Subchronic Intranasal Administration of NeuroEPO Reduces Long-Term Consequences of Severe Traumatic Brain Injury in Male Rats
Abstract
1. Introduction
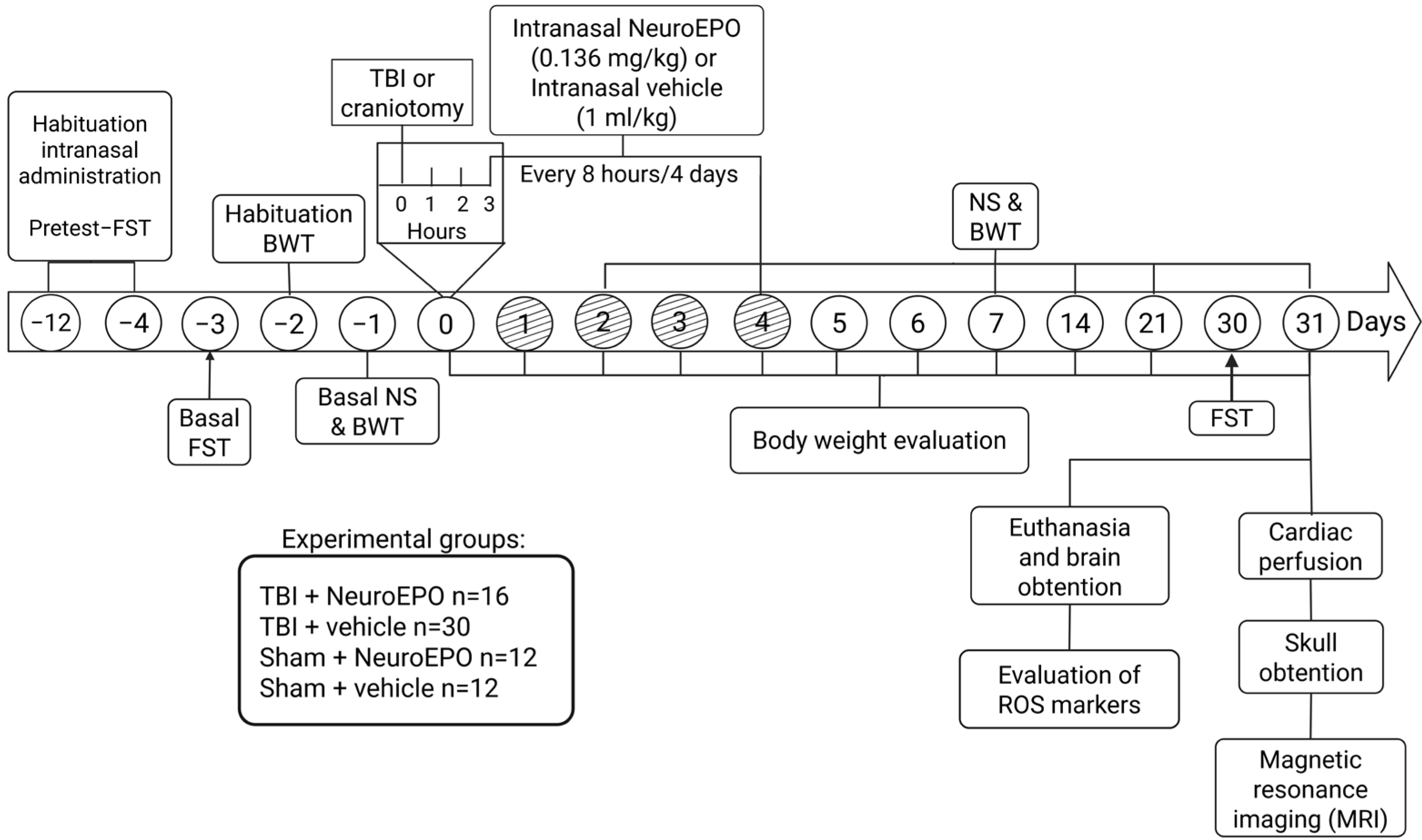
2. Materials and Methods
2.1. Animals
2.2. Induction of Severe TBI
2.3. Experimental Groups
2.4. Evaluation of Sensoriomotor Function
2.5. Evaluation of Depression-like Behaviors
2.6. Evaluation of Oxidative Stress Markers in Brain Structures
- (a)
- Estimation of ROS production
- (b)
- Analysis of malondialdehyde production
- (c)
- Evaluation of catalase activity
2.7. Analysis of Brain Volume by Ex Vivo MRI
2.8. Statistical Analysis
3. Results
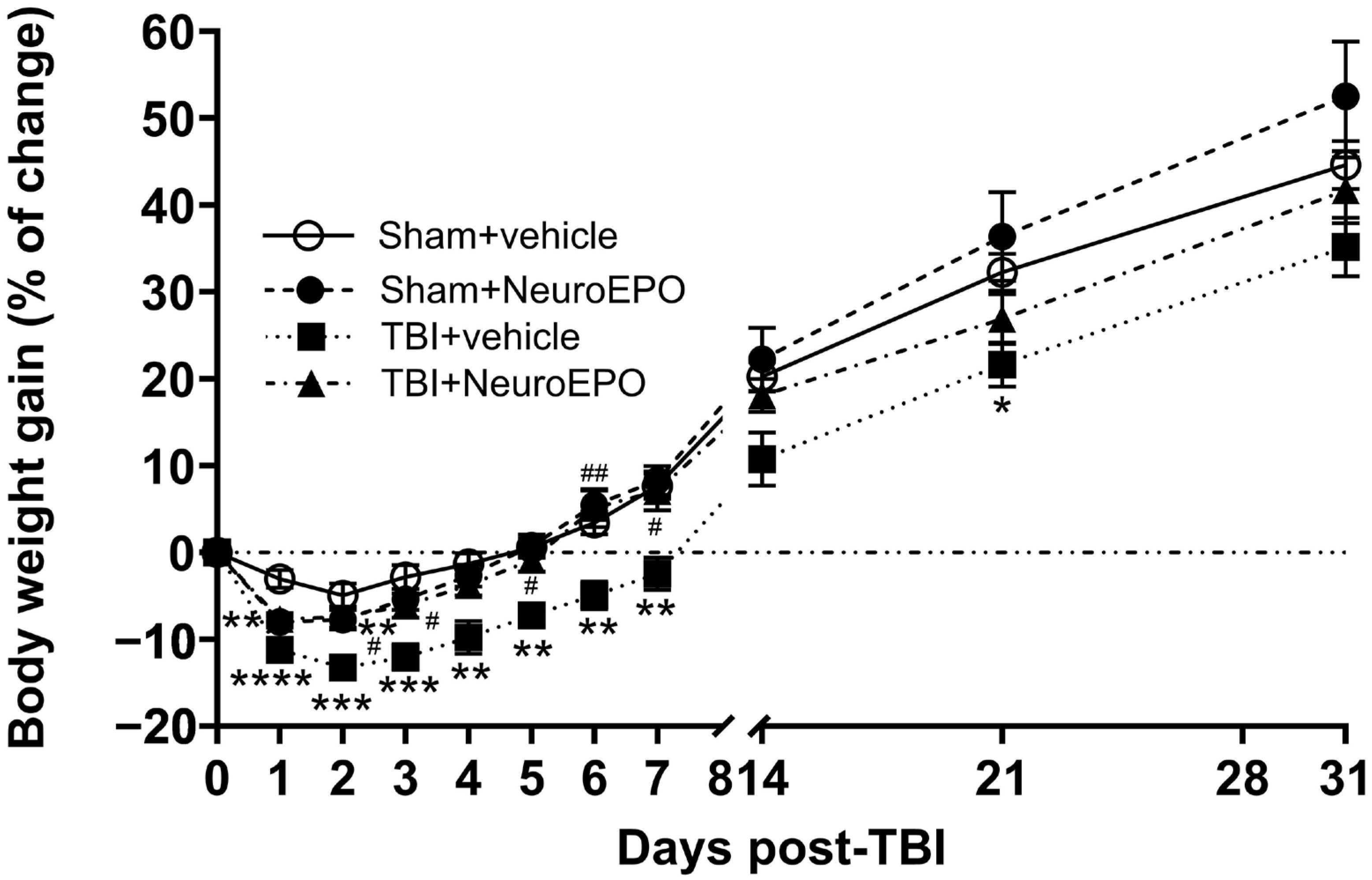
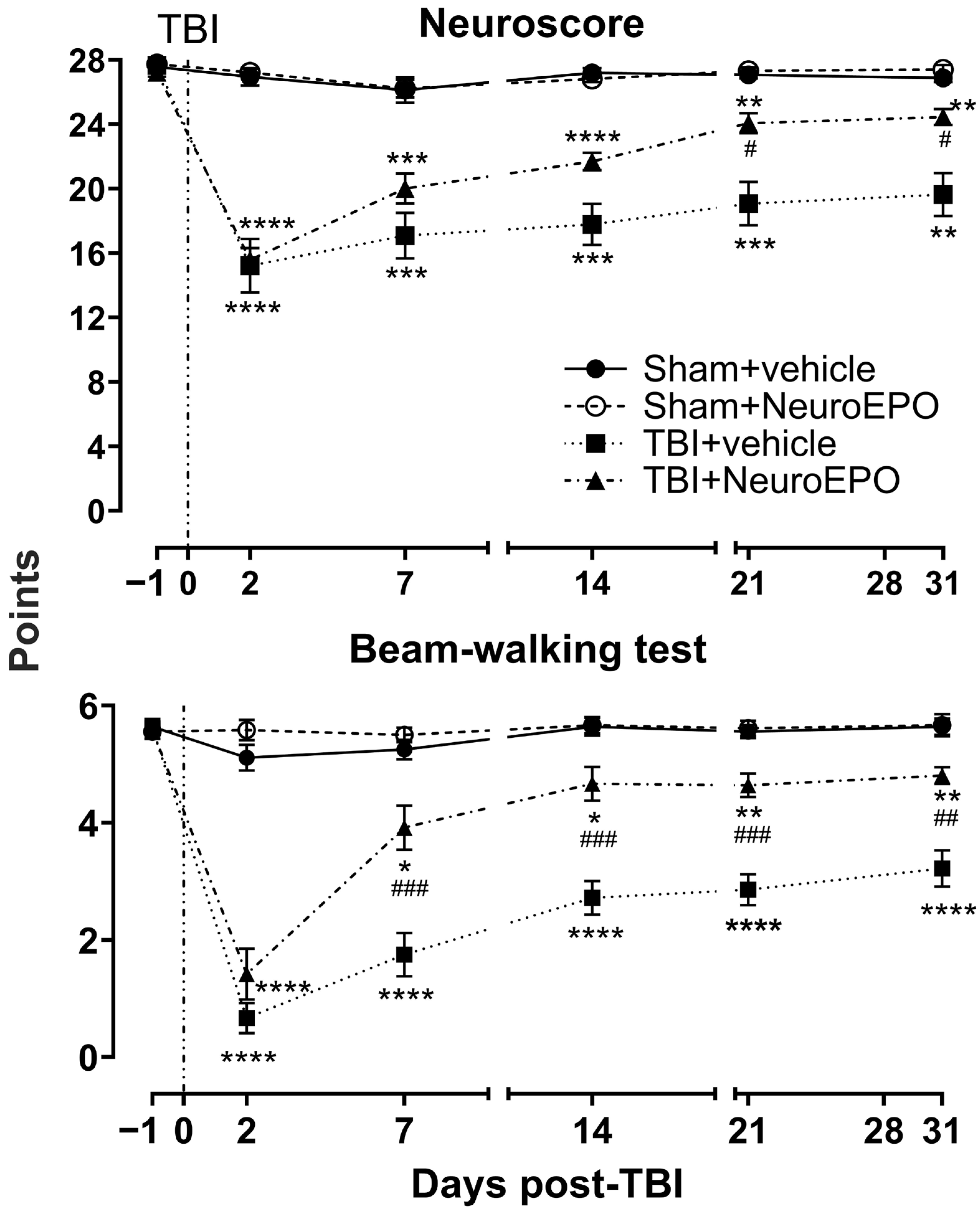
3.1. Intranasal Administration of NeuroEPO Lessens Mortality Rate and Improves Body Weight Gain and Sensorimotor Recovery After a Severe TBI
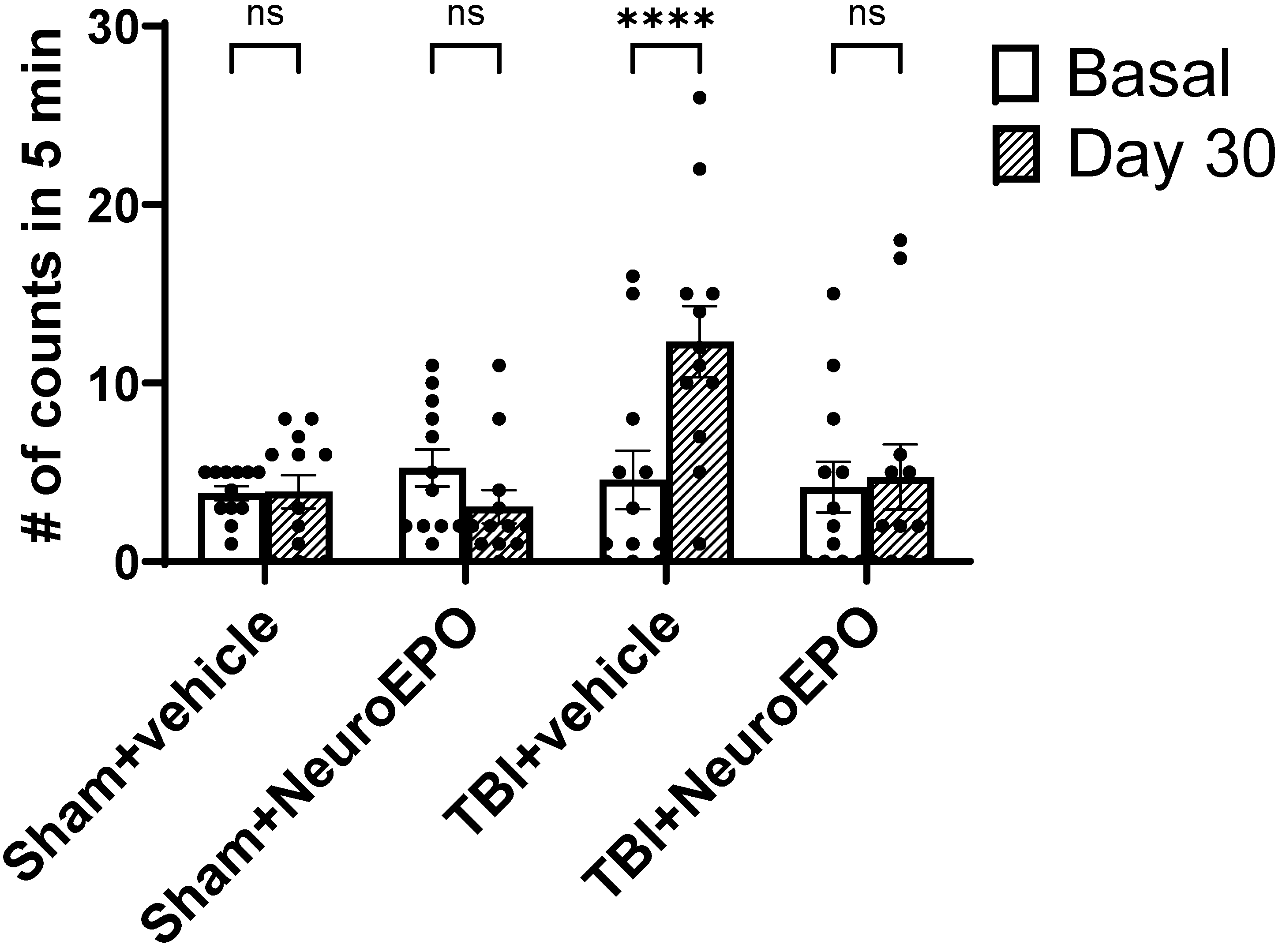
3.2. Intranasal Administration of NeuroEPO Reduces Depression-like Behavior Induced by Severe TBI
3.3. Intranasal Administration of NeuroEPO Avoids TBI-Induced REDOX Imbalance in Different Brain Areas
3.4. Intranasal Administration of NeuroEPO Prevents TBI-Induced Brain Atrophy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBA | Thiobarbituric acid |
| TRIS | Tris(hydroxymethyl)aminomethane |
| HEPES | N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid |
| PBS | phosphate buffer solution |
| s.c. | subcutaneous |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
| LFPI | lateral fluid percussion injury model |
| NS | Neuroscore |
| TBI | traumatic brain injury |
| SE | standard error |
| RM | repeated-measures |
References
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Karel, M.; Lund, D. Epidemiology and Pathophysiology of Traumatic Brain Injury. In Traumatic Brain Injury; CRC Press: Boca Raton, FL, USA, 2015; pp. 34–65. [Google Scholar]
- Haarbauer-Krupa, J.; Pugh, M.J.; Prager, E.M.; Harmon, N.; Wolfe, J.; Yaffe, K. Epidemiology of Chronic Effects of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Castañeda, C.; Huerta de la Cruz, S.; Martínez-Aguirre, C.; Orozco-Suárez, S.A.; Rocha, L. Cannabidiol Reduces Short- and Long-Term High Glutamate Release after Severe Traumatic Brain Injury and Improves Functional Recovery. Pharmaceutics 2022, 14, 1609. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Martin, R.M.; Wright, M.J.; Lutkenhoff, E.S.; Ellingson, B.M.; Van Horn, J.D.; Tubi, M.; Alger, J.R.; McArthur, D.L.; Vespa, P.M. Traumatic Hemorrhagic Brain Injury: Impact of Location and Resorption on Cognitive Outcome. J. Neurosurg. 2017, 126, 796–804. