regulatory mechanisms and immune response in european sea bass (Dicentrarchus Labrax) fed a plant-based diet. PLoS ONE 2016, 11, e0160332. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Xavier, R.; Severino, R.; Silva, S.M. Signatures of dysbiosis in fish microbiomes in the context of aquaculture. Rev. Aquac. 2024, 16, 706–731. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Cai, G.H.; Yang, H.L.; Nie, Q.J.; Liu, Z.Y.; Sun, Y.Z. New insights on intestinal microorganisms and carbohydrate metabolism in fish. Aquac. Int. 2024, 32, 2151–2170. [Google Scholar] [CrossRef]
- Zhu, B.R.; Lei, L.; Sun, Y.M.; Shi, X.J.; Fu, K.Y.; Hua, J.H.; Martyniuk, C.J.; Han, J.; Yang, L.H.; Zhou, B.S. Niclosamide exposure at environmentally relevant concentrations efficaciously inhibited the growth and disturbed the liver-gut axis of adult male zebrafish. Environ. Sci. Technol. 2022, 56, 11516–11526. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, Z.; Ran, C.; He, S.; Yang, Y.; Du, Z.; Zhou, Z. The hepatotoxicity of palmitic acid in zebrafish involves the intestinal microbiota. J. Nutr. 2018, 148, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Wang, B.K.; Pu, C.C.; Chang, K.; Cheng, Y.F.; Sun, R.Y.; Qi, Q.; Xu, R.Y.; Chen, J.L.; Zhang, C.N. Protective effects of sulforaphane on inflammation, oxidative stress and intestinal dysbacteriosis induced by triphenyltin in Cyprinus carpio haematopterus. Fish Shellfish Immunol. 2023, 142, 109135. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Z.; Chen, M.; Qu, Y.; Li, J.; Zhou, A.; Zou, J. Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Sci. Total Environ. 2019, 657, 1194–1204. [Google Scholar] [CrossRef]
- Li, Z.; Yu, E.; Wang, G.; Yu, D.; Zhang, K.; Gong, W.; Xie, J. Broad bean (Vicia faba L.) induces intestinal inflammation in Grass carp (Ctenopharyngodon idellus C. et V) by increasing relative abundances of intestinal gram-negative and flagellated bacteria. Front. Microbiol. 2018, 9, 1913. [Google Scholar] [CrossRef]
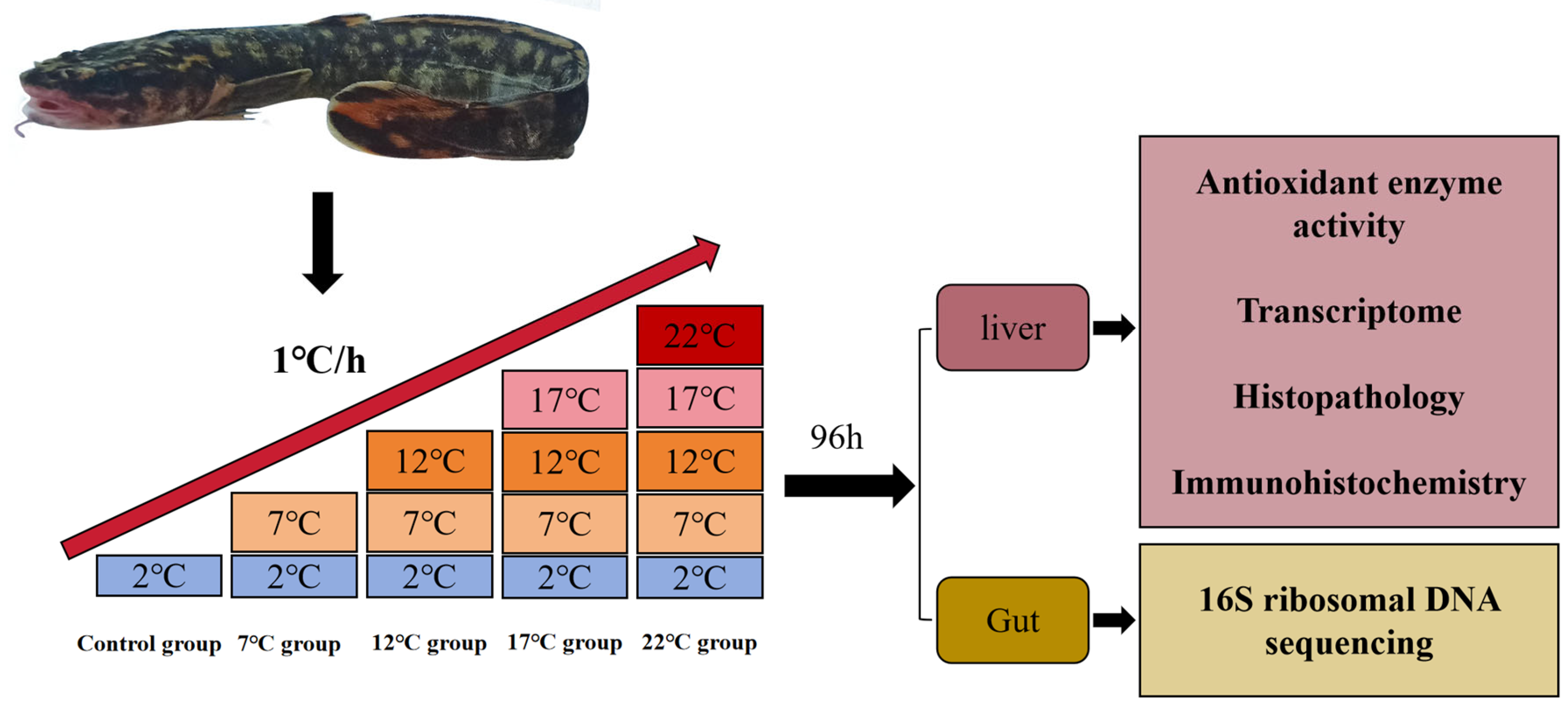
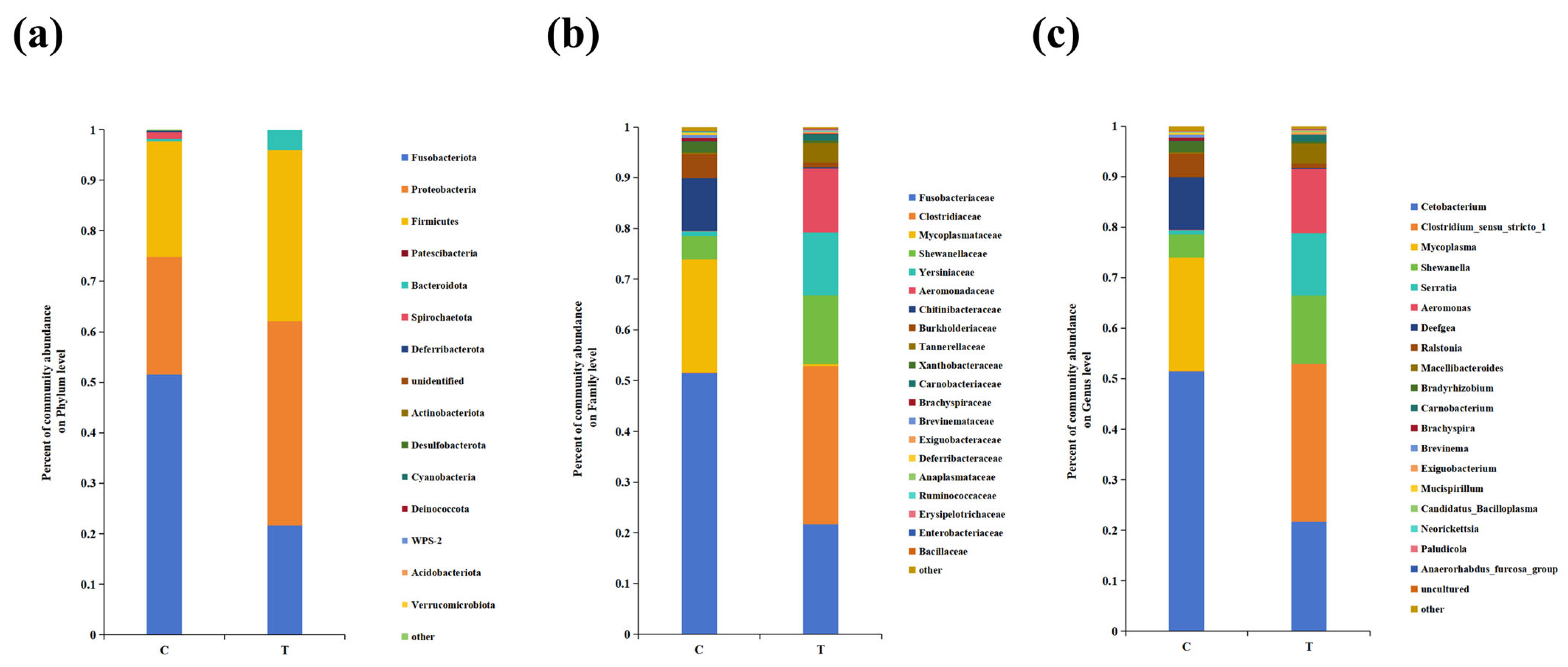
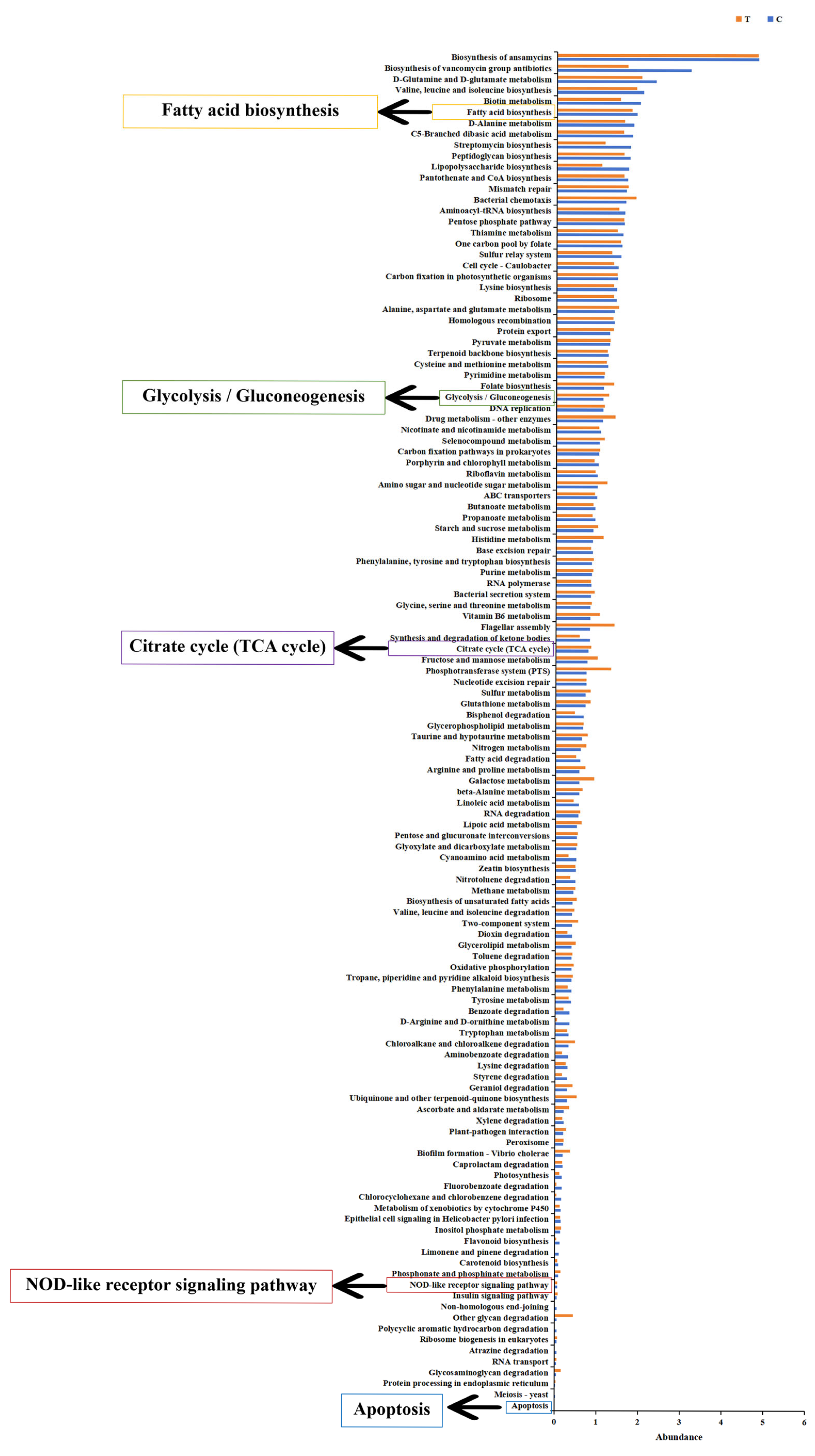


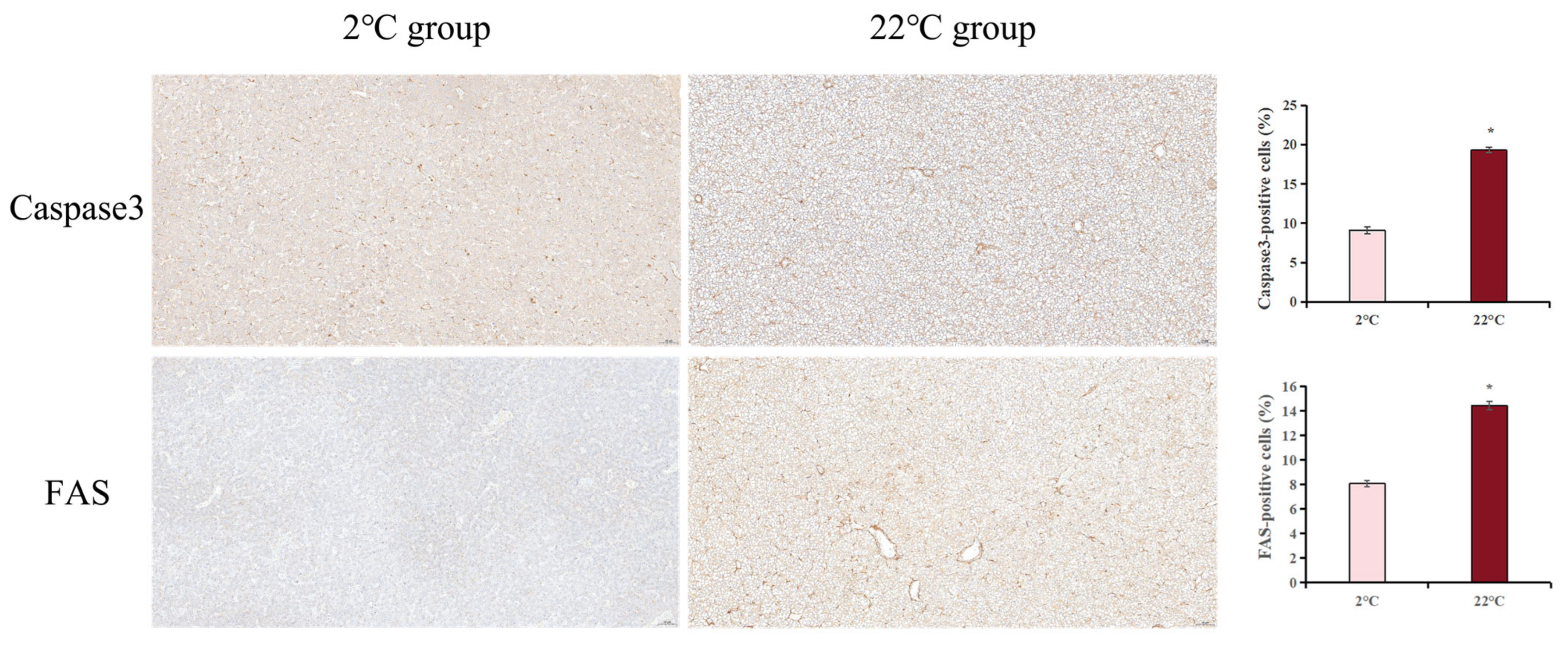
