The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
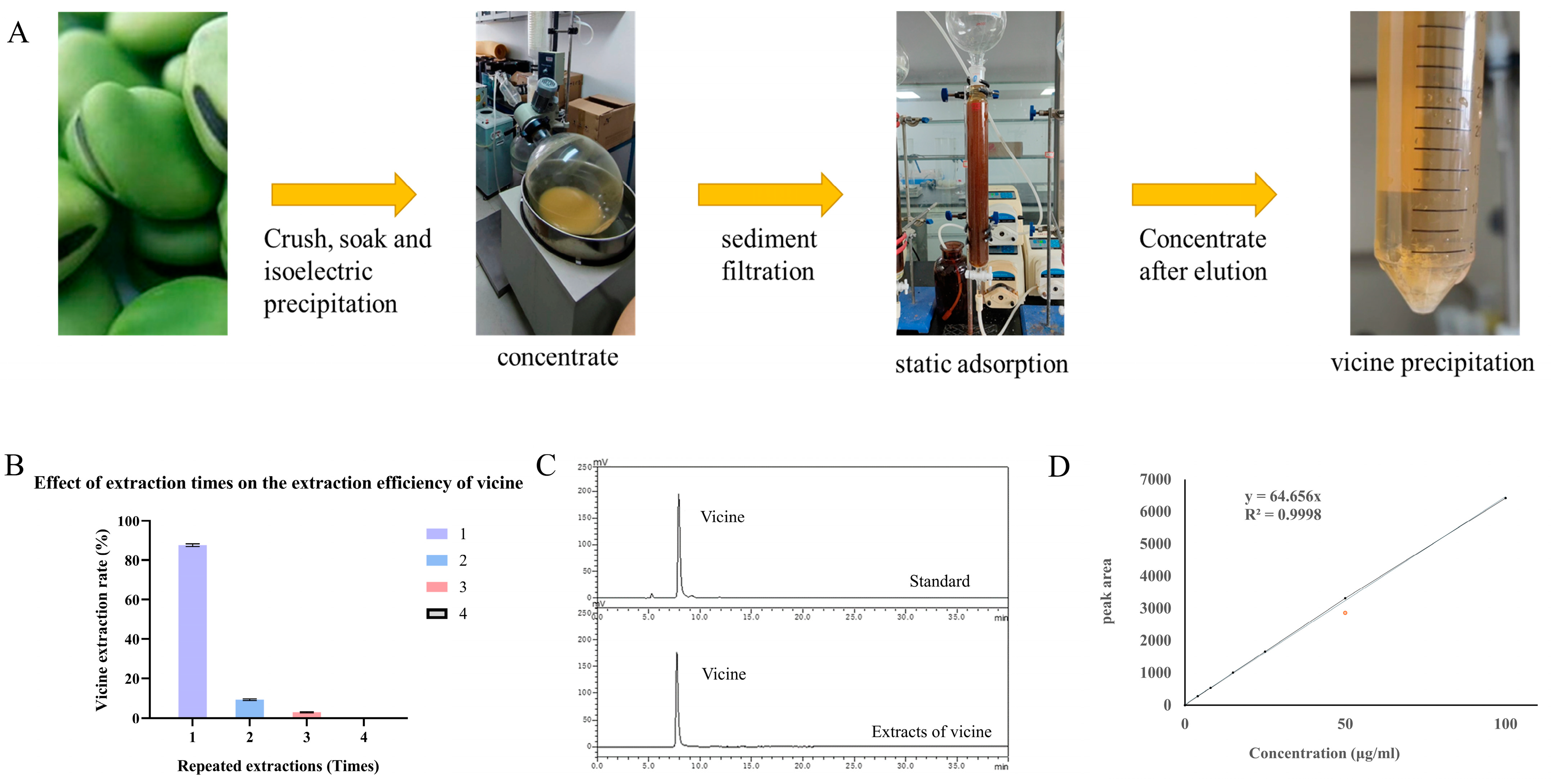
2.2. Extraction and Purification of Vicine
2.3. High Performance Liquid Chromatography (HPLC) Analysis of Vicine
2.4. Preparation of Vicine Feed
2.5. Feeding Trial
2.6. Sampling Procedures
2.7. Measurement of Textural Quality Parameters and Collagen Content
2.8. Histological Analysis
2.9. Tissue Biochemical Analysis
2.10. Quantitative Real-Time PCR
2.11. Intestinal Microbial Sequencing and Analysis
2.12. Statistical Analyses
3. Results
3.1. Extraction, Purification, and Identification of Vicine
3.2. Growth Performance and Muscle Texture, Biochemistry and Microstructure of Grass Carp After Feeding on Vicine
