In Vivo Evidence of Melatonin’s Protective Role in Alkylating-Agent-Induced Pulmonary Toxicity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- -
- Focused on in vivo experimental models;
- -
- Involved the co-administration of melatonin and an alkylating agent;
- -
- Specifically evaluated pulmonary toxicity or lung-related outcomes.
2.3. Study Selection
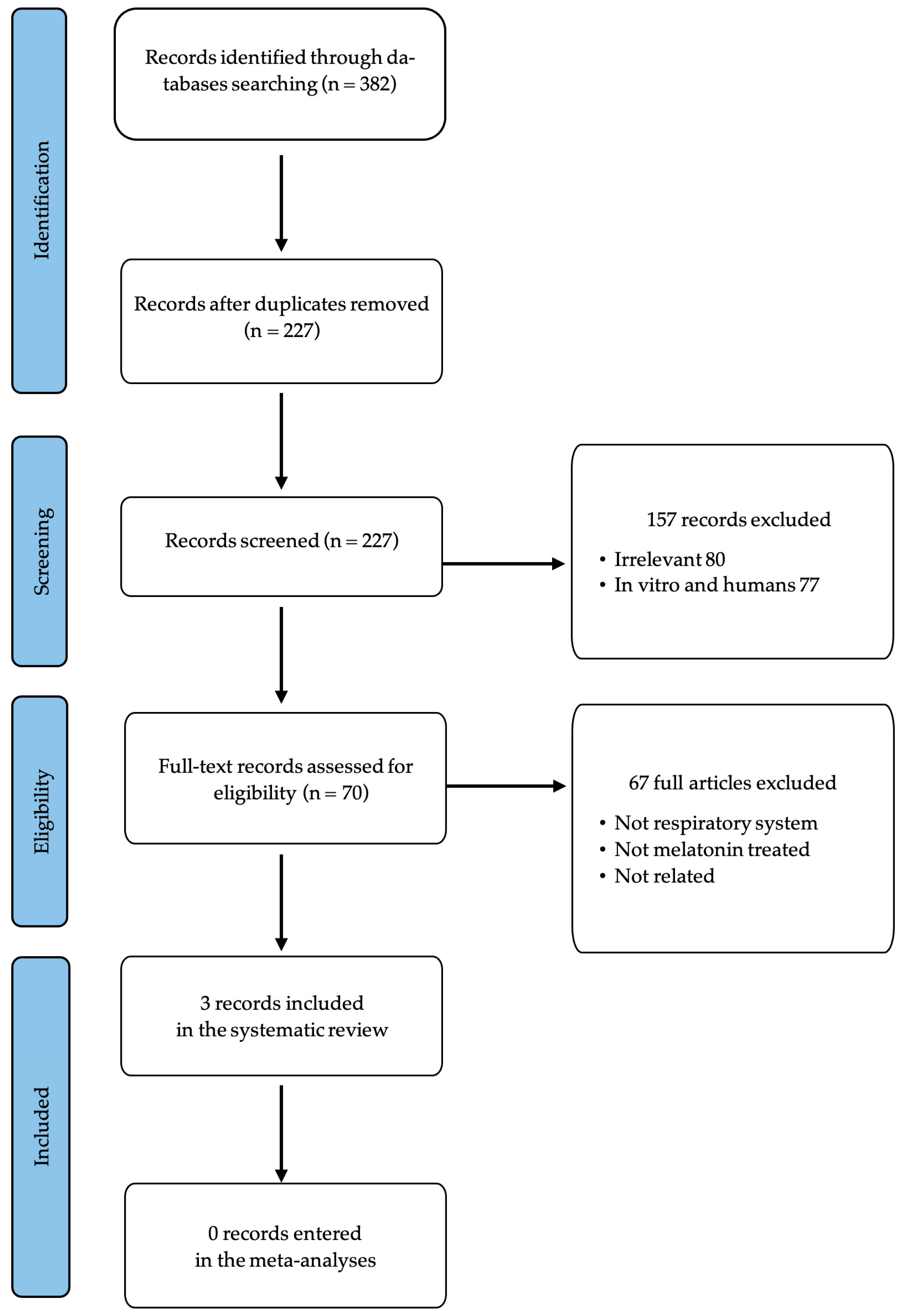
3. Results
3.1. Selection and Identification of Relevant Studies
3.2. Characteristics of Studies
3.3. Comparative Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic Potential of Nitrogen Mustard Based Hybrid Molecules. Front. Pharmacol. 2018, 9, 1453. [Google Scholar] [CrossRef]
- Owens, M.; Thyagarajan, A.; Travers, J.B.; Sahu, R.P. Mechanistic Insights and Pharmacological Approaches for Nitrogen and Sulfur Mustards and Their Implications as Therapeutic Agents. J. Appl. Toxicol. 2025. [Google Scholar] [CrossRef]
- Highley, M.S.; Landuyt, B.; Prenen, H.; Harper, P.G.; De Bruijn, E.A. The Nitrogen Mustards. Pharmacol. Rev. 2022, 74, 552–599. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Long-term Respiratory Effects of Mustard Vesicants. Toxicol. Lett. 2020, 319, 168–174. [Google Scholar] [CrossRef]
- Malaviya, R.; Sunil, V.R.; Venosa, A.; Vayas, K.N.; Heck, D.E.; Laskin, J.D.; Laskin, D.L. Inflammatory mechanisms of pulmonary injury induced by mustards. Toxicol. Lett. 2016, 244, 2–7. [Google Scholar] [CrossRef]
- Bellomo, A.; Herbert, J.; Kudlak, M.J.; Laskin, J.D.; Gow, A.J.; Laskin, D.L. Identification of early events in nitrogen mustard pulmonary toxicity that are independent of infiltrating inflammatory cells using precision cut lung slices. Toxicol. Appl. Pharmacol. 2024, 486, 116941. [Google Scholar] [CrossRef] [PubMed]
- Sunil, V.R.; Vayas, K.N.; Abramova, E.V.; Rancourt, R.; Cervelli, J.A.; Malaviya, R.; Goedken, M.; Venosa, A.; Gow, A.J.; Laskin, J.D.; et al. Lung injury, oxidative stress and fibrosis in mice following exposure to nitrogen mustard. Toxicol. Appl. Pharmacol. 2020, 387, 114798. [Google Scholar] [CrossRef]