| Temperature Groups | Mortality (%) |
|---|---|
| 2 °C | 0% |
| 7 °C | 0% |
| 12 °C | 0% |
| 17 °C | 15% |
| 22 °C | 67% |
| Gene Name | Gene Description | log2 (Fold Change) | Regulation Trend | KEGG Pathway |
|---|---|---|---|---|
| scd1 | stearoyl-CoA desaturase | −6.8926 | down | PPAR signaling pathway |
| fabp | fatty acid-binding protein | −3.7339 | down | |
| cpt-1 | fatty acid-binding protein | −3.8176 | down | |
| hk1 | hexokinase-1 | −2.0128 | down | Glycolysis/Gluconeogenesis |
| hk2 | hexokinase-2 | −2.7132 | down | |
| alya | ATP-citrate synthase | −1.4418 | down | Citrate cycle (TCA cycle) |
| aco2 | aconitate hydratase | −1.5861 | down | |
| idh1 | isocitrate dehydrogenase | −1.3379 | down | |
| ogdhl | 2-oxoglutarate dehydrogenase-like | −1.9731 | down | |
| sdha | succinate dehydrogenase | −0.93966 | down | |
| stat1a | signal transducer and activator of transcription 1a | 2.2886 | up | NOD-like receptor signaling pathway |
| irf9 | interferon regulatory factor 9 | 1.4744 | up | |
| ifnα/β | interferon alpha/beta | 4.7766 | up | |
