Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
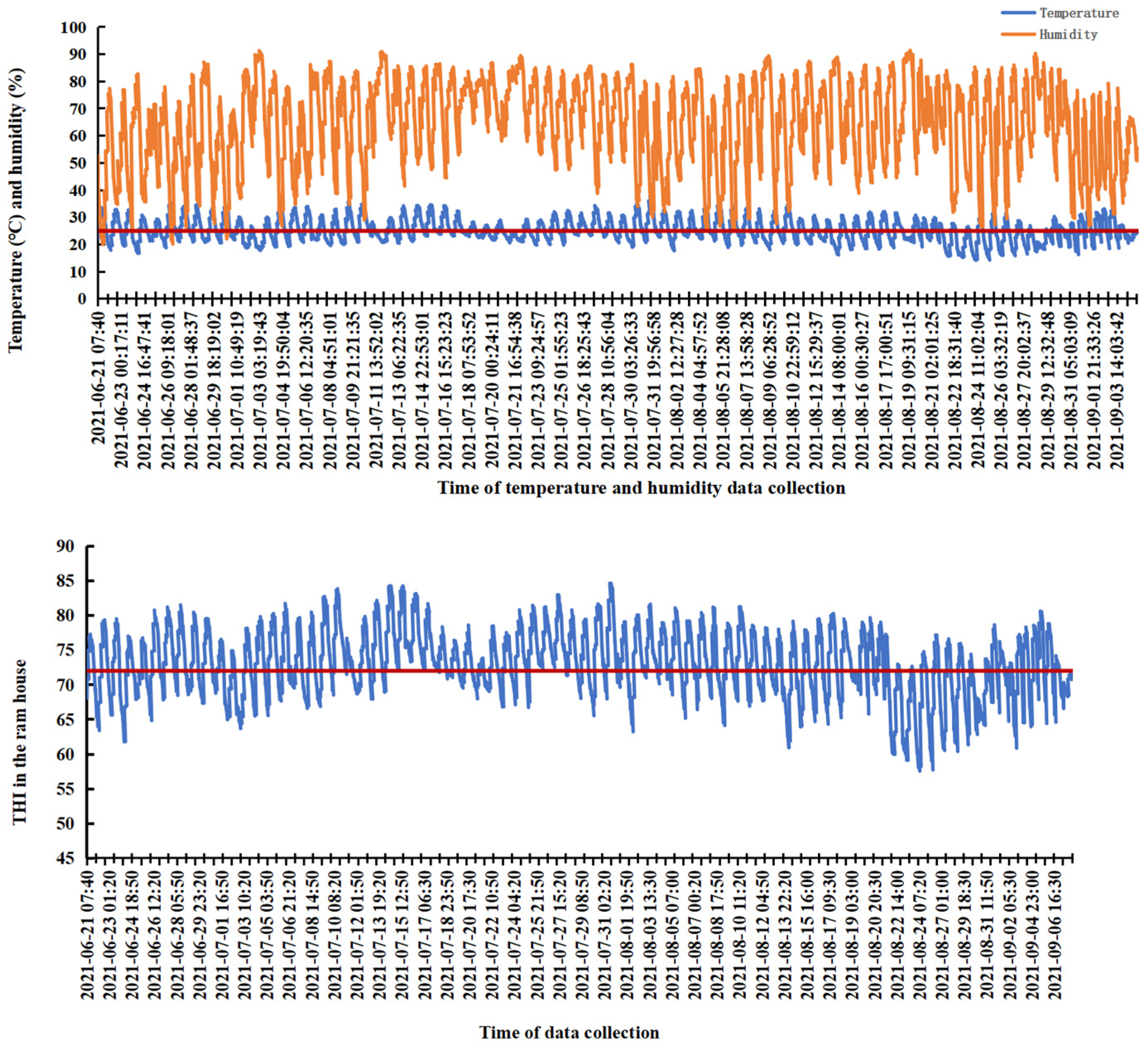
2.2. Collection of Temperature and Humidity Data
2.3. Collection of Blood and Semen Samples
2.4. Evaluation of Sperm Quality
2.5. Determination of the Oxidative Stress Parameters and Hormone Levels
2.6. Targeted Tryptophan Metabolite Analysis
2.7. Statistical Analysis
3. Results
3.1. Heat Stress of Rams Housed During the Summer Season
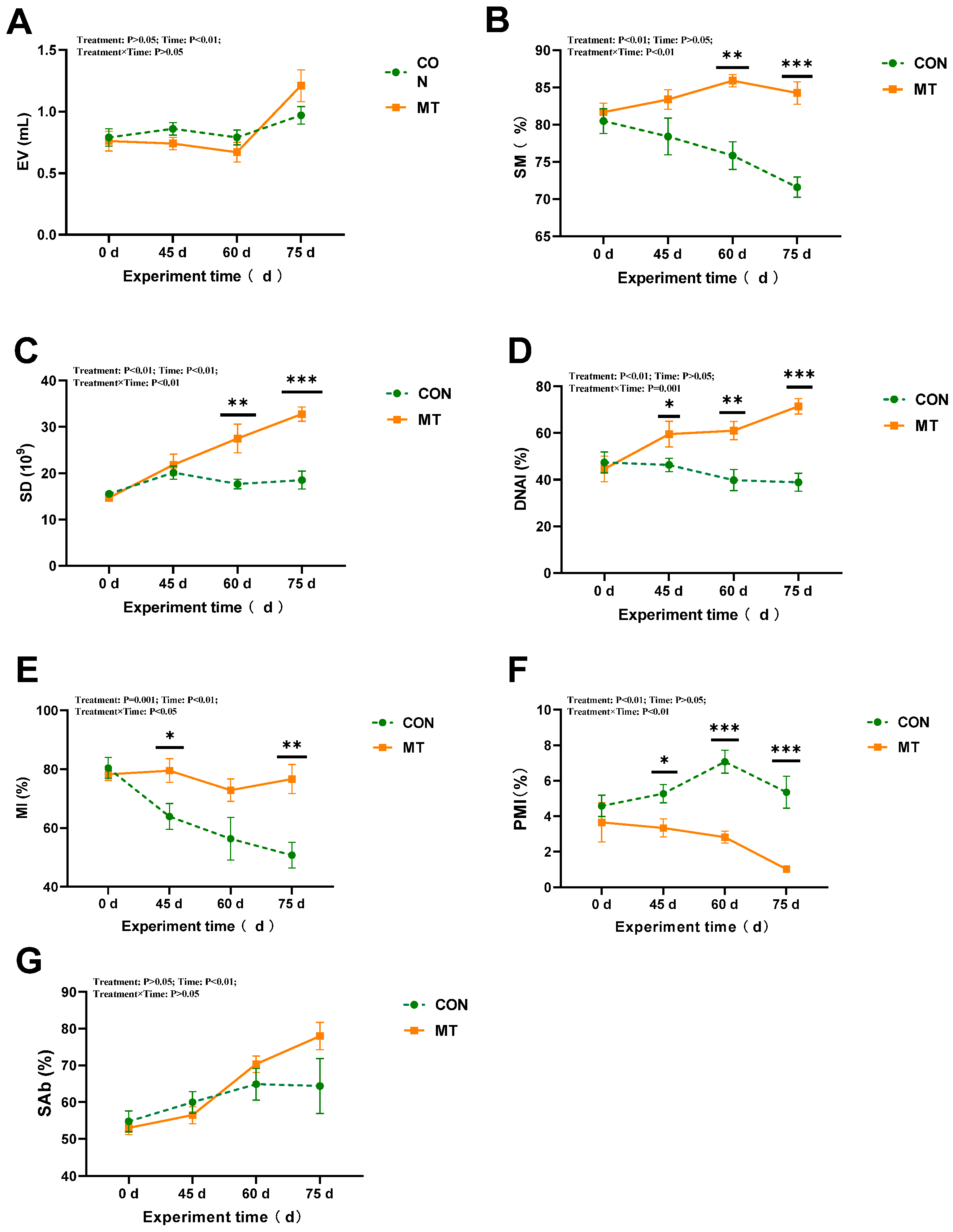
3.2. The Effect of Melatonin Implantation on the Semen Quality of Rams During the Summer Season
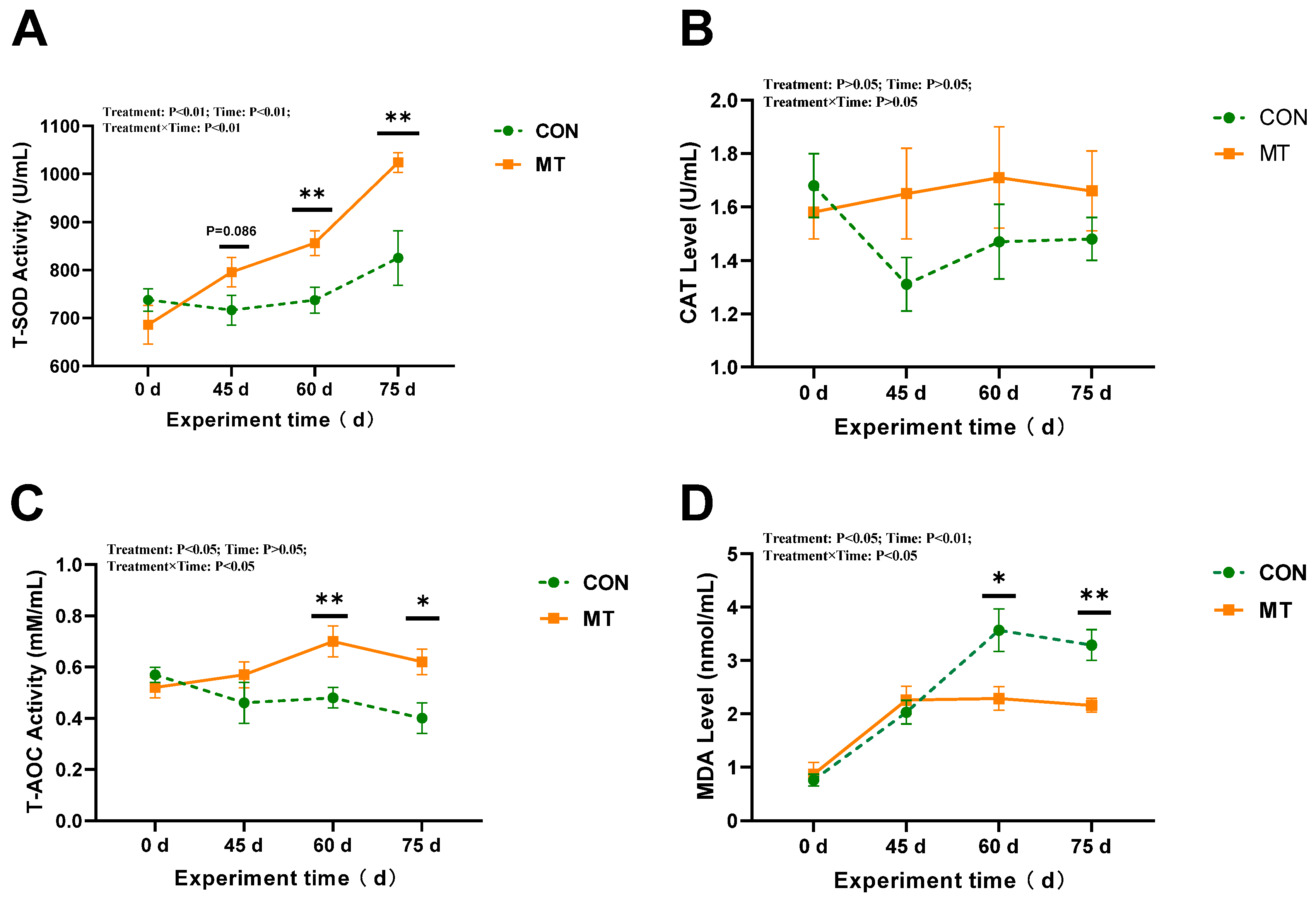
3.3. The Impact of Melatonin on Antioxidant Capacity in Serum and Seminal Plasma of Rams During the Summer Season
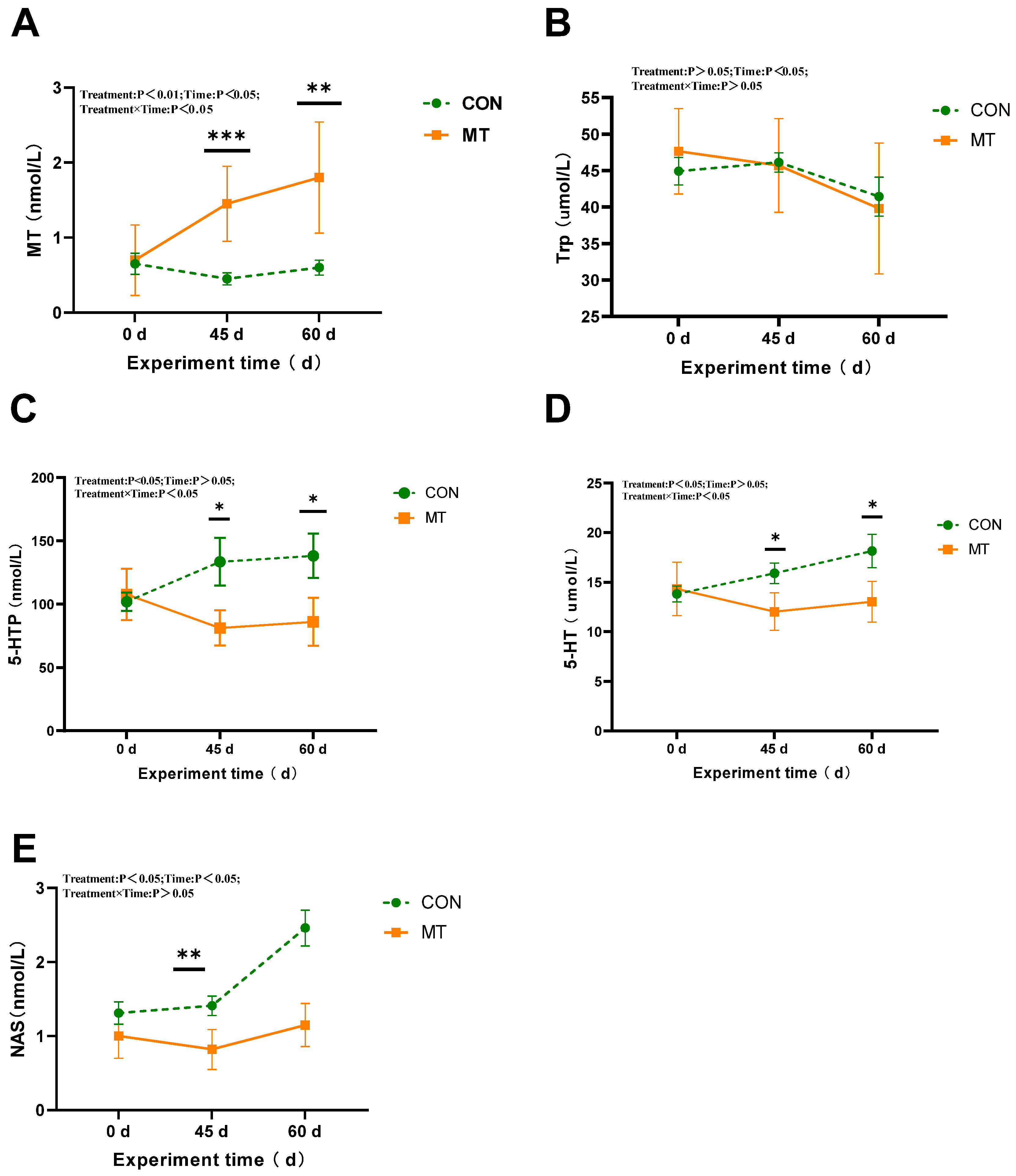
3.4. The Impact of Melatonin on Concentrations of Endocrine Hormones in the Serum and Seminal Plasma of Rams During the Summer Season
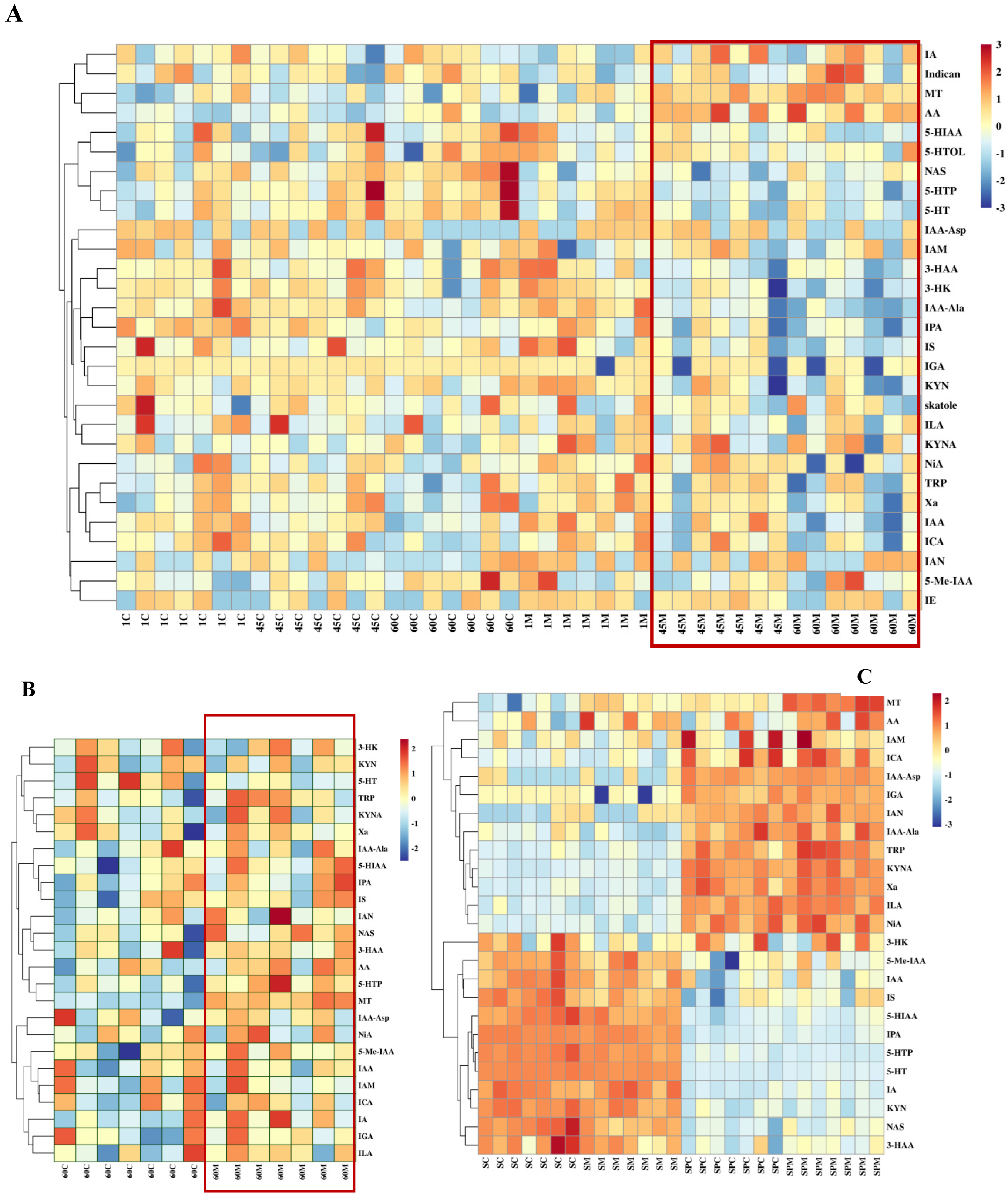
3.5. The Influence of Melatonin on the Key Tryptophan Metabolites Involved in Melatonin Biosynthesis in the Serum and Seminal Plasma of Rams
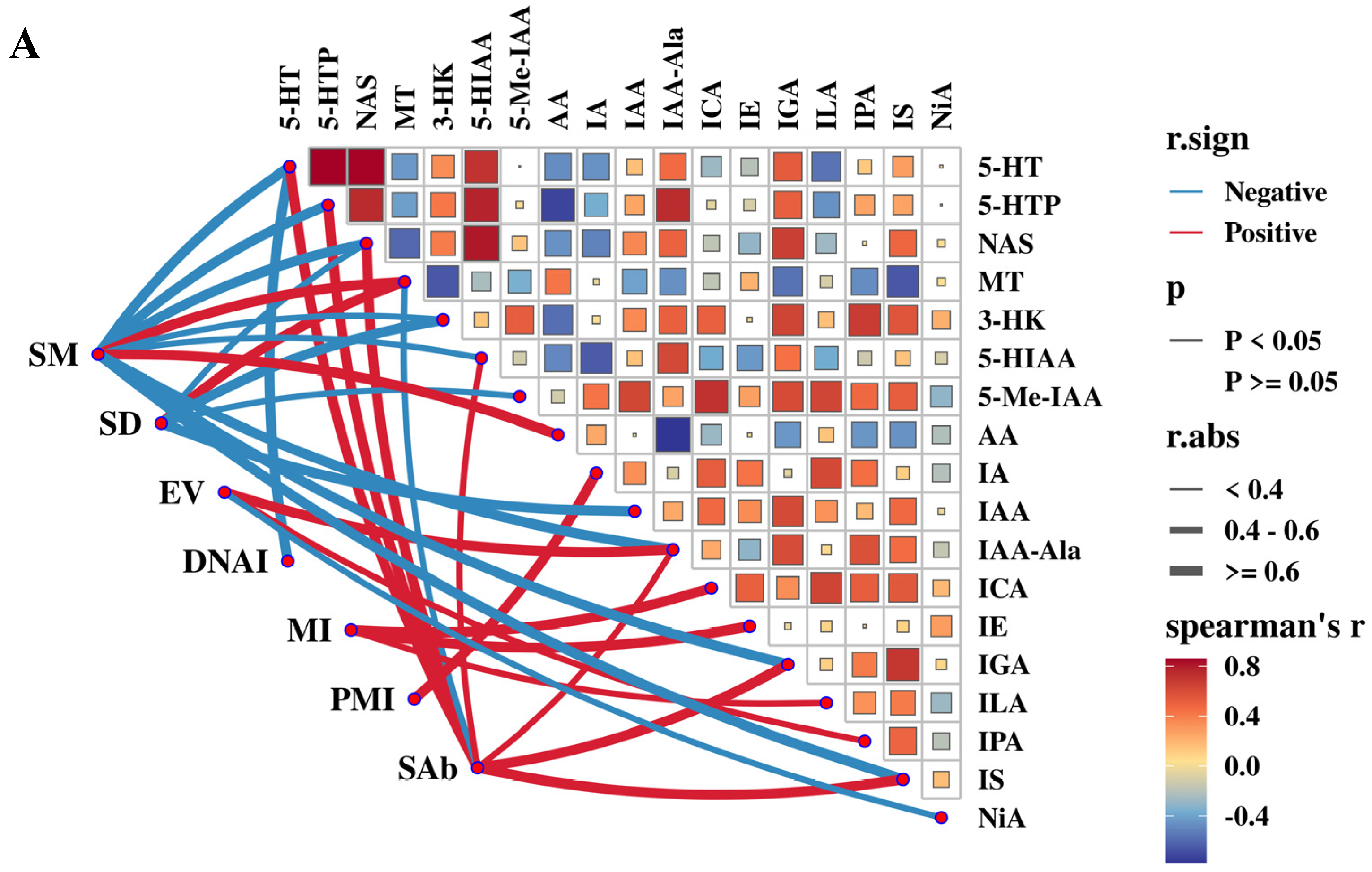
3.6. Correlation Analysis Between Semen Quality Parameters and Antioxidant Capacity and Endocrine Hormone Levels in the Serum and Seminal Plasma of Rams
3.7. Correlation Analysis Between Semen Quality Parameters and Tryptophan Metabolites in the Serum or Seminal Plasma of Rams
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarlós, P.; Egerszegi, I.; Balogh, O.; Molnár, A.; Cseh, S.; Rátky, J. Seasonal changes of scrotal circumference, blood plasma testosterone concentration and semen characteristics in Racka rams. Small Rumin. Res. 2013, 111, 90–95. [Google Scholar] [CrossRef]
- Barragán, A.L.; Avendaño-Reyes, L.; Mellado-Bosque, M.; Meza-Herrera, C.A.; Vicente-Pérez, R.; Castañeda, V.J.; Díaz-Molina, R.; Macías-Cruz, U. Seasonal heat stress compromises testicular thermoregulation and semen quality of Dorper rams raised in a desert climate. J. Therm. Biol. 2023, 118, 103737. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.; Safdarian, M.; Hashemi, M. Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams. Small Rumin. Res. 2004, 53, 133–139. [Google Scholar] [CrossRef]
- Rathore, A.K. Acrosomal abnormality in ram spermatozoa due to heat stress. Br. Vet. J. 1970, 126, 440–443. [Google Scholar] [CrossRef]
- Rathore, A.K.; Yeates, N.T. Morphological changes in ram spermatozoa due to heat stress. Vet. Rec. 1967, 81, 343–344. [Google Scholar] [CrossRef]
