Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Management
2.2. Acute Toxicity Test (96 h-LC50 Determination)
2.3. Chronic Exposure Experimental Design
2.4. Physiological and Biochemical Assays
2.5. Gene Quantification Analysis
2.5.1. RNA Extraction
2.5.2. RNA Quality Assessment
2.5.3. cDNA Synthesis
2.6. qRT-PCR Primer Design
2.7. Statistical Analysis
3. Results
3.1. The Results of 96 h-LC50 Determination of Juvenile Chinese Perch
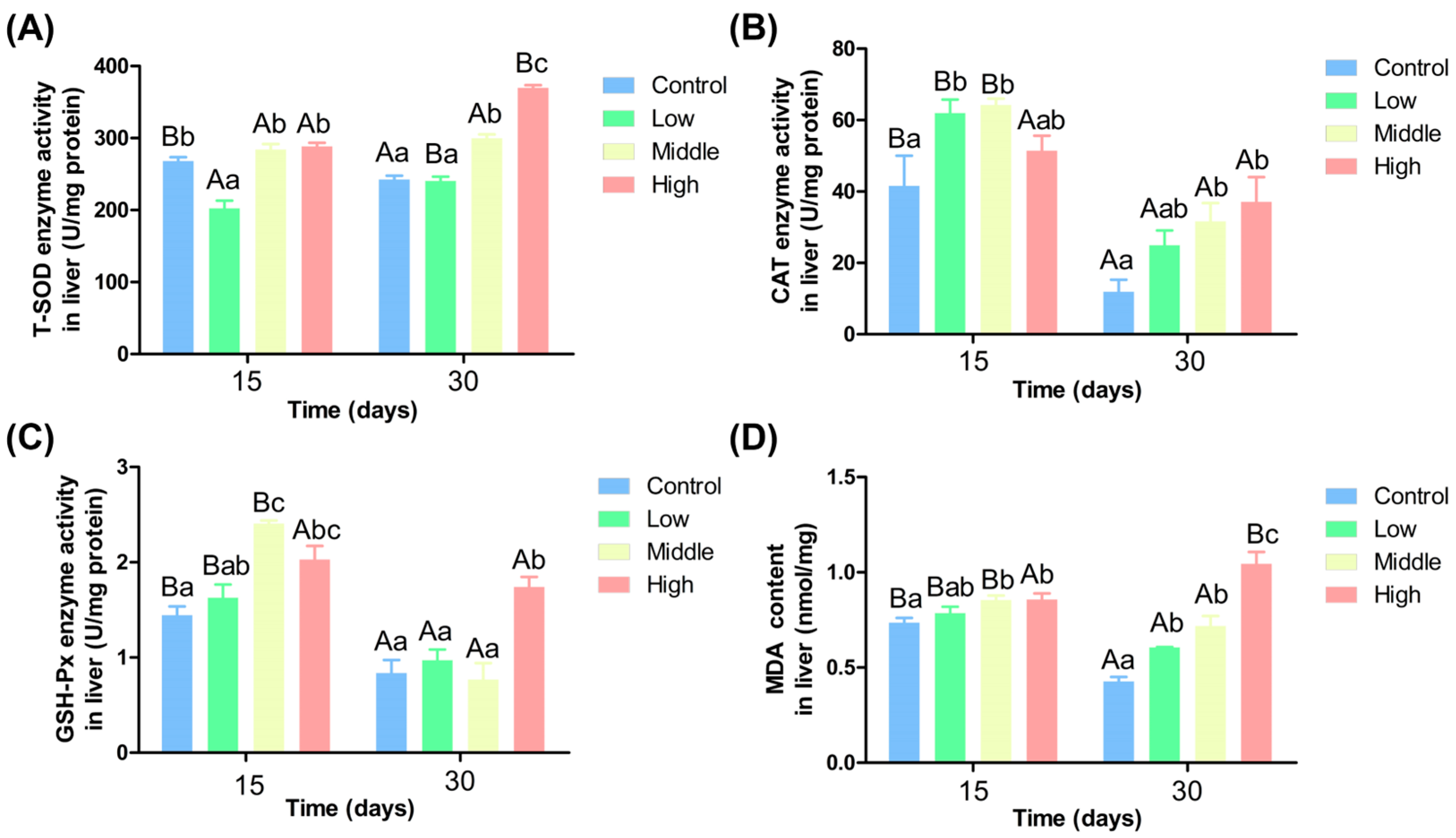
3.2. Different Ammonia Nitrogen Level Affect Chinese Perch Antioxidant Activity
3.3. The Influence of Different Ammonia Nitrogen Levels on Chinese Perch Blood Biochemical
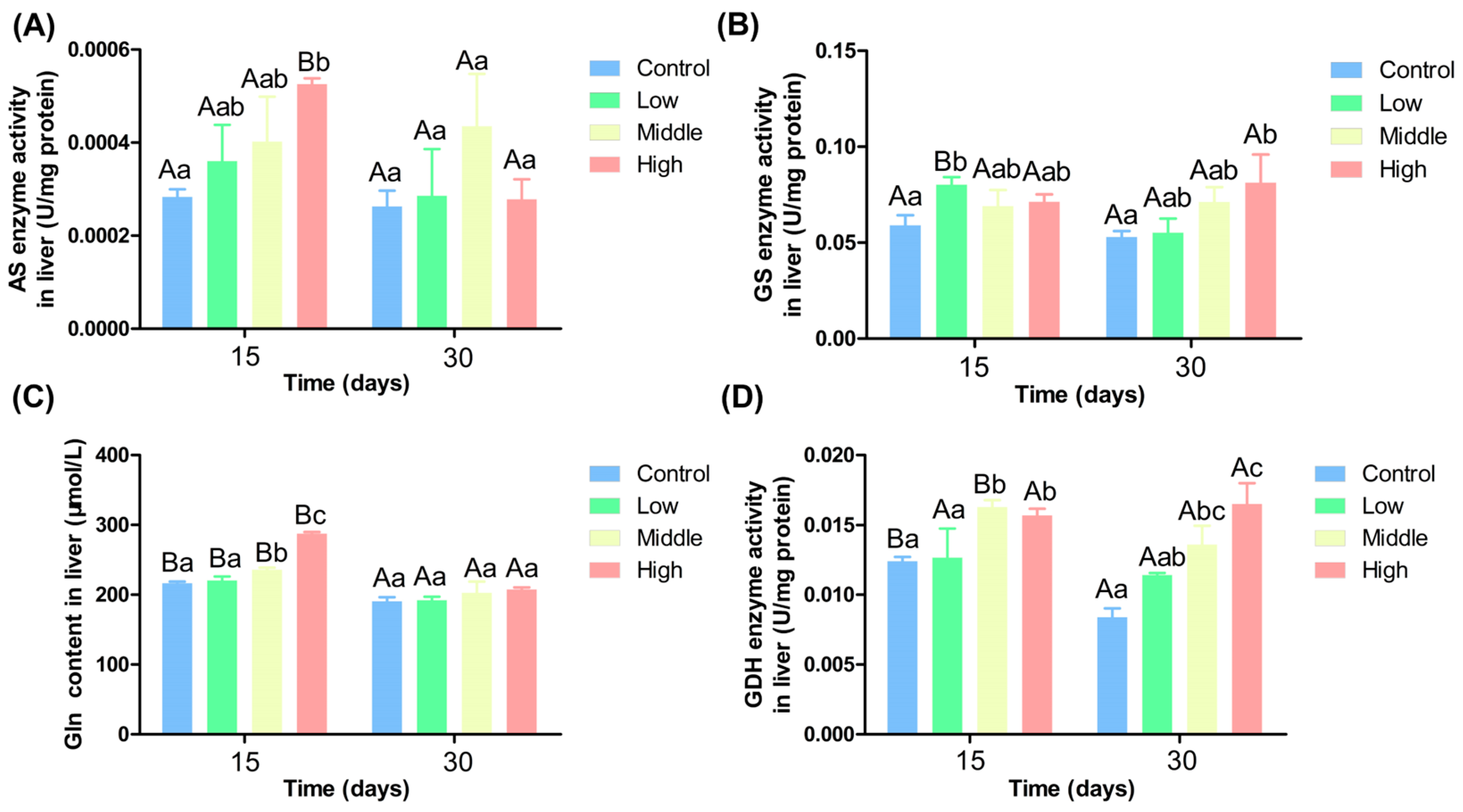
3.4. Effects of Different Ammonia Nitrogen Levels on Liver Enzyme Activities of Chinese Perch
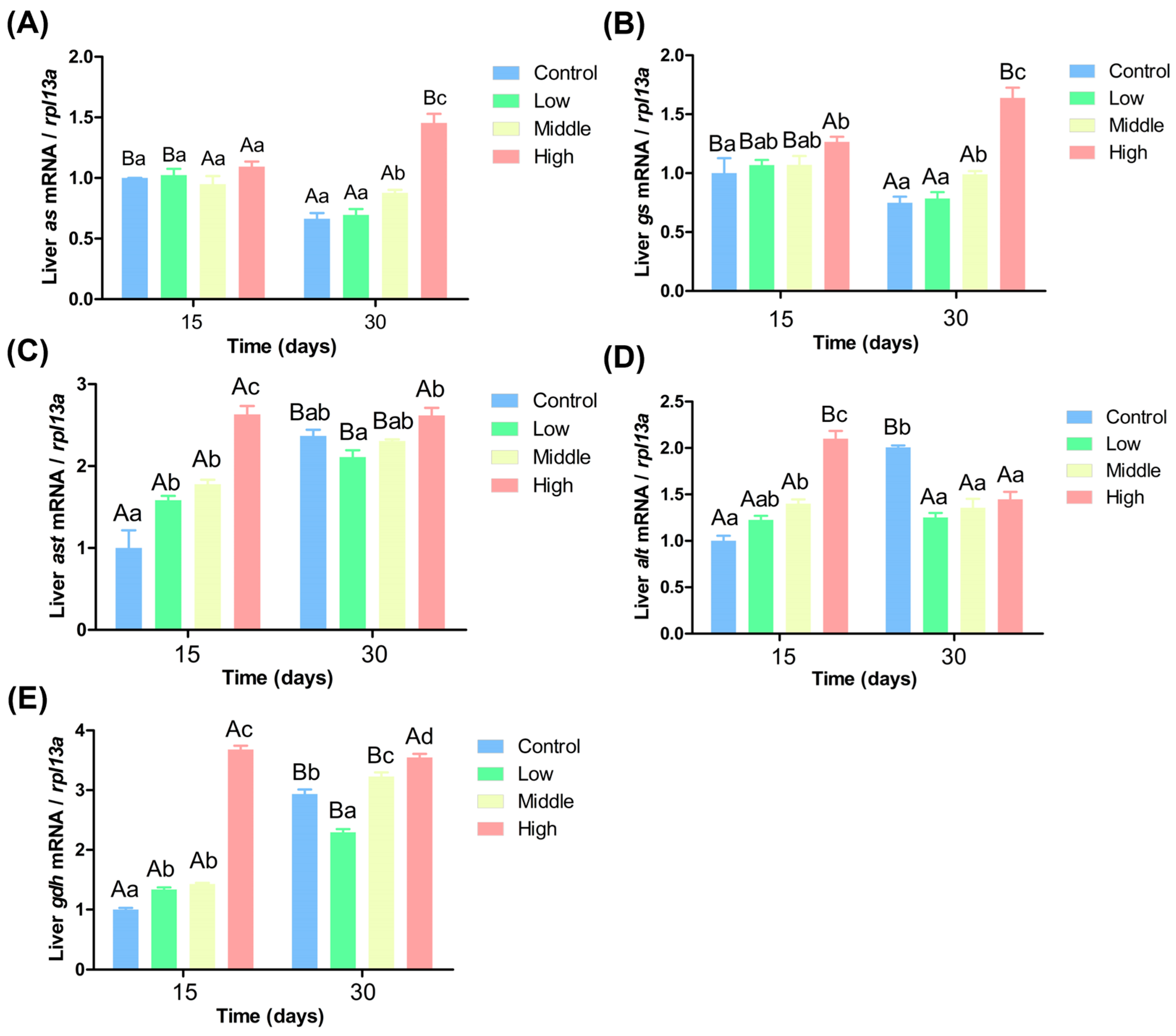
3.5. Effects of Different Ammonia Nitrogen Levels on Gene Expression in the Liver of Chinese Perch
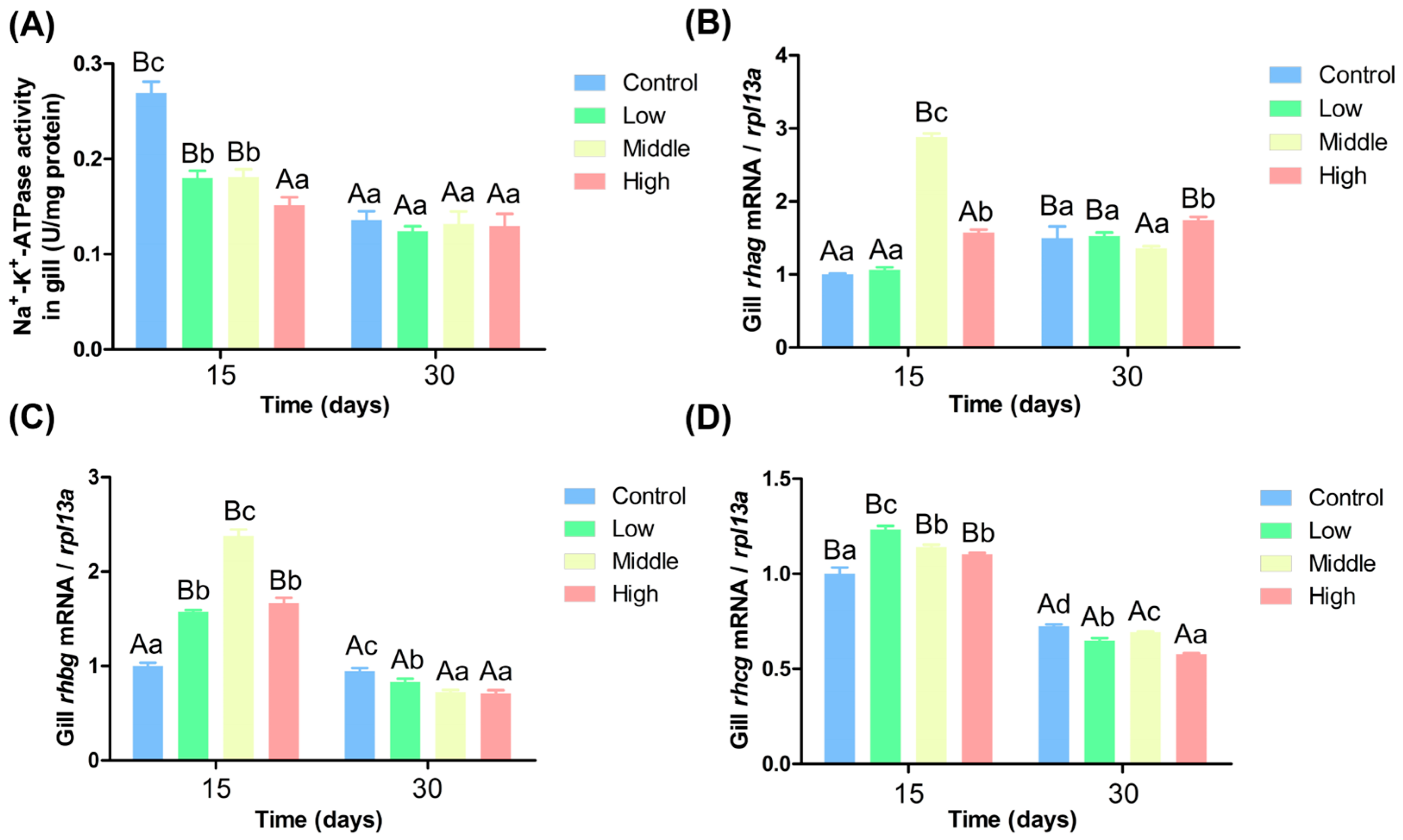
3.6. Impact of Varying Ammonia Nitrogen Concentrations on the Activity and Expression of Gill Filase in Chinese Perch
3.7. Effects of Different Levels of Ammonia Nitrogen on the AMPK Pathway of Chinese Perch
4. Discussion
4.1. Experiment on the Acute Toxicity of Ammonia Nitrogen to Chinese Perch