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal Pathology in Traumatic Brain Injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Lorenzo, J.M.; Molina, I.; Jiménez, A. Association between Serum Malondialdehyde Levels and Mortality in Patients with Severe Brain Trauma Injury. J. Neurotrauma 2015, 32, 1–6. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, A.; Egea-Guerrero, J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative Stress in Traumatic Brain Injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Jorge, R.E.; Arciniegas, D.B. Mood Disorders After TBI. Psychiatr. Clin. N. Am. 2014, 37, 13–29. [Google Scholar] [CrossRef]
- Crenn, P.; Hamchaoui, S.; Bourget-Massari, A.; Hanachi, M.; Melchior, J.-C.; Azouvi, P. Changes in Weight after Traumatic Brain Injury in Adult Patients: A Longitudinal Study. Clin. Nutr. 2014, 33, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Sackheim, A.M.; Stockwell, D.; Villalba, N.; Haines, L.; Scott, C.L.; Russell, S.; Hammack, S.E.; Freeman, K. Traumatic Brain Injury Impairs Sensorimotor Function in Mice. J. Surg. Res. 2017, 213, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.C.; de Rooij, R.; Kuhl, E. The Shrinking Brain: Cerebral Atrophy Following Traumatic Brain Injury. Ann. Biomed. Eng. 2019, 47, 1941–1959. [Google Scholar] [CrossRef]
- Tani, J.; Wen, Y.-T.; Hu, C.-J.; Sung, J.-Y. Current and Potential Pharmacologic Therapies for Traumatic Brain Injury. Pharmaceuticals 2022, 15, 838. [Google Scholar] [CrossRef]
- Nichol, A.; French, C.; Little, L.; Haddad, S.; Presneill, J.; Arabi, Y.; Bailey, M.; Cooper, D.J.; Duranteau, J.; Huet, O.; et al. Erythropoietin in Traumatic Brain Injury (EPO-TBI): A Double-Blind Randomised Controlled Trial. Lancet 2015, 386, 2499–2506. [Google Scholar] [CrossRef]
- Said, M.F.; Islam, A.A.; Massi, M.N. Prihantono Effect of Erythropoietin Administration on the Expression of Brain-Derived Neurotrophic Factor, Stromal Cell-Derived Factor-1, and Neuron-Specific Enolase in Traumatic Brain Injury: A Literature Review. Ann. Med. Surg. 2021, 69, 102666. [Google Scholar] [CrossRef]
- Blixt, J.; Gunnarson, E.; Wanecek, M. Erythropoietin Attenuates the Brain Edema Response after Experimental Traumatic Brain Injury. J. Neurotrauma 2018, 35, 671–680. [Google Scholar] [CrossRef]
- Hartley, C.E.; Varma, M.; Fischer, J.P.; Riccardi, R.; Strauss, J.A.; Shah, S.; Zhang, S.; Yang, Z.-J. Neuroprotective Effects of Erythropoietin on Acute Metabolic and Pathological Changes in Experimentally Induced Neurotrauma. J. Neurosurg. 2008, 109, 708–714. [Google Scholar] [CrossRef]
- Evans, P.; Persinger, M.A. Erythropoietin and Mild Traumatic Brain Injury: Neuroprotective Potential and Dangerous Side-Effects. J. Biol. Sci. 2010, 10, 739–746. [Google Scholar] [CrossRef][Green Version]