- El-Zeftawy, M.; Mahmoud, G.B.; Hassan, M. Impact of thermal stress exposure on seminal quality, antioxidant defence system, TNF-alpha and TIMP-3 in Ossimi ram. Reprod. Domest. Anim. 2020, 55, 870–881. [Google Scholar] [CrossRef]
- Ko, S.H. Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods. Nutrients 2024, 16, 3032. [Google Scholar] [CrossRef] [PubMed]
- Madian, H.A.; Hassan, M.A.E.; El-Hadad, E.S.; Saad, M.F.; El-Shhat, A.M.; Eliraqy, E.Z. Semen Production, Testosterone Profile, and Testicular Histology of Heat Stressed Egyptian Geese Administrated with L-Arginine. J. Anim. Poult. Prod. 2023, 14, 135–141. [Google Scholar] [CrossRef]
- Edens, S.W.W. Heat-stress response of broiler cockerels to manipulation of the gonadal steroids, testosterone and estradiol. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 629–633. [Google Scholar] [CrossRef]
- Anjali; Vk, G.; Sarma, L.; Tripathi, M.; Verma, M.R.; Verma, V.; Pathak, M.C.; Samad, H.A.; Maurya, V.P.; Chouhan, V.S.; et al. Thyroid hormone dynamics of Tharparkar and Sahiwal cattle during induced heat stress. Trop. Anim. Health Prod. 2023, 55, 57. [Google Scholar] [CrossRef]
- Bueno, J.; Gotardo, L.; Dos, S.A.; Litz, F.H.; Olivieri, O.; Alves, R.; Moraes, C.A.; de Mattos, N.M. Effect of cyclic heat stress on thyroidal hormones, thyroid histology, and performance of two broiler strains. Int. J. Biometeorol. 2020, 64, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.J.; Washburn, K.W.; Huston, T.M. Involvement of the thyroid gland in the response of young chickens to heat stress. Poult. Sci. 1984, 63, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Cebrian, I.; Asumpcao, M.E.; Perez-Pe, R.; Abecia, J.A.; Forcada, F.; Cebrian-Perez, J.A.; Muino-Blanco, T. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol. 2010, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Cabrera, J.; D’Arpa, D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv. Exp. Med. Biol. 1999, 467, 379–387. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Pool, K.R.; Rickard, J.P.; de Graaf, S.P. Melatonin improves the motility and DNA integrity of frozen-thawed ram spermatozoa likely via suppression of mitochondrial superoxide production. Domest. Anim. Endocrinol. 2021, 74, 106516. [Google Scholar] [CrossRef]
- Shayestehyekta, M.; Mohammadi, T.; Soltani, L.; PooyanMehr, M. Effect of different concentrations of melatonin on ram epididymal spermatozoa recovered post-mortem under oxidative stress conditions and storage at 4 degrees C. Reprod. Domest. Anim. 2022, 57, 1520–1528. [Google Scholar] [CrossRef]