4.2. Different Ammonia Nitrogen Conditions on the Chinese Perch Resisting Oxidative Stress and the Effects of Liver Enzyme Activity
4.3. Different Ammonia Nitrogen Conditions Affect Chinese Perch Blood Physiological Indexes
4.4. Impact of Varying Ammonia Nitrogen Conditions on Enzyme Activity and Gene Expression in Chinese Perch
4.5. Effects of Different Ammonia-Nitrogen Conditions on Ammonia Excretion and Transformation in Chinese Perch
4.6. Effects of Different Ammonia Nitrogen Conditions on the AMPK Pathway in Chinese Perch
4.7. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACP | Acid phosphatase |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AS | Asparagine synthase |
| AST | Aspartate aminotransferase |
| CAT | Catalase |
| eEF2 | Eukaryotic elongation factor 2 |
| eEF2k | Eukaryotic elongation factor 2 Kinase |
| GDH | Glutamate dehydrogenase |
| Gln | Glutamine |
| GS | Glutamine synthetase |
| GSH-Px | Glutathione peroxidase |
| LKB1 | Liver kinase B1 |
| MDA | Malondialdehyde |
| Rhag | Rh a glycoprotein |
| Rhbg | Rh b glycoprotein |
| Rhcg | Rh c glycoprotein |
| TP | Total Protein |
| T-SOD | Total Superoxide Dismutase |
References
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Bai, J.J.; Ye, X.; Hu, Y.C.; Li, S.J.; Yu, L.Y. Polymorphisms in the 5′ flanking region of the insulin-like growth factor I gene are associated with growth traits in largemouth bass Micropterus salmoides. Fish Sci. 2009, 75, 351–358. [Google Scholar] [CrossRef]