- Metzger, M.L.; Billett, A.; Link, M.P. The impact of drug shortages on children with cancer—The example of mechlorethamine. N. Engl. J. Med. 2012, 367, 2461–2463. [Google Scholar] [CrossRef]
- Bai, H.; Padron, A.S.; Deng, Y.; Liao, Y.J.; Murray, C.J.; Ontiveros, C.; Kari, S.J.; Kancharla, A.; Kornepati, A.V.R.; Garcia, M.; et al. Pharmacological tumor PDL1 depletion with chlorambucil treats ovarian cancer and melanoma: Improves antitumor immunity and renders anti-PDL1-resistant tumors anti-PDL1-sensitive through NK cell effects. J. Immunother. Cancer 2023, 11, e004871. [Google Scholar] [CrossRef]
- Dhillon, S. Melphalan Flufenamide (Melflufen): First Approval. Drugs 2021, 81, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Gil-Martin, E.; De Los Rios, C.; Egea, J.; Lopez-Munoz, F.; Pita, R.; Juberias, A.; Torrado, J.J.; Serrano, D.R.; Reiter, R.J.; et al. Melatonin as Modulator for Sulfur and Nitrogen Mustard-Induced Inflammation, Oxidative Stress and DNA Damage: Molecular Therapeutics. Antioxidants 2023, 12, 397. [Google Scholar] [CrossRef]
- Romero, A.; Ramos, E.; Lopez-Munoz, F.; De Los Rios, C.; Egea, J.; Gil-Martin, E.; Pita, R.; Torrado, J.J.; Serrano, D.R.; Juberias, A. Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option? Diseases 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Marco-Contelles, J.; Ramos, E.; Del Pino, J.; Romero, A. Toxicity induced by chemical warfare agents: Insights on the protective role of melatonin. Chem. Biol. Interact. 2013, 206, 134–142. [Google Scholar] [CrossRef]
- Ucar, M.; Korkmaz, A.; Reiter, R.J.; Yaren, H.; Oter, S.; Kurt, B.; Topal, T. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol. Lett. 2007, 173, 124–131. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Ahmadi, A.; Jafarinejhad, M.; Hasani-Nourian, Y. Protective Effects of Melatonin against Cyclophosphamide-induced Oxidative Lung Toxicity in Mice. Drug Res. 2015, 65, 281–286. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Van den Akker, O.R.; Peters, G.Y.; Bakker, C.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Generalized Systematic Review Registration Form. MetaArXiv 2023. preprint. Available online: https://osf.io/z9kdc (accessed on 10 March 2025).