- Bhalothia, S.K.; Mehta, J.S.; Kumar, T.; Prakash, C.; Talluri, T.R.; Pal, R.S.; Kumar, A. Melatonin and canthaxanthin enhances sperm viability and protect ram spermatozoa from oxidative stress during liquid storage at 4 degrees C. Andrologia 2022, 54, e14304. [Google Scholar] [CrossRef]
- Ge, W.B.; Xiao, L.F.; Duan, H.W.; Li, Z.S.; Jiang, Y.T.; Yang, S.S.; Hu, J.J.; Zhang, Y.; Zhao, X.X. Melatonin protects against lipopolysaccharide-induced epididymitis in sheep epididymal epithelial cells in vitro. Immunol. Lett. 2019, 214, 45–51. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Liu, L.; Wan, Y.; Wang, F. Melatonin alleviated oxidative stress induced by energy restriction on sheep Leydig cells through Sirt1/Sod2 pathway. Theriogenology 2021, 173, 83–92. [Google Scholar] [CrossRef]
- Tsantarliotou, M.P.; Kokolis, N.A.; Smokovitis, A. Melatonin administration increased plasminogen activator activity in ram spermatozoa. Theriogenology 2008, 69, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Pool, K.R.; Rickard, J.P.; Pini, T.; de Graaf, S.P. Exogenous melatonin advances the ram breeding season and increases testicular function. Sci. Rep. 2020, 10, 9711. [Google Scholar] [CrossRef]
- El-Shalofy, A.S.; Shahat, A.M.; Hedia, M.G. Effects of melatonin administration on testicular hemodynamics, echotexture, steroids production, and semen parameters during the non-breeding season in Ossimi rams. Theriogenology 2022, 184, 34–40. [Google Scholar] [CrossRef]
- Kokolis, N.; Theodosiadou, E.; Tsantarliotou, M.; Rekkas, C.; Goulas, P.; Smokovitis, A. The effect of melatonin implants on blood testosterone and acrosin activity in spermatozoa of the ram. Andrologia 2000, 32, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Chang, S.; De, A.K.; Baruah, K.K.; Khate, K.; Vupru, K.; Mitra, A. Slow release exogenous melatonin modulates scrotal circumference and testicular parameters, libido, endocrinological profiles and antioxidant and oxidative stress profiles in mithun. Theriogenology 2020, 154, 1–10. [Google Scholar] [CrossRef]
- Abbas, M.; Khan, M.I.; Hameed, N.; Rehman, A.; Mohsin, I.; Bilal, M.; Shahzad, M. Melatonin along with eCG improves fresh semen quality and plasma concentrations of melatonin and testosterone during non-breeding season in Beetal bucks. Small Rumin. Res. 2021, 205, 106569. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Gao, B.; Wang, H.J.; He, J.G.; Chen, H.S.; Hu, Z.L.; Long, L.H.; Chen, J.G.; Wang, F. Melatonin Ameliorates Depressive-Like Behaviors in Ovariectomized Mice by Improving Tryptophan Metabolism via Inhibition of Gut Microbe Alistipes Inops. Adv. Sci. 2024, 11, e2309473. [Google Scholar] [CrossRef]