| nod1 | nucleotide-binding oligomerization domain-containing protein 1 isoform X1 | 1.434 | up | |
| caspase1 | caspase b | 2.0607 | up | |
| ripk2 | receptor-interacting serine/threonine-protein kinase 2 | −1.9997 | down | |
| tab | TAK1-binding protein | −1.2793 | down | |
| map3k7 | mitogen-activated protein kinase kinase kinase 7 | 3.2473 | up | |
| mapk | itogen-activated protein kinase | 1.6597 | up | |
| tnf-α | tumor necrosis factor a | 3.298 | up | Toll-like receptor signaling pathway |
| tlr2 | toll-like receptor 2 | 2.3241 | up | |
| myd88 | myeloid differentiation primary response protein MyD88 | 1.3693 | up | |
| akt | RAC-gamma serine/threonine-protein kinase | 1.9379 | up | |
| prf | perforin | 2.3632 | up | Apoptosis |
| dffb | DNA fragmentation factor subunit beta | −1.6189 | down | |
| caspase8 | caspase 8 | −1.3553 | down | |
| caspase3 | caspase 3 | 1.3808 | up | |
| sptan1 | spectrin alpha chain, non-erythrocytic 1 | 0.98599 | up | |
| atm | serine-protein kinase ATM | 2.0906 | up | |
| fas | tumor necrosis factor receptor superfamily member 6 | 1.5171 | up | |
| bid | BH3 interacting domain death agonist | −1.2813 | down | |
| ip3r | inositol 1,4,5-triphosphate receptor type 1 | 2.2898 | up | |
| calpain1 | calpain-1 catalytic subunit | 1.1952 | up | |
| bax | apoptosis regulator BAX | −1.2334 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, C.; Li, Y.; Wang, R.; Han, H.; Zhang, Y.; Ma, B. Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish. Antioxidants 2025, 14, 947. https://doi.org/10.3390/antiox14080947
Zhai C, Li Y, Wang R, Han H, Zhang Y, Ma B. Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish. Antioxidants. 2025; 14(8):947. https://doi.org/10.3390/antiox14080947
Chicago/Turabian StyleZhai, Cunhua, Yutao Li, Ruoyu Wang, Haoxiang Han, Ying Zhang, and Bo Ma. 2025. "Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish" Antioxidants 14, no. 8: 947. https://doi.org/10.3390/antiox14080947
APA StyleZhai, C., Li, Y., Wang, R., Han, H., Zhang, Y., & Ma, B. (2025). Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish. Antioxidants, 14(8), 947. https://doi.org/10.3390/antiox14080947