- Bucking, C. A broader look at ammonia production, excretion, and transport in fish: A review of impacts of feeding and the environment. J. Comp. Physiol. B 2017, 187, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.F.; Ip, Y.K. Excretory nitrogen metabolism and defence against ammonia toxicity in air-breathing fishes. J. Fish Biol. 2014, 84, 603–638. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Zou, Z.; Long, C.; Lin, J.; Zeng, J.; Hou, J.; Han, L.; Jiang, Y.; Li, S. Identification of SNPs and candidate genes associate with growth performance in all-female mandarin fish (Siniperca chuatsi) by a genome-wide association study. Aquaculture 2024, 586, 740778. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Sun, Z.; Fang, Z.; Gong, Y.; Huang, X.; Zhang, H.; Chen, N.; Li, S. Effects of dietary lipid and protein levels on growth, body composition, antioxidant capacity, and flesh quality of mandarin fish (Siniperca chuatsi). Aquacult. Int. 2025, 33, 78. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Liu, Y.; Tian, Q.; Wang, Z.; Zhu, D.; Qian, Z.; Yi, Y.; Hu, J.; Li, Y.; et al. Dissolved oxygen and ammonia affect ammonia production via GDH/AMPK signaling pathway and alter flesh quality in Chinese perch (Siniperca chuatsi). Fish Physiol. Biochem. 