- Ahmed, H.; Jahan, S.; Khan, A.; Khan, L.; Ullah, H.; Riaz, M.; Ullah, K.; Ullah, F. Supplementation of l-tryptophan (an aromatic amino acid) in tris citric acid extender enhances post-thaw progressive motility, plasmalemma, mitochondrial membrane potential, acrosome, and DNA integrities, and in vivo fertility rate of buffalo (Bubalus bubalis) bull spermatozoa. Cryobiology 2020, 92, 117–123. [Google Scholar] [PubMed]
- Wang, J.; Chen, M.; Yao, Y.; Zhu, M.; Jiang, Y.; Duan, J.; Yuan, Y.; Li, L.; Chen, M.; Sha, J. Characterization of Metabolic Patterns in Mouse Spermatogenesis and Its Clinical Implications in Humans. Int. J. Mol. Sci. 2025, 26, 1001. [Google Scholar] [CrossRef]
- Zhang, X.Y. Semen Quality and Testicular Antioxidant Properties of Hu Sheep in Summer and Winter. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2024. (In Chinese). [Google Scholar]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Small Ruminants. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academies Press: Washington, DC, USA, 2006; ISBN 9780309102131. [Google Scholar] [CrossRef]
- Yang, C.H.; Duan, C.H.; Wu, Z.Y.; Li, Y.; Luan, Y.Y.; Fu, X.J.; Zhang, C.X.; Zhang, W. Effects of melatonin administration to cashmere goats on cashmere production and hair follicle characteristics in two consecutive cashmere growth cycles. Domest. Anim. Endocrinol. 2021, 74, 106534. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; Lopez-Gatius, F.; Bech-Sabat, G.; Santolaria, P.; Yaniz, J.L.; Nogareda, C.; De Rensis, F.; Lopez-Bejar, M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007, 67, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, K.; Duan, G.; Yin, W.; Lei, M.; Yan, Y.; Ren, Y.; Zhang, C. Folic Acid and Taurine Alleviate the Impairment of Redox Status, Immunity, Rumen Microbial Composition and Fermentation of Lambs under Heat Stress. Animals 2024, 14, 998. [Google Scholar] [CrossRef] [PubMed]
- Moses, D.F.; Heras, M.A.D.L.; Valcárcel, A.; Pérez, L.; Baldassarre, H. Use of computerized motility analyzer for the evaluation of frozen-thawed ram spermatozoa. Andrologia 1995. [CrossRef] [PubMed]