- Garcia Rodriguez, J.C.; Rama, R. Neuro-EPO by Nasal Route as a Neuroprotective Therapy in Brain Ischemia. In Acute Ischemic Stroke; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Garzón, F.; Coimbra, D.; Parcerisas, A.; Rodriguez, Y.; García, J.C.; Soriano, E.; Rama, R. NeuroEPO Preserves Neurons from Glutamate-Induced Excitotoxicity. J. Alzheimer’s Dis. 2018, 65, 1469–1483. [Google Scholar] [CrossRef]
- García-Rodríguez, J.C.; Sosa Teste, I. The Nasal Route as a Potential Pathway for Delivery of Erythropoietin in the Treatment of Acute Ischemic Stroke in Humans. Sci. World J. 2009, 9, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Sosa, I.; Cruz, J.; Santana, J.; Mengana, Y.; García-Salman, J.D.; Muñoz, A.; Ozuna, T.G.; García, J.C. Paso de la Molécula de Eritropoyetina Humana Recombinante Con Bajo Contenido de Ácido Siálico al Sistema Nervioso Central por la vía Intranasal en los Modelos del Meriones Unguiculatus y el Primate no Humano Macaca Fascicularis. Rev. Salud Anim. 2008, 30, 39–44. [Google Scholar]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Santos-Morales, O.; Díaz-Machado, A.; Jiménez-Rodríguez, D.; Pomares-Iturralde, Y.; Festary-Casanovas, T.; González-Delgado, C.A.; Pérez-Rodríguez, S.; Alfonso-Muñoz, E.; Viada-González, C.; Piedra-Sierra, P.; et al. Nasal Administration of the Neuroprotective Candidate NeuroEPO to Healthy Volunteers: A Randomized, Parallel, Open-Label Safety Study. BMC Neurol. 2017, 17, 129. [Google Scholar] [CrossRef]
- Rodríguez Cruz, Y.; Yuneidys Mengana, T.; Muñoz Cernuda, A.; Subirós Martines, N.; González-Quevedo, A.; Sosa Testé, I.; García Rodríguez, J.C. Treatment with Nasal Neuro-EPO Improves the Neurological, Cognitive, and Histological State in a Gerbil Model of Focal Ischemia. Sci. World J. 2010, 10, 2288–2300. [Google Scholar] [CrossRef]
- Rama, R.; Garzón, F.; Rodríguez-Cruz, Y.; Maurice, T.; García-Rodríguez, J.-C. Neuroprotective Effect of Neuro-EPO in Neurodegenerative Diseases: “Alea Jacta Est. ” Neural Regen. Res. 2019, 14, 1519. [Google Scholar] [CrossRef]
- García-Llano, M.; Pedroso-Ibáñez, I.; Morales-Chacón, L.; Rodríguez-Obaya, T.; Pérez-Ruiz, L.; Sosa-Testé, I.; Amaro-González, D.; Bringas-Vega, M.L. Short-Term Tolerance of Nasally-Administered NeuroEPO in Patients with Parkinson Disease. MEDICC Rev. 2021, 23, 49–54. [Google Scholar] [CrossRef]
- Rodriguez-Labrada, R.; Ortega-Sanchez, R.; Hernández Casaña, P.; Santos Morales, O.; Padrón-Estupiñan, M.d.C.; Batista-Nuñez, M.; Jiménez Rodríguez, D.; Canales-Ochoa, N.; Peña Acosta, A.; Medrano Montero, J.; et al. Erythropoietin in Spinocerebellar Ataxia Type 2: Feasibility and Proof-of-Principle Issues from a Randomized Controlled Study. Mov. Disord. 2022, 37, 1516–1525. [Google Scholar] [CrossRef]