2024, 50, 1237–1249. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Tissue Structure in Fish Exposed to Ammonia Nitrogen: A Review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef]
- Wajsbrot, N.; Gasith, A.; Diamant, A.; Popper, D.M. Chronic toxicity of ammonia to juvenile gilthead seabream Sparus aurata and related histopathological effects. J. Fish. Biol. 1993, 42, 321–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, L.; Zou, Z.; Su, J.; Liu, L. Effects of dietary carbohydrate levels on growth and ammonia excretion in Chinese perch (Siniperca chuatsi) at low water temperatures. Int. J. Mol. Sci. 2025, 26, 4638. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Oxidative stress, neurotoxicity, and non-specific immune responses in juvenile red sea bream, Pagrus major, exposed to different waterborne selenium concentrations. Chemosphere 2015, 135, 46–52. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Qian, Y.; Shi, G.; Wang, R. Ammonia toxicity in the yellow catfish (Pelteobagrus fulvidraco): The mechanistic insight from physiological detoxification to poisoning. Fish Shellfish Immunol. 2020, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Wang, R.; Qian, Y. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol. 2018, 79, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.Ç.K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef]
- Rodrigues, R.V.; Romano, L.A.; Schwarz, M.; Delbos, B.C.; Sampaio, L.A. Acute tolerance and histopathological effects of ammonia on juvenile maroon clownfish Premnas biaculeatus (Block 1790). Aquac. Res. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Sun, H.; Lü, K.; Minter, E.J.A.; Chen, Y.; Yang, Z.; Montagnes, D.J.S. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard. Mater. 2012, 221–222, 213–219. [Google Scholar] [CrossRef]
- He, S.; Liang, X.-F.; Sun, J.; Li, L.; Yu, Y.; Huang, W.; Qu, C.-M.; Cao, L.; Bai, X.-L.; Tao, Y.-X. Insights into food preference in hybrid F1 of Siniperca chuatsi (♀) × Siniperca scherzeri (♂) mandarin fish through transcriptome analysis. BMC Genom. 2013, 14, 601. [Google Scholar] [CrossRef]
- Parvathy, A.J.; Das, B.C.; Jifiriya, M.J.; Varghese, T.; Pillai, D.; Rejish Kumar, V.J. Ammonia induced toxico-physiological responses in fish and management interventions. Rev. Aquacult. 2023, 15, 452–479. [Google Scholar] [CrossRef]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Huff, L.; Delos, C.; Gallagher, K.; Beaman, J.J. Aquatic Life Ambient Water Quality Criteria for Ammonia—Freshwater; U.S. Environmental Protection Agency: Washington, DC, USA, 2013; pp. 5–6.