- Macit, E.; Yaren, H.; Aydin, I.; Kunak, Z.I.; Yaman, H.; Onguru, O.; Uysal, B.; Korkmaz, A.; Turel, S.; Kenar, L. The protective effect of melatonin and S-methylisothiourea treatments in nitrogen mustard induced lung toxicity in rats. Environ. Toxicol. Pharmacol. 2013, 36, 1283–1290. [Google Scholar] [CrossRef]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Segal, G.M.; Duckert, L.G. Reversible mechlorethamine-associated hearing loss in a patient with Hodgkin’s disease. Cancer 1986, 57, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef]
- Feng, J.; Ma, W.W.; Li, H.X.; Pei, X.Y.; Deng, S.L.; Jia, H.; Ma, W.Z. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front Endocrinol. 2022, 13, 895095. [Google Scholar] [CrossRef]
- Goudarzi, M.; Khodayar, M.J.; Hosseini Tabatabaei, S.M.T.; Ghaznavi, H.; Fatemi, I.; Mehrzadi, S. Pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam. Clin. Pharmacol. 2017, 31, 625–635. [Google Scholar] [CrossRef]
- Pimenta, G.F.; Awata, W.M.C.; Orlandin, G.G.; Silva-Neto, J.A.; Assis, V.O.; da Costa, R.M.; Bruder-Nascimento, T.; Tostes, R.C.; Tirapelli, C.R. Melatonin prevents overproduction of reactive oxygen species and vascular dysfunction induced by cyclophosphamide. Life Sci. 2024, 338, 122361. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bao, X.; Kong, H.; Yang, J.; Li, Y.; Sun, Z. Melatonin Protects Against Cyclophosphamide-induced Premature Ovarian Failure in Rats. Hum. Exp. Toxicol. 2022, 41, 9603271221127430. [Google Scholar] [CrossRef] [PubMed]
- Chabra, A.; Shokrzadeh, M.; Naghshvar, F.; Salehi, F.; Ahmadi, A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum. Exp. Toxicol. 2014, 33, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, L.; Liu, D.; Zhang, X.; Di, S.; Li, W.; Zhang, J.; Reiter, R.J.; Han, J.; Li, X.; et al. Utilizing Melatonin to Alleviate Side Effects of Chemotherapy: A Potentially Good Partner for Treating Cancer with Ageing. Oxidative Med. Cell Longev. 2020, 2020, 6841581. [Google Scholar] [CrossRef]
- Egea, J.; Lopez-Munoz, F.; Fernandez-Capetillo, O.; Reiter, R.J.; Romero, A. Alkylating Agent-Induced Toxicity and Melatonin-Based Therapies. Front. Pharmacol. 2022, 13, 873197. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Ramos, E.; Egea, J.; Lopez-Munoz, F.; Gil-Martin, E.; Romero, A. Therapeutic Potential of Melatonin Counteracting Chemotherapy-Induced Toxicity in Breast Cancer Patients: A Systematic Review. Pharmaceutics 2023, 15, 1616. [Google Scholar] [CrossRef]
- Businaro, R.; Maggi, E.; Armeli, F.; Murray, A.; Laskin, D.L. Nutraceuticals as potential therapeutics for vesicant-induced pulmonary fibrosis. Ann. N. Y. Acad. Sci. 2020, 1480, 5–13. [Google Scholar] [CrossRef]
- Cruz-Hernandez, A.; Mendoza, R.P.; Nguyen, K.; Harder, A.; Evans, C.M.; Bauer, A.K.; Tewari-Singh, N.; Brown, J.M. Mast Cells Promote Nitrogen Mustard-Mediated Toxicity in the Lung Associated with Proinflammatory Cytokine and Bioactive Lipid Mediator Production. Toxicol. Sci. 2021, 184, 127–141. [Google Scholar] [CrossRef]