- Sosa, S.; Bringas, G.; Urrutia, N.; Peñalver, A.I.; López, D.; González, E.; Fernández, A.; Hernández, Z.M.; Viña, A.; Peña, Y.; et al. NeuroEPO plus (NeuralCIM®) in Mild-to-Moderate Alzheimer’s Clinical Syndrome: The ATHENEA Randomized Clinical Trial. Alzheimers Res. Ther. 2023, 15, 215. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A.L. Traumatic Brain Injury in the Rat: Characterization of a Lateral Fluid-Percussion Model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- Immonen, R.J.; Kharatishvili, I.; Gröhn, H.; Pitkänen, A.; Gröhn, O.H.J. Quantitative MRI Predicts Long-Term Structural and Functional Outcome after Experimental Traumatic Brain Injury. Neuroimage 2009, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Fine, J.M.; Svitak, A.L.; Faltesek, K.A. Intranasal Administration of CNS Therapeutics to Awake Mice. J. Vis. Exp. 2013, e4440. [Google Scholar] [CrossRef]
- Teste, I.S.; Tamos, Y.M.; Cruz, Y.R.; Cernada, A.M.; Rodríguez, J.C.; Martínez, N.S.; Antich, R.M.C.; González-Quevedo, A.; Rodríguez, J.C.G. Dose Effect Evaluation and Therapeutic Window of the Neuro-EPO Nasal Application for the Treatment of the Focal Ischemia Model in the Mongolian Gerbil. Sci. World J. 2012, 2012, 607498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurice, T.; Mustafa, M.-H.; Desrumaux, C.; Keller, E.; Naert, G.; García-Barceló, M.d.l.C.; Rodríguez Cruz, Y.; Garcia Rodríguez, J.C. Intranasal Formulation of Erythropoietin (EPO) Showed Potent Protective Activity against Amyloid Toxicity in the Aβ 25-35 Non-Transgenic Mouse Model of Alzheimer’s Disease. J. Psychopharmacol. 2013, 27, 1044–1057. [Google Scholar] [CrossRef]
- Pierce, J.E.S.; Smith, D.H.; Trojanowski, J.Q.; McIntosh, T.K. Enduring Cognitive, Neurobehavioral and Histopathological Changes Persist for up to One Year Following Severe Experimental Brain Injury in Rats. Neuroscience 1998, 87, 359–369. [Google Scholar] [CrossRef]
- Luong, T.N.; Carlisle, H.J.; Southwell, A.; Patterson, P.H. Assessment of Motor Balance and Coordination in Mice Using the Balance Beam. J. Vis. Exp. 2011, 49, e2376. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; Fernández-Guasti, A.; López-Rubalcava, C. Antidepressant-Like Effect of Different Estrogenic Compounds in the Forced Swimming Test. Neuropsychopharmacology 2003, 28, 830–838. [Google Scholar] [CrossRef]
- Ng, N.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Catalase. In Plant-Microbe Interactions; Humana: New York, NY, USA, 2021; pp. 113–115. [Google Scholar]
- Awasthi, D.; Church, D.F.; Torbati, D.; Carey, M.E.; Pryor, W.A. Oxidative Stress Following Traumatic Brain Injury in Rats. Surg. Neurol. 1997, 47, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Fiorin, F.; Cunha do Espírito Santo, C.; Santos do Nascimento, R.; França, A.P.; Freire Royes, L.F. Behavioral Deficits after Mild Traumatic Brain Injury by Fluid Percussion in Rats. Neurosci. Lett. 2024, 818, 137550. [Google Scholar] [CrossRef]