- Murthy, C.R.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Suzuki, H.; Yanaka, A.; Shibahara, T.; Matsui, H.; Nakahara, A.; Tanaka, N.; Muto, H.; Momoi, T.; Uchiyama, Y. Ammonia-induced apoptosis is accelerated at higher pH in gastric surface mucous cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G986–G995. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ning, F.; Zhang, J.; Tang, Y.; Teng, X. NF-κB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic lymphocytes. Sci. Total Environ. 2020, 729, 139017. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, J.; Sun, Q.; Xu, Y.; Teng, X. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol. Environ. Saf. 2020, 203, 110974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Du, M.; Fang, J.; Gao, Y.; Mao, Y.; Chen, Q.; Lin, F.; Jiang, Z. Response of Yesso scallop Patinopecten yessoensis to acute temperature challenge: Physiological and biochemical parameters. J. Ocean. Limnol. 2019, 37, 321–329. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.J.; Kim, K.I.; Kim, S.K.; Kim, J.H. Toxic effects of nitrogenous compounds (ammonia, nitrite, and nitrate) on acute toxicity and antioxidant responses of juvenile olive flounder, Paralichthys olivaceus. Environ. Toxicol. Pharmacol. 2019, 67, 73–78. [Google Scholar] [CrossRef]
- Li, C.; Ni, D.; Song, L.; Zhao, J.; Zhang, H.; Li, L. Molecular cloning and characterization of a catalase gene from Zhikong scallop Chlamys farreri. Fish. Shellfish. Immun. 2008, 24, 26–34. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Loumbourdis, N.S. Exposure of the frog Rana ridibunda to copper: Impact on two biomarkers, lipid peroxidation, and glutathione. Bull. Environ. Contam. Toxicol. 2002, 69, 885–891. [Google Scholar] [CrossRef]
- Guan, M.; Zhang, D.; Tang, D. Effects of chronic ammonia nitrogen stress on growth and liver antioxidant and immune indexes of juvenile Sturgeon sturgeon. S. China Fish. Sci. 2020, 16, 36–42. [Google Scholar]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Phys. A 2013, 166, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rama, S.; Manjabhat, S.N. Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 2014, 107, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xu, Z.; Zhang, H.; Mei, J.; Xie, J. The effects of acute exposure to ammonia on oxidative stress, hematological parameters, flesh quality, and gill morphological changes of the large yellow croaker (Larimichthys crocea). Animals 2023, 13, 2534. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Wu, H.; Gao, J.; Wu, J.; Xiong, Z.; Feng, Z.; Xie, M.; Li, S.; Xie, Z. The effect of short-term artificial feed domestication on the expression of oxidative-stress-related genes and antioxidant capacity in the liver and gill tissues of Mandarin fish (Siniperca chuatsi). Genes 2024, 15, 487. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Tang, S.; Liu, X.; Wu, T.; Zhao, J. Study on acute toxicity of ammonia nitrogen to juvenile tilapia “Neogyphus”. Aquat. Sci. 2017, 6, 42–46. [Google Scholar]
- Kim, J.-H.; Kang, J.-C. Changes in hematological parameters, plasma cortisol, and acetylcholinesterase of juvenile rockfish, Sebastes schlegelii supplemented with the dietary ascorbic acid. Aquacult. Rep. 2016, 4, 80–85. [Google Scholar] [CrossRef][Green Version]
- Habte-Tsion, H.-M.; Liu, B.; Ge, X.; Xu, P.; Ren, M.; Zhou, Q.; Pan, L.; Chen, R. Effects of Dietary Protein Level on Growth Performance, Muscle Composition, Blood Composition, and Digestive Enzyme Activity of Wuchang Bream (Megalobrama amblycephala) Fry. Isr. J. Aquacult. Bamid. 2013, 65, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, K.; Wang, G.; Mo, W.; Huang, Y.; Cao, J. Metabolic changes, antioxidant status, immune response and resistance to ammonia stress in juvenile yellow catfish (Pelteobagrus fulvidraco) fed diet supplemented with sodium butyrate. Aquaculture 2021, 536, 736441. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Hoseinifar, S.H.; Van Doan, H. Antioxidant, enzymatic and hematological responses of common carp (Cyprinus carpio) fed with myrcene- or menthol-supplemented diets and exposed to ambient ammonia. Aquaculture 2019, 506, 246–255. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, S.; Zhu, J.; Miao, L.; Ren, M.; Lin, Y.; Ge, X.; Sun, S. Growth performance, physiological response and histology changes of juvenile blunt snout bream, Megalobrama amblycephala exposed to chronic ammonia. Aquaculture 2019, 506, 424–436. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Tarkhani, R. Effect of short-term treatment with potassium permanganate on stress markers and blood biochemistry in goldfish Carassius auratus. Aquac. Res. 2013, 44, 869–875. [Google Scholar] [CrossRef]