- Espinoza, J.A.; Schulz, M.A.; Sanchez, R.; Villegas, J.V. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia 2009, 41, 51–54. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Traganos, F.; Sharpless, T.; Melamed, M.R. Thermal denaturation of DNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp. Cell Res. 1975, 90, 411–428. [Google Scholar] [CrossRef]
- Alcay, S.; Berk Toker, M.; Gokce, E.; Ustuner, B.; Tekin Onder, N.; Sagirkaya, H.; Nur, Z.; Kemal Soylu, M. Successful ram semen cryopreservation with lyophilized egg yolk-based extender. Cryobiology 2015, 71, 329–333. [Google Scholar] [CrossRef]
- Hu, P.; Yan, X.; Zeng, Y.; Jiang, Z.; Liu, J.; Feng, W. An UPLC-MS/MS method for targeted analysis of microbial and host tryptophan metabolism after administration of polysaccharides from Atractylodes macrocephala Koidz. in ulcerative colitis mice. J. Pharm. Biomed. Anal. 2023, 235, 115585. [Google Scholar] [CrossRef]
- Tömösi, F.; Kecskeméti, G.; Cseh, E.K.; Szabó, E.; Rajda, C.; Kormány, R.; Szabó, Z.; Vécsei, L.; Janáky, T. A validated UHPLC-MS method for tryptophan metabolites: Application in the diagnosis of multiple sclerosis. J. Pharm. Biomed. Anal. 2020, 185, 113246. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Chniter, M.; Najar, T.; Ghram, A. Meta-analysis of some physiologic, metabolic and oxidative responses of sheep exposed to environmental heat stress. Livest. Sci. 2019, 229, 179–187. [Google Scholar] [CrossRef]
- Wang, Q.; Yi, H.; Ou, J.; Wang, R.; Wang, M.; Wang, P.; He, X.; Tang, W.; Chen, J.; Yu, Y.; et al. Environmental Heat Stress Decreases Sperm Motility by Disrupting the Diurnal Rhythms of Rumen Microbes and Metabolites in Hu Rams. Int. J. Mol. Sci. 2024, 25, 11161. [Google Scholar] [CrossRef]
- Fayez, E.; Samir, H.; Youssef, F.S.; Salama, A.; ElSayed, M.A. Administration of melatonin nanoparticles improves testicular blood flow, echotexture of testicular parenchyma, scrotal circumference, and levels of estradiol and nitric oxide in prepubertal ossimi rams under summer heat stress. Vet. Res. Commun. 2024, 48, 3953–3965. [Google Scholar] [CrossRef]
- Zhao, F.; Whiting, S.; Lambourne, S.; Aitken, R.J.; Sun, Y.P. Melatonin alleviates heat stress-induced oxidative stress and apoptosis in human spermatozoa. Free Radic. Biol. Med. 2021, 164, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of Mammalian Photoperiodism and the Critical Role of the Pineal Gland and Melatonin. J. Biol. Rhythm. 2001, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Almeida, O.F.; Arendt, J. Role of melatonin and circadian rhythms in seasonal reproduction in rams. J. Reprod. Fertil. Suppl. 1981, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Palacín, I.; Abecia, J.; Forcada, F.; Casao, A.; Cebrián, J.; Muiño, T.; Palacios, C.; Pontes, J.M. Effects of exogenous melatonin treatment on out-of-season ram fertility. Ital. J. Anim. Sci. 2008, 7, 199–206. [Google Scholar] [CrossRef]