- Kahriman, A.; Bouley, J.; Bosco, D.A.; Salman Shazeeb, M.; Henninger, N. Differential Association of Baseline Body Weight and Body-Weight Loss with Neurological Deficits, Histology, and Death after Repetitive Closed Head Traumatic Brain Injury. Neurosci. Lett. 2022, 771, 136430. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.-L.; Xu, X.-H.; Sun, Z.-Y.; Gu, J.-W.; Gao, F.-B. Brain Structure Alterations and Cognitive Impairment Following Repetitive Mild Head Impact: An in Vivo MRI and Behavioral Study in Rat. Behav. Brain Res. 2018, 340, 41–48. [Google Scholar] [CrossRef]
- Lagarto, A.; Bueno, V.; Guerra, I.; Valdés, O.; Couret, M.; López, R.; Vega, Y. Absence of Hematological Side Effects in Acute and Subacute Nasal Dosing of Erythropoietin with a Low Content of Sialic Acid. Exp. Toxicol. Pathol. 2011, 63, 563–567. [Google Scholar] [CrossRef]
- McPherson, J.I.; Prakash Krishnan Muthaiah, V.; Kaliyappan, K.; Leddy, J.J.; Personius, K.E. Temporal Expression of Brainstem Neurotrophic Proteins Following Mild Traumatic Brain Injury. Brain Res. 2024, 1835, 148908. [Google Scholar] [CrossRef]
- Venkatraman, A.; Edlow, B.L.; Immordino-Yang, M.H. The Brainstem in Emotion: A Review. Front. Neuroanat. 2017, 11, 15. [Google Scholar] [CrossRef]
- Firsching, R.; Woischneck, D.; Diedrich, M.; Klein, S.; Rückert, A.; Wittig, H.; Döhring, W. Early Magnetic Resonance Imaging of Brainstem Lesions after Severe Head Injury. J. Neurosurg. 1998, 89, 707–712. [Google Scholar] [CrossRef]
- Xueping, C.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar]
- Ataizi, Z.S.; Ozkoc, M.; Kanbak, G.; Karimkhani, H.; Burukoglu Donmez, D.; Ustunisik, N.; Ozturk, B. Evaluation of the Neuroprotective Role of Boric Acid in Preventing Traumatic Brain Injury-Mediated Oxidative Stress. Turk. Neurosurg. 2019, 31, 493–499. [Google Scholar] [CrossRef]
- Goss, J.R.; Taffe, K.M.; Kochanek, P.M.; DeKosky, S.T. The Antioxidant Enzymes Glutathione Peroxidase and Catalase Increase Following Traumatic Brain Injury in the Rat. Exp. Neurol. 1997, 146, 291–294. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Oxidative Stress, the Blood–Brain Barrier and Neurodegenerative Diseases: The Critical Beneficial Role of Dietary Antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef]