- David, M.; Mushigeri, S.B.; Shivakumar, R.; Philip, G.H. Response of Cyprinus carpio (Linn) to sublethal concentration of cypermethrin: Alterations in protein metabolic profiles. Chemosphere 2004, 56, 347–352. [Google Scholar] [CrossRef]
- Asthana, S.; Fatma, F. Effect of ammonia inhalation on serum protein of Albino rat. Natl. Acad. Sci. Lett. 2008, 31, 117–119. [Google Scholar]
- Li, B.; Fan, Q.; Yang, K.; Zhang, L.; Guo, H.; Wang, Q.; Gao, Y.; Zhu, S.; Fang, W. Effects of chronic ammonia nitrogen stress on feeding, growth and blood indexes of Pelteobagrus pelteobagrus. J. Environ. Biol. 2011, 17, 824–828. [Google Scholar]
- Stebbing, A. Tolerance and hormesis—Increased resistance to copper in hydroids linked to hormesis. Mar. Environ. Res. 2002, 54, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.H.; Masroor, F.; Kang, J.C. Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquac. Res. 2005, 36, 898–905. [Google Scholar] [CrossRef]
- Jeney, G.; Nemcsok, J.; Jeney, Z.; Olah, J. Acute effect of sublethal ammonia concentrations on common carp (Cyprinus carpio L.). II. Effect of ammonia on blood plasma transaminases (GOT, GPT), G1DH enzyme activity, and ATP value. Aquaculture 1992, 104, 149–156. [Google Scholar] [CrossRef]
- Han, C.Y.; Zheng, Q.M.; Chen, G.D.; Liu, L.X. Effect of ammonia-nitrogen stress on non-specific immunity of hybrid Tilapia (Oreochromis niloticus × Oreochromis areus). S. China Fish. Sci. 2014, 10, 47–52. [Google Scholar]
- Nawata, C.M.; Hung, C.C.Y.; Hung, C.C.Y.; Tsui, T.K.N.; Wilson, J.M.; Wright, P.A.; Wood, C.M. Ammonia excretion in rainbow trout (Oncorhynchus mykiss): Evidence for Rh glycoprotein and H+-ATPase involvement. Physiol. Genom. 2007, 31, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Randall, D.J.; Donowitz, M.; Vogl, A.W.; Ip, A.K. Immunolocalization of ion-transport proteins to branchial epithelium mitochondria-rich cells in the mudskipper (Periophthalmodon schlosseri). J. Exp. Biol. 2000, 203 Pt 15, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.C.; Tsui, K.N.T.; Wilson, J.M.; Nawata, C.M.; Wood, C.M.; Wright, P.A. Rhesus glycoprotein gene expression in the mangrove killifish Kryptolebias marmoratus exposed to elevated environmental ammonia levels and air. J. Exp. Biol. 2007, 210, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhang, B.; Chng, Y.R.; Ong, J.L.; Chew, S.F.; Wong, W.P.; Lam, S.H.; Nakada, T.; Ip, Y.K. Ammonia exposure affects the mRNA and protein expression levels of certain Rhesus glycoproteins in the gills of climbing perch. J. Exp. Biol. 2017, 220, 2916–2931. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, S.; Gopal, K. Inhibition of Na+-K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic. Biochem. Phys. 2008, 92, 57–60. [Google Scholar] [CrossRef]
- Ip, Y.; Chew, S.; Randall, D.J. Ammonia toxicity, tolerance, and excretion. Fish Physiol. 2001, 20, 109–148. [Google Scholar]
- Altman, B.J.; Stine, Z.E.; Dang, C.V.J.N.R.C. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; da Silva Lima, F.; Sartori, T.; Santos, A.C.A.; Rogero, M.M.; Fock, R.A. Glutamine metabolism and its effects on immune response: Molecular mechanism and gene expression. Nutrire 2016, 41, 14. [Google Scholar] [CrossRef]
- Simpson, N.E.; Tryndyak, V.P.; Pogribna, M.; Beland, F.A.; Pogribny, I.P. Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics 2012, 7, 1413–1420. [Google Scholar] [CrossRef]
- Wong, C.; Qian, Y.; Yu, J. Interplay between epigenetics and metabolism in oncogenesis: Mechanisms and therapeutic approaches. Oncogene 2017, 36, 3359–3374. [Google Scholar] [CrossRef]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Marth, J.D.; Hart, G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress: A survival response of mammalian cells. J. Biol. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef]