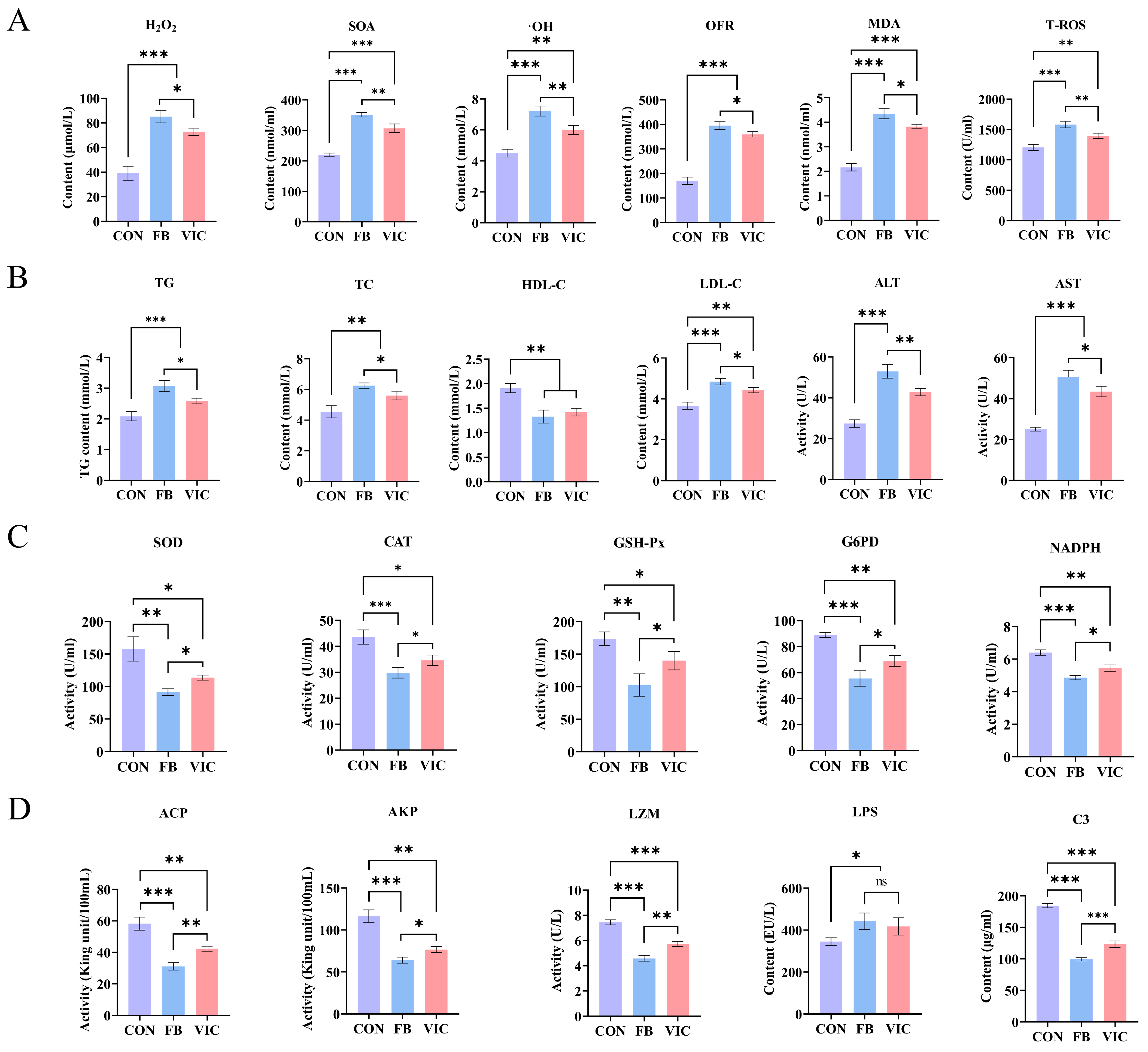
3.3. Effects of Vicine on Blood Biochemistry of Grass Carp
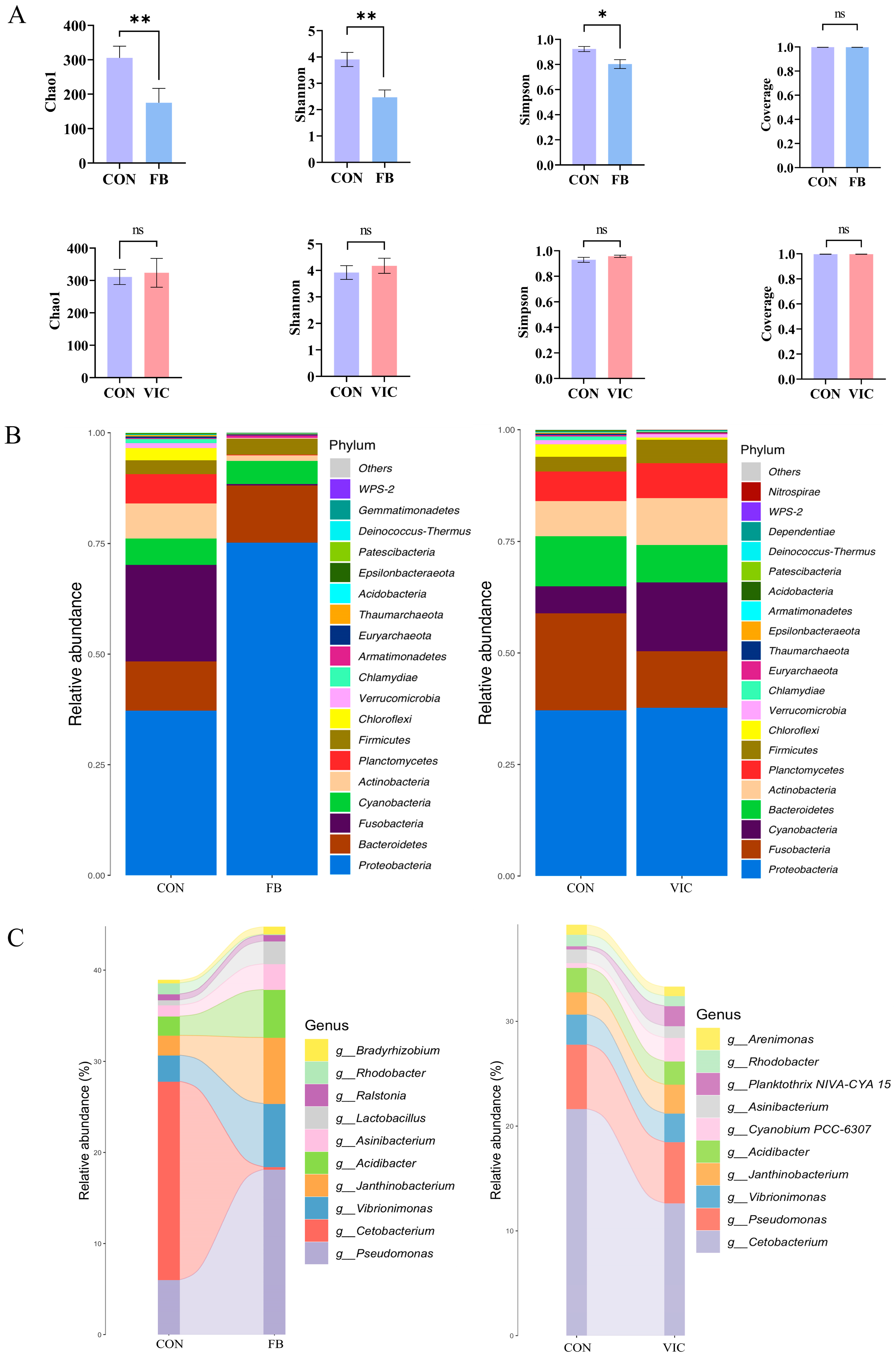
3.4. Effects of Vicine on Biochemical Parameters, Microstructure and Microbiota of the Intestine of Grass Carp
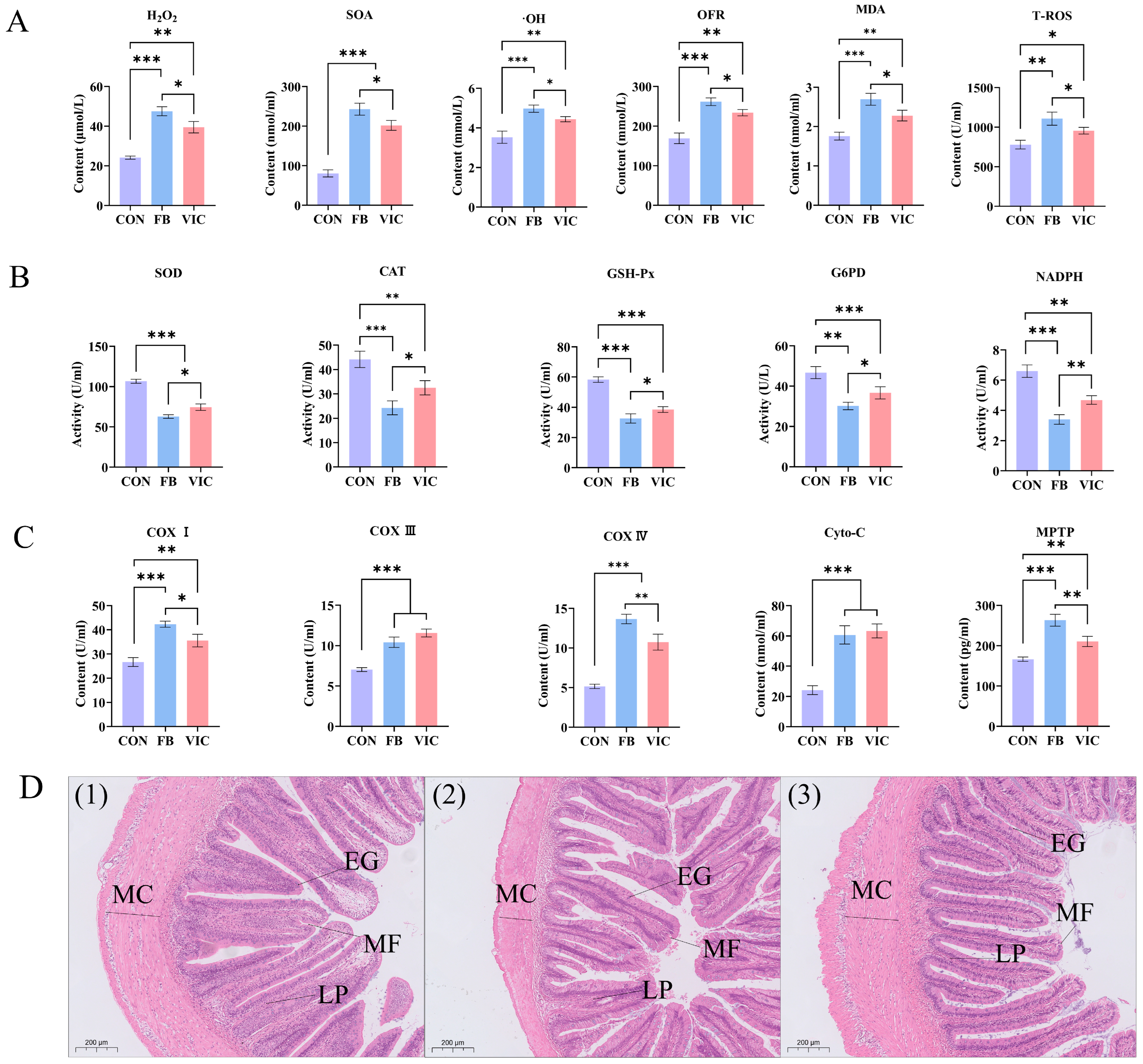
3.5. Effects of Vicine on Biochemistry and Microstructure of Hepatopancreas of Grass Carp
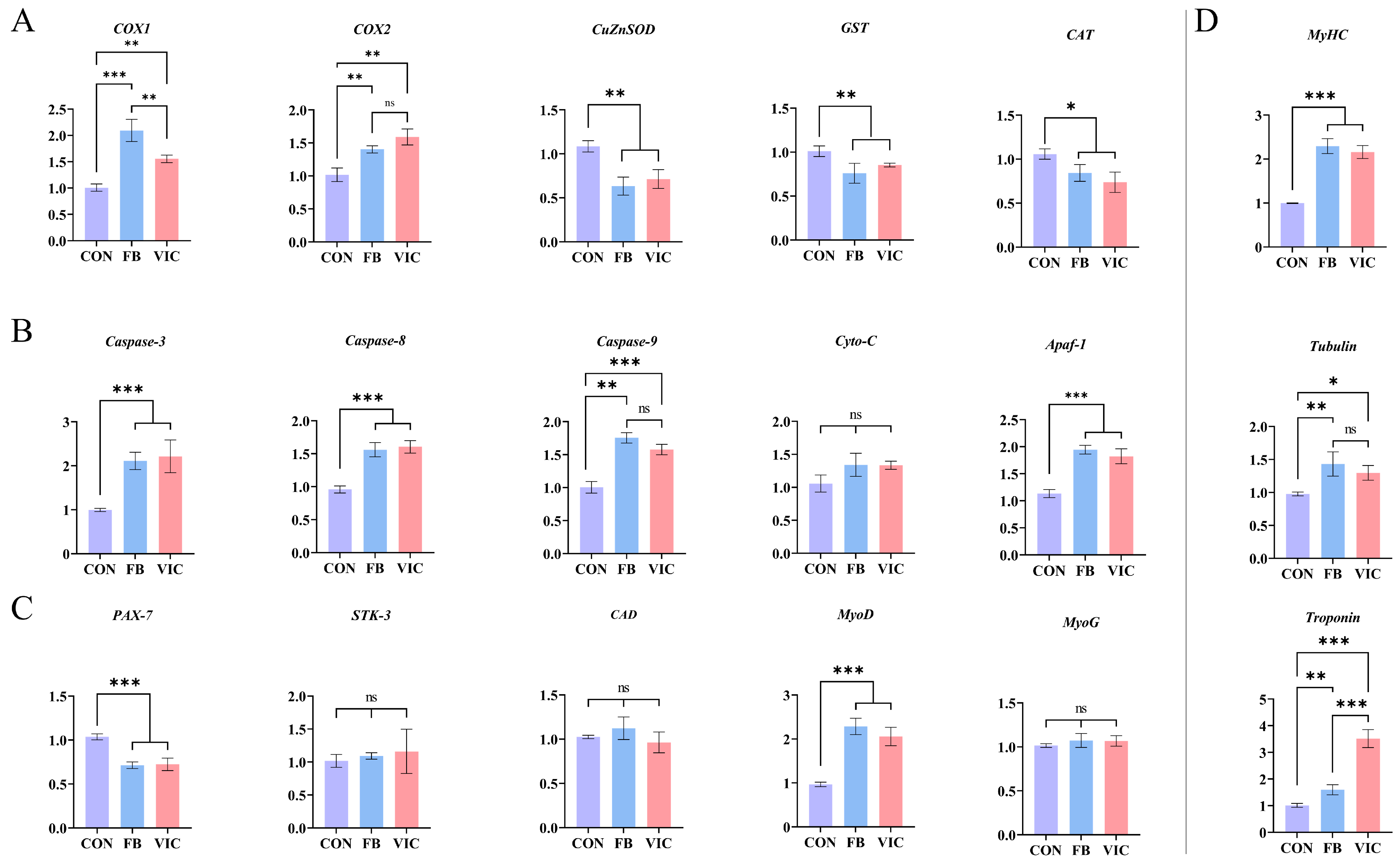
3.6. Quantitative Real-Time PCR of Grass Carp Muscle
4. Discussion
4.1. Extraction Effect of Vicine
4.2. Potential Mode of Action of Vicine in Causing Changes in Muscle Texture