- López-Preza, F.I.; Huerta de la Cruz, S.; Santiago-Castañeda, C.; Silva-Velasco, D.L.; Beltran-Ornelas, J.H.; Tapia-Martínez, J.; Sánchez-López, A.; Rocha, L.; Centurión, D. Hydrogen Sulfide Prevents the Vascular Dysfunction Induced by Severe Traumatic Brain Injury in Rats by Reducing Reactive Oxygen Species and Modulating ENOS and H2S-Synthesizing Enzyme Expression. Life Sci. 2023, 312, 121218. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Yadav, V.; Marks, D.L. Hypothalamic Dysfunction and Multiple Sclerosis: Implications for Fatigue and Weight Dysregulation. Curr. Neurol. Neurosci. Rep. 2016, 16, 98. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-Gut Axis Dysfunction in the Pathogenesis of Traumatic Brain Injury. J. Clin. Investig. 2021, 131, e143777. [Google Scholar] [CrossRef]
- Foley, N.; Marshall, S.; Pikul, J.; Salter, K.; Teasell, R. Hypermetabolism Following Moderate to Severe Traumatic Acute Brain Injury: A Systematic Review. J. Neurotrauma 2008, 25, 1415–1431. [Google Scholar] [CrossRef]
- Munivenkatappa, A.; Agrawal, A. Role of Thalamus in Recovery of Traumatic Brain Injury. J. Neurosci. Rural. Pract. 2016, 07, S076–S079. [Google Scholar] [CrossRef]
- Jacob, Y.; Morris, L.S.; Verma, G.; Rutter, S.B.; Balchandani, P.; Murrough, J.W. Altered Hippocampus and Amygdala Subregion Connectome Hierarchy in Major Depressive Disorder. Transl. Psychiatry 2022, 12, 209. [Google Scholar] [CrossRef]
- Roddy, D.; Kelly, J.R.; Farrell, C.; Doolin, K.; Roman, E.; Nasa, A.; Frodl, T.; Harkin, A.; O’Mara, S.; O’Hanlon, E.; et al. Amygdala Substructure Volumes in Major Depressive Disorder. Neuroimage Clin. 2021, 31, 102781. [Google Scholar] [CrossRef] [PubMed]
- Bringas Vega, M.L.; Pedroso Ibáñez, I.; Razzaq, F.A.; Zhang, M.; Morales Chacón, L.; Ren, P.; Galan Garcia, L.; Gan, P.; Virues Alba, T.; Lopez Naranjo, C.; et al. The Effect of Neuroepo on Cognition in Parkinson’s Disease Patients Is Mediated by Electroencephalogram Source Activity. Front. Neurosci. 2022, 16, 841428. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mason, S.; Lecky, F.; Dawson, J. Prevalence of Depression after TBI in a Prospective Cohort: The SHEFBIT Study. Brain Inj. 2018, 32, 84–90. [Google Scholar] [CrossRef]
- Raghupathi, R.; Graham, D.I.; MCINTOSH, T.K. Apoptosis After Traumatic Brain Injury. J. Neurotrauma 2000, 17, 927–938. [Google Scholar] [CrossRef]
- Fernando, G.; Yamila, R.; Cesar, G.; Ramón, R. Neuroprotective Effects of NeuroEPO Using an In Vitro Model of Stroke. Behav. Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Brines, M.; Cerami, A. The Receptor That Tames the Innate Immune Response. Mol. Med. 2012, 18, 486–496. [Google Scholar] [CrossRef]
- Gao, Y.; Mengana, Y.; Cruz, Y.R.; Muñoz, A.; Testé, I.S.; García, J.D.; Wu, Y.; Rodríguez, J.C.G.; Zhang, C. Different Expression Patterns of Ngb and EPOR in the Cerebral Cortex and Hippocampus Revealed Distinctive Therapeutic Effects of Intranasal Delivery of Neuro-EPO for Ischemic Insults to the Gerbil Brain. J. Histochem. Cytochem. 2011, 59, 214–227. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Drug Delivery to the Brain via the Nasal Route of Administration: Exploration of Key Targets and Major Consideration Factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef]