- Malaviya, R.; Venosa, A.; Hall, L.; Gow, A.J.; Sinko, P.J.; Laskin, J.D.; Laskin, D.L. Attenuation of acute nitrogen mustard-induced lung injury, inflammation and fibrogenesis by a nitric oxide synthase inhibitor. Toxicol. Appl. Pharmacol. 2012, 265, 279–291. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.J.; Lopes, R.H.; Lamy-Freund, M.T. Permeability of pure lipid bilayers to melatonin. J. Pineal Res. 1995, 19, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, J. Effects of melatonin on protecting against lung injury. Exp. Ther. Med. 2021, 21, 228. [Google Scholar] [CrossRef]
- Atasever, A.; Tekin, S.; Bolat, I.; Bolat, M.; Dag, Y.; Cina, R.B.; Sengul, E.; Yildirim, S.; Warda, M.; Celebi, F. The effects of melatonin on oxidative stress, inflammation, apoptosis and Nrf2/HO-1 in acrylamide-induced lung injury in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Steiniger, B.S. Human spleen microanatomy: Why mice do not suffice. Immunology 2015, 145, 334–346. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Menczel Schrire, Z.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2022, 72, e12782. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Walkin, L.; Herrick, S.E.; Summers, A.; Brenchley, P.E.; Hoff, C.M.; Korstanje, R.; Margetts, P.J. The role of mouse strain differences in the susceptibility to fibrosis: A systematic review. Fibrogenesis Tissue Repair. 2013, 6, 18. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kumar, A.; Saha, N.; Chaudhury, N.K. PK-PD based optimal dose and time for orally administered supra-pharmacological dose of melatonin to prevent radiation induced mortality in mice. Life Sci. 2019, 219, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Frouz Asadi, M.; Ziaei, A.; Vatankhah, M.; Safa, O.; Kamali, M.; Fathalipour, M.; Mahmoodi, M.; Hassanipour, S. Efficacy and safety of oral melatonin in patients with severe COVID-19: A randomized controlled trial. Inflammopharmacology 2023, 31, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Pandi-Perumal, S.R.; Pupko, H.; Kennedy, J.L.; Cardinali, D.P. Melatonin as an Add-On Treatment of COVID-19 Infection: Current Status. Diseases 2021, 9, 64. [Google Scholar] [CrossRef]
- Galley, H.F.; Allen, L.; Colin, P.J.; Galt, S.P.; Webster, N.R. Dose assessment of melatonin in sepsis (DAMSEL2) study: Pharmacokinetics of two doses of oral melatonin in patients with sepsis. J. Pineal Res. 2022, 73, e12830. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhou, X.; Lu, X.; Liu, X.; Li, G.; Long, J. Melatonin Attenuates Sepsis-Induced Acute Lung Injury Through Improvement of Epithelial Sodium Channel-Mediated Alveolar Fluid Clearance via Activation of SIRT1/SGK1/Nedd4-2 Signaling Pathway. Front. Pharmacol. 2020, 11, 590652. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Su, X.; Li, Y.; Wang, Y.; Fang, R.; Guo, Y.; Jin, T.; Shan, H.; Zhao, X.; et al. Melatonin prevents lung injury by regulating apelin 13 to improve mitochondrial dysfunction. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, W.; Wu, Y.; Xu, W.; Hu, S.; Zhang, Y.; Xu, H.; Deng, H.; Chen, Y.; Wu, L.; et al. Melatonin Alleviates Acute Respiratory Distress Syndrome by Inhibiting Alveolar Macrophage NLRP3 Inflammasomes Through the ROS/HIF-1alpha/GLUT1 Pathway. Lab. Investig. 2023, 103, 100266. [Google Scholar] [CrossRef]