4.3. Effects of Vicine on Intestinal and Hepatopancreatic Health of Grass Carp
4.4. Vicine May Indirectly Affect Muscle Texture Through the Gut–Muscle Axis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.-L.; Kaneko, G.; Wang, X.-J.; Xie, J.; Tian, J.-J.; Zhang, K.; Wang, G.-J.; Yu, D.-G.; Li, Z.-F.; Gong, W.-B. Effects of four faba bean extracts on growth parameters, textural quality, oxidative responses, and gut characteristics in grass carp. Aquaculture 2020, 516, 734620. [Google Scholar] [CrossRef]
- Yu, E.; Fu, B.; Wang, G.; Li, Z.; Ye, D.; Jiang, Y.; Ji, H.; Wang, X.; Yu, D.; Ehsan, H. Proteomic and metabolomic basis for improved textural quality in crisp grass carp (Ctenopharyngodon idellus C. et V) fed with a natural dietary pro-oxidant. Food Chem. 2020, 325, 126906. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, L.; Qi, B.; Wu, Y.; Hu, X.; Lin, W.; Hao, S.; Huang, H. Quality evaluation of crisp grass carp (Ctenopharyngodon idellus C. ET V) based on instrumental texture analysis and cluster analysis. Food Anal. Methods 2015, 8, 2107–2114. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Fu, B.; Xie, J.; Wang, G.; Tian, J.; Xia, Y.; Yu, E. Textural quality, growth parameters and oxidative responses in Nile tilapia (Oreochromis niloticus) fed faba bean water extract diet. PeerJ 2022, 10, e13048. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; He, Z.; Mi, J.; Luo, J.; Jin, M.; Zhou, Q.; Nie, G. Effects of faba bean (Vicia faba L.) on fillet quality of Yellow River carp (Cyprinus carpio) via the oxidative stress response. Food Chem. 2022, 388, 132953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Yu, Y.E.; Xie, X.J.; Wang, W.G.; Yu, Y.D.; Li, L.Z.; Gong, G.W.; Wang, W.H.; Wei, W.N.; Xia, X.Y. Intestinal microflora dynamic change, serum enzyme and growth performance of the grass carp (Ctenopharyngodon idellus) at different stages of feeding broad bean (Vicia faba). J. Agric. Biotechnol. 2015, 23, 151–160. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20163230302 (accessed on 20 October 2016).