- Kleinridders, A.; Ferris, H.A.; Reyzer, M.L.; Rath, M.; Soto, M.; Manier, M.L.; Spraggins, J.; Yang, Z.; Stanton, R.C.; Caprioli, R.M.; et al. Regional Differences in Brain Glucose Metabolism Determined by Imaging Mass Spectrometry. Mol. Metab. 2018, 12, 113–121. [Google Scholar] [CrossRef]
- Gribnau, A.; van Zuylen, M.L.; Coles, J.P.; Plummer, M.P.; Hermanns, H.; Hermanides, J. Cerebral Glucose Metabolism Following TBI: Changes in Plasma Glucose, Glucose Transport and Alternative Pathways of Glycolysis—A Translational Narrative Review. Int. J. Mol. Sci. 2024, 25, 2513. [Google Scholar] [CrossRef]
| Markers | Experimental Groups | Side | Cerebral Structures | ||||
|---|---|---|---|---|---|---|---|
| Cortex | Hippocampus | Amygdala | Thalamus | Hypothalamus | |||
| DCF (ng DCF formed/mg of protein/h | Sham+vehicle n = 6 | Ipsi- | 2822 ± 578.8 | 3092 ± 549.9 | 4390 ± 2010 | 1963 ± 226.3 | 1565 ± 333.8 |
| Contra- | 4044 ± 756.3 | 3903 ± 847.9 | 4055 ± 1641 | ||||
| Sham+NeuroEPO n = 6 | Ipsi- | 4561 ± 1562 | 4080 ± 972.3 | 4262 ± 1809 | 3393 ± 1204 | 3993 ± 1594 | |
| Contra- | 4824 ± 1218 | 3633 ± 725.8 | 5718 ± 2153 | ||||
| TBI+vehicle n = 6 | Ipsi- | 11,150 ± 1253 ** | 11,331 ± 1267 **** | 13,928 ± 1899 ** | 3393 ± 1204 *** | 10,392 ± 1248 **** | |
| Contra- | 14,051 ± 3171 *** | 12,272 ± 1754 **** | 14,384 ± 1588 *** | ||||
| TBI+NeuroEPO n = 6 | Ipsi- | 3746 ± 976.2 ## | 4737 ± 1142 ### | 11,851 ± 865.7 * | 4863 ± 1873 # | 4444 ± 2076 ### | |
| Contra- | 5411 ± 1331 ## | 4251 ± 1040 #### | 10,687 ± 1810 | ||||
| Catalase (U/mL/mg of protein) | Sham+vehicle n = 6 | Ipsi- | 3.72 ± 1.20 | 7.36 ± 1.91 | 13.42 ± 2.10 | 5.26 ± 0.80 | 5.48 ± 1.94 |
| Contra- | 4.90 ± 1.34 | 6.16 ± 1.87 | 13.74 ± 2.16 | ||||
| Sham+NeuroEPO n = 6 | Ipsi- | 3.15 ± 1.20 | 5.15 ± 0.85 | 8.92 ± 0.48 | 5.17 ± 1.75 | 6.34 ± 1.33 | |
| Contra- | 3.11 ± 0.52 | 5.10 ± 0.57 | 13.53 ± 3.35 | ||||
| TBI+vehicle n = 6 | Ipsi- | 30.53 ± 8.97 ** | 25.13 ± 9.27 ** | 40.88 ± 3.26 *** | 37.48 ± 9.50 **** | 43.83 ± 6.71 **** | |
| Contra- | 27.43 ± 9.37 ** | 20.23 ± 4.97 ** | 30.45 ± 6.67 * | ||||
| TBI+NeuroEPO n = 6 | Ipsi- | 3.86 ± 1.01 ## | 8.18 ± 3.21 ## | 25.06 ± 6.93 | 6.29 ± 1.54 #### | 10.72 ± 2.06 #### | |
| Contra- | 2.99 ± 0.22 ## | 6.95 ± 1.41 ## | 27.10 ± 4.62 | ||||
| MDA (nmoles of MDA/mg of protein) | Sham+vehicle n = 6 | Ipsi- | 167.7 ± 12.60 | 151.2 ± 13.31 | 142.1 ± 8.84 | 72.66 ± 13.84 | 85.41 ± 14.99 |
| Contra- | 142.5 ± 10.75 | 173.6 ± 31.01 | 159.2 ± 9.88 | ||||