| Reference | Animal Model | Sample Size | Melatonin Dosage (i.p.) | Protocol | AA | Duration | Outcome |
|---|---|---|---|---|---|---|---|
| [16] | Wistar rats | 40 | 20 or 40 mg/kg every 12 h for 3 days | Co- and post-administration | 0.5 mg/kg HN2 | 5 days | Reduction in lung MDA levels. Promotion of antioxidant enzyme levels. Prevention of GPx decrease. |
| [25] | Sprague Dawley rats | 27 | 100 mg/kg every 12 h | Co- and post-administration | 3.5 mg/kg HN2 | 3 days | Reduction in proinflammatory cytokines (TNF-α, IL-1β, and NOX). |
| [17] | NMRI mice | 30 | 2.5, 5, 10, or 20 mg/kg every 24 h | Pre-treatment | 200 mg/kg CP | 7 days | Reduction in an elevated lipid peroxidation level. Inhibition of GSH depletion. Promotion of antioxidant enzyme activities (SOD, CAT). |
| Animal Model | Reference | Melatonin Daily Dose | Administration Period | Daily HED for a 60 kg Adult |
|---|---|---|---|---|
| Mouse | [17] | 2.5 mg/kg every 24 h | 7 days | 12 mg |
| 5 mg/kg every 24 h | 7 days | 24 mg | ||
| 10 mg/kg every 24 h | 7 days | 49 mg | ||
| 20 mg/kg every 24 h | 7 days | 97 mg | ||
| Rat | [16] | 20 mg/kg every 12 h for 3 days | 5 days | 195 mg |
| [16] | 40 mg/kg every 12 h for 3 days | 5 days | 389 mg | |
| [25] | 100 mg/kg every 12 h | 3 days | 973 mg |
| Reference | [16] | [25] | [17] |
| Protocol | HN2, 0.5 mg/kg/MLT, 20 or 40 mg/kg every 12 h for 3 days | HN2, 3.5 mg/kg/MLT, 100 mg/kg every 12 h | CP, 200 mg/kg/MLT, 2.5, 5, 10, or 20 mg/kg every 24 h |
| Histopathological evaluation after melatonin administration | Reduction in edema Reduction in alveolar hemorrhage Reduction in inflammatory cell infiltration Improvement of airway pathology | Reduction in alveolar epithelial injury Reduction in inflammation Reduction in interalveolar septal thickening | Reduction in alveolar epithelial injury Reduction in inflammation Reduction in interalveolar septal thickening |
| Alkylating Agent | Common Toxicities | Non-Pulmonary Toxicities | Pulmonary Toxicities |
|---|---|---|---|
| HN2 |
|
|
|
| CP |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sola, E.; Morales-García, J.A.; López-Muñoz, F.; Ramos, E.; Romero, A. In Vivo Evidence of Melatonin’s Protective Role in Alkylating-Agent-Induced Pulmonary Toxicity: A Systematic Review. Antioxidants 2025, 14, 712. https://doi.org/10.3390/antiox14060712
Sola E, Morales-García JA, López-Muñoz F, Ramos E, Romero A. In Vivo Evidence of Melatonin’s Protective Role in Alkylating-Agent-Induced Pulmonary Toxicity: A Systematic Review. Antioxidants. 2025; 14(6):712. https://doi.org/10.3390/antiox14060712
Chicago/Turabian StyleSola, Emma, Jose A. Morales-García, Francisco López-Muñoz, Eva Ramos, and Alejandro Romero. 2025. "In Vivo Evidence of Melatonin’s Protective Role in Alkylating-Agent-Induced Pulmonary Toxicity: A Systematic Review" Antioxidants 14, no. 6: 712. https://doi.org/10.3390/antiox14060712
APA StyleSola, E., Morales-García, J. A., López-Muñoz, F., Ramos, E., & Romero, A. (2025). In Vivo Evidence of Melatonin’s Protective Role in Alkylating-Agent-Induced Pulmonary Toxicity: A Systematic Review. Antioxidants, 14(6), 712. https://doi.org/10.3390/antiox14060712