- Gan, L.; Li, X.X.; Pan, Q.; Wu, S.L.; Feng, T.; Ye, H. Effects of replacing soybean meal with faba bean meal on growth, feed utilization and antioxidant status of juvenile grass carp, Ctenopharyngodon idella. Aquac. Nutr. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Fu, B.; Yu, E.; Wang, G.; Xie, J.; Li, Z.; Zhang, K.; Gong, W.; Tian, J. Effects of feeding broad bean on fat accumulation and metabolism in grass carp (Ctenopharyngodon idellus) liver. J. Shanghai Ocean Univ. 2020, 29, 45–54. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Zhang, J.-M.; Xie, J.; Wang, G.-J.; Tian, J.-J.; Liu, L.-P.; Luo, M.-Y.; Yu, E.-M. Effects of faba bean water extract and vitamin C/E on muscle texture, nutrition composition and oxidative stress in grass carp (Ctenopharyngodon idella). J. Fish. China 2023, 47, 118–132. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20230428808 (accessed on 6 July 2023).
- Chen, L.; Kaneko, G.; Li, Y.; Xie, J.; Wang, G.; Li, Z.; Tian, J.; Zhang, K.; Gong, W.; Xia, Y. Reactive oxygen species (ROS)-mediated regulation of muscle texture in grass carp fed with dietary oxidants. Aquaculture 2021, 544, 737150. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. Lwt 2021, 138, 110796. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Lu, Q.; Zhang, H.; Lin, L.; Li, Q. Tannic acid reduced the growth performance, antioxidant, and immune functions of the Nile tilapia (Oreochromis niloticus). Aquaculture 2024, 596, 741872. [Google Scholar] [CrossRef]
- Zhong, J.-R.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Phytic acid disrupted intestinal immune status and suppressed growth performance in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019, 92, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity and gut microbiota community on rice field eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Marquardt, R.R.; Kemp, G. Variation in vicine and convicine concentration of faba bean genotypes. Can. J. Plant Sci. 1982, 62, 589–592. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Gautam, M.; Lampi, A.-M.; Ollilainen, V.; Stoddard, F.; Sontag-Strohm, T.; Salovaara, H.; Piironen, V. Determination of vicine and convicine from faba bean with an optimized high-performance liquid chromatographic method. Food Res. Int. 2015, 76, 168–177. [Google Scholar] [CrossRef]
- Arbid, M.S.S.; Marquardt, R.R. Favism-like effects of divicine and isouramil in the rat: Acute and chronic effects on animal health, mortalities, blood parameters and ability to exchange respiratory gases. J. Sci. Food Agric. 1988, 43, 75–90. [Google Scholar] [CrossRef]
- Getachew, F.; Vandenberg, A.; Smits, J. A practical toxicity bioassay for vicine and convicine levels in faba bean (Vicia faba). J. Sci. Food Agric. 2018, 98, 5105–5111. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef]
- Jamalian, J.; Aylward, F.; Hudson, B.J.F. Favism-inducing toxins in broad beans (Vicia faba): Estimation of the vicine contents of broad bean and other legume samples. Qual. Plant. 1977, 27, 207–211. [Google Scholar] [CrossRef]
- Hegazy, M.I.; Marquardt, R.R. Metabolism of vicine and convicine in rat tissues: Absorption and excretion patterns and sites of hydrolysis. J. Sci. Food Agric. 1984, 35, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.R.; Muduuli, D.S.; Frohlich, A.A. Purification and some properties of vicine and convicine isolated from faba bean (Vicia faba L.) protein concentrate. J. Agric. Food Chem. 1983, 31, 839–844. [Google Scholar] [CrossRef]
- Purves, R.W.; Khazaei, H.; Vandenberg, A. Quantification of vicine and convicine in faba bean seeds using hydrophilic interaction liquid chromatography. Food Chem. 2018, 240, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; El-Din, M.G.S.; Selim, M.A.-F.; Owis, A.I.; Abouzid, S.F. Mass spectrometry-based metabolites profiling of nutrients and anti-nutrients in major legume sprouts. Food Biosci. 2021, 39, 100800. [Google Scholar] [CrossRef]