- Pallett, L.J.; Dimeloe, S.; Sinclair, L.V.; Byrne, A.J.; Schurich, A. A glutamine ‘tug-of-war’: Targets to manipulate glutamine metabolism for cancer immunotherapy. Immunother. Adv. 2021, 1, ltab010. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.; Walsh, P.J. Biochemical and environmental perspectives on nitrogen metabolism in fishes. Experientia 1992, 48, 583–593. [Google Scholar] [CrossRef]
- Wee, N.L.; Tng, Y.Y.; Cheng, H.T.; Lee, S.M.; Chew, S.F.; Ip, Y. Ammonia toxicity and tolerance in the brain of the African sharptooth catfish, Clarias gariepinus. Aquat. Toxicol. 2007, 82, 204–213. [Google Scholar] [CrossRef]
- Hegazi, M.M.; Attia, Z.I.; Hegazi, M.A.; Hasanein, S.S. Metabolic consequences of chronic sublethal ammonia exposure at cellular and subcellular levels in Nile tilapia brain. Aquaculture 2010, 299, 149–156. [Google Scholar] [CrossRef]
- Wicks, B.J.; Randall, D.J. The effect of sub-lethal ammonia exposure on fed and unfed rainbow trout: The role of glutamine in regulation of ammonia. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2002, 132, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.K.; Tay, A.S.; Lee, K.H.; Chew, S.F. Strategies for surviving high concentrations of environmental ammonia in the swamp eel Monopterus albus. Physiol. Biochem. Zool. 2004, 77, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M. Urea and glutamine synthesis: Environmental influences on nitrogen excretion. Fish Physiol. 2001, 20, 239–277. [Google Scholar]
- Wang, G.; Pan, L.; Ding, Y. Defensive strategies in response to environmental ammonia exposure of the sea cucumber Apostichopus japonicus: Glutamine and urea formation. Aquaculture 2014, 432, 278–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhuo, H.; Lin, L.; Li, J.; Fu, S.; Xue, H.; Wen, H.; Zhou, X.; Guo, C. Differential toxicity responses between hepatopancreas and gills in Litopenaeus vannamei under chronic ammonia-N exposure. Animals 2023, 13, 3799. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Yu, P.; Huang, Y.; Cai, M.; Zhang, M.; Zhao, Y. Cold stress regulates lipid metabolism via AMPK signalling in Cherax quadricarinatus. J. Therm. Biol. 2020, 92, 102693. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saikia, S.J. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]



| Gene | Primer | Primer Sequence (5′-3′) | Tm (°C) |
|---|---|---|---|
| gdh | F | CATTTCCTTCCCGTGTT | 58 |
| R | TCTGTCTGCGGAGTTGGT | ||
| ampd | F | CATTTCCTTCCCGTGTT | 58 |
| R | TCTGTCTGCGGAGTTGGT | ||
| ast | F | TCTGTGCTCAGTCGTTCTC | 58 |
| R | AACTCGCTTCAGGTTGTCT | ||
| alt | F | ATGTCCGAGAATGGAGTGT | 58 |
| R | TCAGGGTAGGAGCAGAGC | ||
| gs | F | TGGATTGATGGAACTGGAGAG | 58 |
| R | CCACTCAGGCAGGTCTTC | ||
| as | F | TGCTGCTACACTGGTGAAG | 58 |
| R | GCGATGATGTCTGGACTGT | ||
| ampk | F | GGGATGCAAACCAAGATG | 58 |
| R | ACAGACCCAGAGCGGAGA | ||
| eef2 | F | TCTGCTGTTATCCCGCCT | 58 |
| R | TCGCCATCACTCCTCCTCT | ||
| lkb1 | F | GACGGGGCACTTAAAATC | 58 |
| R | GTGTTACTCCAGCAGACCAAA | ||
| rpl13a | F | CACCCTATGACAAGAGGAAGC | 59 |
| R | TGTGCCAGACGCCCAAG | ||
| rhag | F | TGATTGGATTAGTGGCTGGCATA | 58 |
| R | GTGGACACCGCAGGTATCTT | ||
| rhbg | F | AAGACGCAGCAACCAACAT | 58 |
| R | CCAAGGCACCGAAGAGGAT | ||
| rhcg | F | ACATCCAGAACTCCACTCTT | 60 |
| R | AGATGACACCACAGCAGAA |
| Ammonia Nitrogen Concentration (mg/L) | Mortality (%) |
|---|---|
| 10.39 ± 0.40 | 0 a |
| 11.84 ± 0.43 | 20 ± 0 b |
| 13.46 ± 0.54 | 60 ± 5.77 c |
| 15.03 ± 0.42 | 100 ± 0 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, R.; He, M.; Su, J.; Liu, L. Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms. Antioxidants 2025, 14, 768. https://doi.org/10.3390/antiox14070768
Li Y, Yang R, He M, Su J, Liu L. Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms. Antioxidants. 2025; 14(7):768. https://doi.org/10.3390/antiox14070768
Chicago/Turabian StyleLi, Yan, Ru Yang, Minghui He, Jianmei Su, and Liwei Liu. 2025. "Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms" Antioxidants 14, no. 7: 768. https://doi.org/10.3390/antiox14070768
APA StyleLi, Y., Yang, R., He, M., Su, J., & Liu, L. (2025). Chronic Ammonia Stress in Chinese Perch (Siniperca chuatsi): Oxidative Response, Nitrogen Metabolism, and Multi-Enzyme-Mediated Molecular Detoxification Defense Mechanisms. Antioxidants, 14(7), 768. https://doi.org/10.3390/antiox14070768