- Shahat, A.; Thundathil, J.; Kastelic, J. Melatonin improves testicular hemodynamics and sperm quality in rams subjected to mild testicular heat stress. Anim. Reprod. Sci. 2022, 247, 107119. [Google Scholar] [CrossRef]
- França, L.R.; Becker-Silva, S.C.; Chiarini-Garcia, H. The length of the cycle of seminiferous epithelium in goats (Capra hircus). Tissue Cell 1999, 31, 274. [Google Scholar] [CrossRef]
- Robaire, B.; Hinton, B.T. The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 705. [Google Scholar] [CrossRef]
- Makris, A.; Alevra, A.I.; Exadactylos, A.; Papadopoulos, S. The Role of Melatonin to Ameliorate Oxidative Stress in Sperm Cells. Int. J. Mol. Sci. 2023, 24, 15056. [Google Scholar] [CrossRef]
- Kumar, T.; Kumar, P.; Saini, N.; Bhalothia, S.K.; Prakash, C.; Mahla, A.S.; Kumar, A. Shielding effect of melatonin improves seminal quality and oxidative stress indices during chilled storage of ram semen. Trop. Anim. Health Prod. 2022, 54, 197. [Google Scholar] [CrossRef]
- El-Sherbiny, H.R.; Hashem, N.M.; Abdelnaby, E.A. Coat color affects the resilience against heat stress impacts on testicular hemodynamics, reproductive hormones, and semen quality in Baladi goats. BMC Vet. Res. 2023, 19, 107. [Google Scholar] [CrossRef]
- Minton, E.J.; Wettemann, P.R.; Meyerhoeffer, C.D.; Hintz, L.R.; Turman, J.E. Serum luteinizing hormone and testosterone in bulls during exposure to elevated ambient temperature. J. Anim. Sci. 1981, 53, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Einarsson, S.; Lundström, K.; Hakkarainen, J. Endocrine effects of heat stress in boars. Acta Vet. Scand. 1983, 24, 305. [Google Scholar] [CrossRef] [PubMed]
- May, J.D.; Deaton, J.W.; Reece, F.N.; Branton, S.L. Effect of acclimation and heat stress on thyroid hormone concentration. Poult. Sci. 1986, 65, 1211–1213. [Google Scholar] [CrossRef]
- Kumar, V.; Lincoln, G.A. Effects of a one-hour light pulse on the timing of the circadian rhythm in melatonin secretion in rams. J. Pineal Res. 1995, 18, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.I.; Claypool, L.E.; Ebling, F.J.P.; Foster, D.L. Entrainment of the melatonin rhythms in early postnatal lambs and their mothers. J. Biol. Rhythm. 1989, 4, 457–465. [Google Scholar] [CrossRef]