- Wang, W.; Sa, Y.; Zhang, W.; Wang, X.; Wang, Y.; Yuan, H.; Li, L.; Ding, S.; Wilson, G.; Ma, X. Purification and separation of caffeoyl spermidine derivatives from goji leaf tea with ion exchange resin and the mechanisms involved. Food Bioprod. Process. 2024, 147, 53–69. [Google Scholar] [CrossRef]
- Lin, W.L.; Yang, X.Q.; Li, L.H.; Hao, S.X.; Wang, J.X.; Huang, H.; Wei, Y.; Wu, Y.Y. Effect of ultrastructure on changes of textural characteristics between crisp grass carp (Ctenopharyngodon idellus C. Et V) and grass carp (Ctenopharyngodon idellus) inducing heating treatment. J. Food Sci. 2016, 81, E404–E411. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Rahman, N.I.A.; Lam, C.L.; Sulaiman, N.; Abdullah, N.A.H.; Nordin, F.; Ariffin, S.H.Z.; Yazid, M.D. PAX7, a key for myogenesis modulation in muscular dystrophies through multiple signaling pathways: A systematic review. Int. J. Mol. Sci. 2023, 24, 13051. [Google Scholar] [CrossRef]
- Bentzinger, C.; von Maltzahn, J.; Rudnicki, M.A. Extrinsic regulation of satellite cell specification. Stem Cell Res. Ther. 2010, 1, 27. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular mechanisms and genetics of oxidative stress in Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Dick, S.A.; Chang, N.C.; Dumont, N.A.; Bell, R.A.V.; Putinski, C.; Kawabe, Y.; Litchfield, D.W.; Rudnicki, M.A.; Megeney, L.A. Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells. Proc. Natl. Acad. Sci. USA 2015, 112, E5246–E5252. [Google Scholar] [CrossRef]
- Li, Z.; Yu, E.; Wang, G.; Yu, D.; Zhang, K.; Gong, W.; Xie, J. Broad bean (Vicia faba L.) induces intestinal inflammation in grass carp (Ctenopharyngodon idellus C. et V) by increasing relative abundances of intestinal gram-negative and flagellated bacteria. Front. Microbiol. 2018, 9, 1913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, B. The gut-liver axis in health and disease: The role of gut microbiota-derived signals in liver injury and regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-J.; Ji, H.; Wang, Y.-F.; Xie, J.; Wang, G.-J.; Li, Z.-F.; Yu, E.-M.; Yu, D.-G.; Zhang, K.; Gong, W.-B. Lipid accumulation in grass carp (Ctenopharyngodon idellus) fed faba beans (Vicia faba L.). Fish Physiol. Biochem. 2019, 45, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Feng, Q.; Tang, J.; Shen, Q.; Zhou, S. An update on nutritional profile, phytochemical compounds, health benefits, and potential applications in the food industry of pulses seed coats: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1960–1982. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- Dehkordi, M.H.; Tashakor, A.; O’Connell, E.; Fearnhead, H.O. Apoptosome-dependent myotube formation involves activation of caspase-3 in differentiating myoblasts. Cell Death Dis. 2020, 11, 308. [Google Scholar] [CrossRef]
- Simões, I.C.M.; Amorim, R.; Teixeira, J.; Karkucinska-Wieckowska, A.; Carvalho, A.; Pereira, S.P.; Simões, R.F.; Szymanska, S.; Dąbrowski, M.; Janikiewicz, J. The alterations of mitochondrial function during NAFLD progression—An independent effect of mitochondrial ROS production. Int. J. Mol. Sci. 2021, 22, 6848. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Zhang, H.; Zhao, Q.; Si, W.; Qin, Y.; Zhang, J. Dietary Epimedium extract supplementation improves intestinal functions and alters gut microbiota in broilers. J. Anim. Sci. Biotechnol. 2023, 14, 14. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, Z.; Lin, H.; Kong, L.; Ma, J.; Long, Z.; Qin, H.; Liu, L.; Lin, Y.; Li, Z. Effects of mulberry leaf extract on the intestinal health of spotted sea bass (Lateolabrax maculatus). Front. Mar. Sci. 2023, 10, 1185795. [Google Scholar] [CrossRef]