| Sham+NeuroEPO n = 6 | Ipsi- | 172.2 ± 9.85 | 171.0 ± 28.28 | 193.4 ± 34.59 | 102.4 ± 15.17 | 143.8 ± 36.78 | |
| Contra- | 152.6 ± 14.22 | 120.4 ± 7.00 | 155.0 ± 11.19 | ||||
| TBI+vehicle n = 6 | Ipsi- | 554.6 ± 38.2 **** | 850.6 ± 16.09 **** | 418.2 ± 52.03 **** | 213.0 ± 8.01 ** | 369.9 ± 43.09 **** | |
| Contra- | 402.9 ± 50.0 **** | 527.0 ± 86.72 **** | 349.2 ± 47.04 ** | ||||
| TBI+NeuroEPO n = 6 | Ipsi- | 190.3 ± 7.12 #### | 150.4 ± 15.98 #### | 419.0 ± 38.29 **** | 100.1 ± 23.46 # | 160.4 ± 44.73 #### | |
| Contra- | 136.3 ± 12.1 #### | 182.8 ± 19.86 #### | 304.6 ± 59.72 * | ||||
| Structure | Side Evaluated | Experimental Group | |||
|---|---|---|---|---|---|
| Sham+vehicle n = 4 | Sham+NeuroEPO n = 5 | TBI+vehicle n = 6 | TBI+NeuroEPO n = 6 | ||
| Cortex | Ipsi- | 85.3 ± 4.1 | 79.1 ± 2.3 | 65.3 ± 3.2 ** | 84.0 ± 2.0 ### |
| Contra- | 93.1 ± 4.7 | 79.2 ± 4.0 | 63.0 ± 5.4 *** | 80.3 ± 2.8 # | |
| Hippocampus | Ipsi- | 42.3 ± 0.7 | 46.1 ± 6.0 | 29.8 ± 1.5 *** | 44.4 ± 0.6 ### |
| Contra- | 44.0 ± 1.4 | 48.1 ± 6.0 | 35.8 ± 2.9 | 34.4 ± 1.8 | |
| Amygdala | Ipsi- | 23.9 ± 1.3 | 24.1 ± 0.6 | 19.5 ± 0.7 * | 24.1 ± 1.2 ## |
| Contra- | 24.2 ± 1.1 | 24.4 ± 1.1 | 19.4 ± 0.6 * | 23.5 ± 0.9 # | |
| Thalamus | Entire | 11.5 ± 1.0 | 12.8 ± 1.0 | 8.4 ± 0.5 * | 13.7 ± 0.2 ### |
| Hypothalamus | Entire | 20.5 ± 0.1 | 20.5 ± 0.4 | 17.0 ± 0.5 ** | 20.1 ± 0.8 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Preza, F.I.; Nuñez-Lumbreras, M.d.l.A.; Sosa-Testé, I.; Fernández-Guasti, A.; Concha, L.; Rodríguez-Obaya, T.; Rocha, L. Subchronic Intranasal Administration of NeuroEPO Reduces Long-Term Consequences of Severe Traumatic Brain Injury in Male Rats. Antioxidants 2025, 14, 710. https://doi.org/10.3390/antiox14060710
López-Preza FI, Nuñez-Lumbreras MdlA, Sosa-Testé I, Fernández-Guasti A, Concha L, Rodríguez-Obaya T, Rocha L. Subchronic Intranasal Administration of NeuroEPO Reduces Long-Term Consequences of Severe Traumatic Brain Injury in Male Rats. Antioxidants. 2025; 14(6):710. https://doi.org/10.3390/antiox14060710
Chicago/Turabian StyleLópez-Preza, Félix Iván, Maria de los Angeles Nuñez-Lumbreras, Iliana Sosa-Testé, Alonso Fernández-Guasti, Luis Concha, Teresita Rodríguez-Obaya, and Luisa Rocha. 2025. "Subchronic Intranasal Administration of NeuroEPO Reduces Long-Term Consequences of Severe Traumatic Brain Injury in Male Rats" Antioxidants 14, no. 6: 710. https://doi.org/10.3390/antiox14060710
APA StyleLópez-Preza, F. I., Nuñez-Lumbreras, M. d. l. A., Sosa-Testé, I., Fernández-Guasti, A., Concha, L., Rodríguez-Obaya, T., & Rocha, L. (2025). Subchronic Intranasal Administration of NeuroEPO Reduces Long-Term Consequences of Severe Traumatic Brain Injury in Male Rats. Antioxidants, 14(6), 710. https://doi.org/10.3390/antiox14060710