- Welsh, T.H.; Randel, R.D.; Johnson, B.H. Temporal relationships among peripheral blood concentrations of corticosteroids, luteinizing hormone and testosterone in bulls. Theriogenology 1979, 12, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Welsh, T.J.; Johnson, B.H. Stress-induced alterations in secretion of corticosteroids, progesterone, luteinizing hormone, and testosterone in bulls. Endocrinology 1981, 109, 185–190. [Google Scholar] [CrossRef]
- Doerr, P.; Pirke, K.M. Cortisol-induced suppression of plasma testosterone in normal adult males. J. Clin. Endocrinol. Metab. 1976, 43, 622–629. [Google Scholar] [CrossRef]
- Tast, A.; Love, R.J.; Evans, G.; Telsfer, S.; Giles, R.; Nicholls, P.; Voultsios, A.; Kennaway, D.J. The pattern of melatonin secretion is rhythmic in the domestic pig and responds rapidly to changes in daylength. J. Pineal Res. 2001, 31, 294–300. [Google Scholar] [CrossRef]
- Axelrod, J. The pineal gland: A neurochemical transducer. Science 1974, 184, 1341–1348. [Google Scholar] [CrossRef]
- Clariget, R.P.; Forsberg, M.; Rodriguez-Martinez, H. Seasonal variation in live weight, testes size, testosterone, LH secretion, melatonin and thyroxine in Merino and Corriedale rams in a subtropical climate. Acta Vet. Scand. 1998, 39, 35–47. [Google Scholar]
- Zaidan, R.; Geoffriau, M.; Brun, J.; Taillard, J.; Bureau, C.; Chazot, G.; Claustrat, B. Melatonin is able to influence its secretion in humans: Description of a phase-response curve. Neuroendocrinology 1994, 60, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bothorel, B.; Barassin, S.; Saboureau, M.; Perreau, S.; Pévet, P. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. Eur. J. Neurosci. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Sanford, L.M.; Winter, J.S.D.; Palmer, W.M.; Howland, B.E. The profile of LH and testosterone secretion in the ram. Endocrinology 1974, 627, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Aldhous, M.; Franey, C.; English, J.; Arendt, J. The effect of exogenous melatonin on endocrine function in man. Clin. Endocrinol. 1986, 24, 375–382. [Google Scholar] [CrossRef]
- Ninomiya, T.; Iwatani, N.; Tomoda, A.; Miike, T. Effects of exogenous melatonin on pituitary hormones in humans. Clin. Physiol. 2001, 21, 292–299. [Google Scholar] [CrossRef]