- Benie, C.K.D.; Dadié, A.; Guessennd, N.; N’gbesso-Kouadio, N.A.; Kouame, N.z.D.; N’golo, D.C.; Aka, S.; Dako, E.; Dje, K.M.; Dosso, M. Characterization of virulence potential of Pseudomonas aeruginosa isolated from bovine meat, fresh fish, and smoked fish. Eur. J. Microbiol. Immunol. 2017, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, W.; Tan, C.; Li, M.; Li, D.; Zhang, C.; Feng, L.; Chen, Q.; Jiang, J.; Li, Y. Heat stress weakens the skin barrier function in sturgeon by decreasing mucus secretion and disrupting the mucosal microbiota. Front. Microbiol. 2022, 13, 860079. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Silva, C.; Schierwagen, R.; Pohlmann, A.; Magdaleno, F.; Uschner, F.E.; Ryan, P.; Vehreschild, M.J.G.T.; Claria, J.; Latz, E.; Lelouvier, B. Compartmentalization of immune response and microbial translocation in decompensated cirrhosis. Front. Immunol. 2019, 10, 69. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Xv, K.; Zhang, S.; Pang, A.; Wang, T.; Dong, S.; Xv, Z.; Zhang, X.; Liang, J.; Fang, Y.; Tan, B. White feces syndrome is closely related with hypoimmunity and dysbiosis in Litopenaeus vannamei. Aquac. Rep. 2024, 38, 102329. [Google Scholar] [CrossRef]
- Sogge, H.; Rohrlack, T.; Rounge, T.B.; Sønstebø, J.H.; Tooming-Klunderud, A.; Kristensen, T.; Jakobsen, K.S. Gene flow, recombination, and selection in cyanobacteria: Population structure of geographically related Planktothrix freshwater strains. Appl. Environ. Microbiol. 2013, 79, 508–515. [Google Scholar] [CrossRef]
- Li, G.; Jin, B.; Fan, Z. Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxidative Med. Cell. Longev. 2022, 2022, 2151191. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]


| Ingredients | Control | Faba Bean | Vicine |
|---|---|---|---|
| Fish meal/% | 3.00 | 0.30 | 3.00 |
| Chicken meal/% | 3.00 | 0.30 | 3.00 |
| Soybean meal/% | 25.00 | 2.50 | 25.00 |
| Rapeseed meal/% | 22.00 | 2.20 | 22.00 |
| Rice bran/% | 3.00 | 0.30 | 3.00 |
| Wheat flour/% | 36.00 | 3.60 | 36.00 |
| Soybean oil/% | 3.00 | 0.30 | 3.00 |
| Ca(H2PO4)2/% | 2.00 | 0.20 | 2.00 |
| Bentonite/% | 2.00 | 0.20 | 1.40 |
| Premix */% | 1.00 | 0.10 | 1.00 |
| Faba bean/% | 0.00 | 90.00 | 0.00 |
| Vicine/% | 0.00 | 0.00 | 0.60 |
| Total/% | 100.00 | 100.00 | 100.00 |
| Crude protein/% | 29.31 | 30.07 | 29.42 |
| Crude fat/% | 4.92 | 4.77 | 4.89 |
| Calcium/% | 0.67 | 0.76 | 0.67 |
| Moisture/% | 10.90 | 11.50 | 11.02 |
| Crude fiber/g/kg | 28.00 | 30.00 | 28.00 |
| Items | Control | Faba Bean | Vicine |
|---|---|---|---|
| TBW (g) | 525.90 ± 37.10 a | 334.44 ± 12.36 c | 467.74 ± 9.75 b |
| WGR (%) | 192.17 ± 16.72 a | 85.80 ± 6.86 c | 159.85 ± 5.63 b |
| CF (g/cm3) | 2.21 ± 0.22 a | 1.52 ± 0.03 b | 2.22 ± 0.15 a |
| VSI (%) | 5.93 ± 0.61 b | 8.19 ± 0.88 a | 7.71 ± 0.58 a |
| HSI (%) | 1.73 ± 0.09 b | 1.94 ± 0.22 a | 1.98 ± 0.10 a |
| AFI (%) | 3.27 ± 0.15 c | 4.44 ± 0.38 b | 5.15 ± 0.19 a |
| FCR (%) | 2.90 ± 0.25 c | 4.67 ± 0.27 a | 3.47 ± 0.12 b |
| SR (%) | 100 a | 88.00 ± 2.66 a | 100 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Luo, M.; Fu, B.; Kaneko, G.; Tian, J.; Xie, J.; Hou, J.; Yu, E. The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp. Antioxidants 2025, 14, 813. https://doi.org/10.3390/antiox14070813
Zheng X, Luo M, Fu B, Kaneko G, Tian J, Xie J, Hou J, Yu E. The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp. Antioxidants. 2025; 14(7):813. https://doi.org/10.3390/antiox14070813
Chicago/Turabian StyleZheng, Xinyu, Minyi Luo, Bing Fu, Gen Kaneko, Jingjing Tian, Jun Xie, Jilun Hou, and Ermeng Yu. 2025. "The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp" Antioxidants 14, no. 7: 813. https://doi.org/10.3390/antiox14070813
APA StyleZheng, X., Luo, M., Fu, B., Kaneko, G., Tian, J., Xie, J., Hou, J., & Yu, E. (2025). The Naturally Bioactive Vicine Extracted from Faba Beans Is Responsible for the Transformation of Grass Carp (Ctenopharyngodon idella) into Crisp Grass Carp. Antioxidants, 14(7), 813. https://doi.org/10.3390/antiox14070813