- Robinson, M.; Turnbull, S.; Lee, B.Y.; Leonenko, Z. The effects of melatonin, serotonin, tryptophan and NAS on the biophysical properties of DPPC monolayers. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183363. [Google Scholar] [CrossRef]
- Casao, A.; Peña-Delgado, V.; Pérez-Pe, R. From spermatogenesis to fertilisation: The role of melatonin on ram spermatozoa. Domest. Anim. Endocrinol. 2025, 91, 106916. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Hamilton, T.R.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J.A.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef]
- Peña-Delgado, V.; Carvajal-Serna, M.; Miguel-Jiménez, S.; Casao, A.; Pérez-Pe, R. Influence of the polymorphisms of the melatonin receptor 1A (MTNR1A) gene on the distribution of MT1 and MT2 melatonin receptors in ram sperm. Anim. Reprod. Sci. 2022, 247, 107138. [Google Scholar] [CrossRef]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Perez-Pe, R.; Muino-Blanco, T.; Cebrian-Perez, J.A. Identification and immunolocalisation of melatonin MT(1) and MT(2) receptors in Rasa Aragonesa ram spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Simovich, M.J.; Bryant-Thomas, T.; Chyan, Y.J.; Poeggeler, B.; Dubocovich, M.; Bick, R.; Perry, G.; Cruz-Sanchez, F.; Smith, M.A. The neuroprotective activities of melatonin against the Alzheimer β-protein are not mediated by melatonin membrane receptors. J. Pineal Res. 2010, 32, 135–142. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-Q.; Li, X.; Li, J.-H.; Zhou, Y.; Lei, M.-K.; Yin, W.-Q.; Ren, Y.-S.; Yang, C.-H.; Zhang, C.-X. Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season. Antioxidants 2025, 14, 630. https://doi.org/10.3390/antiox14060630
Liu Q-Q, Li X, Li J-H, Zhou Y, Lei M-K, Yin W-Q, Ren Y-S, Yang C-H, Zhang C-X. Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season. Antioxidants. 2025; 14(6):630. https://doi.org/10.3390/antiox14060630
Chicago/Turabian StyleLiu, Qian-Qiu, Xiong Li, Jia-Hao Li, Yang Zhou, Ming-Kai Lei, Wei-Qi Yin, You-She Ren, Chun-He Yang, and Chun-Xiang Zhang. 2025. "Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season" Antioxidants 14, no. 6: 630. https://doi.org/10.3390/antiox14060630
APA StyleLiu, Q.-Q., Li, X., Li, J.-H., Zhou, Y., Lei, M.-K., Yin, W.-Q., Ren, Y.-S., Yang, C.-H., & Zhang, C.-X. (2025). Melatonin Improves Semen Quality by Modulating Oxidative Stress, Endocrine Hormones, and Tryptophan Metabolism of Hu Rams Under Summer Heat Stress and the Non-Reproductive Season. Antioxidants, 14(6), 630. https://doi.org/10.3390/antiox14060630






