Cooperative Interaction of Hyaluronic Acid with Epigallocatechin-3-O-gallate and Xanthohumol in Targeting the NF-κB Signaling Pathway in a Cellular Model of Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures and Treatments
2.3. Cell Viability
2.4. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.5. Nitric Oxide (NO) Determination
2.6. Combination Index (CI) Analysis
2.7. ELISA
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
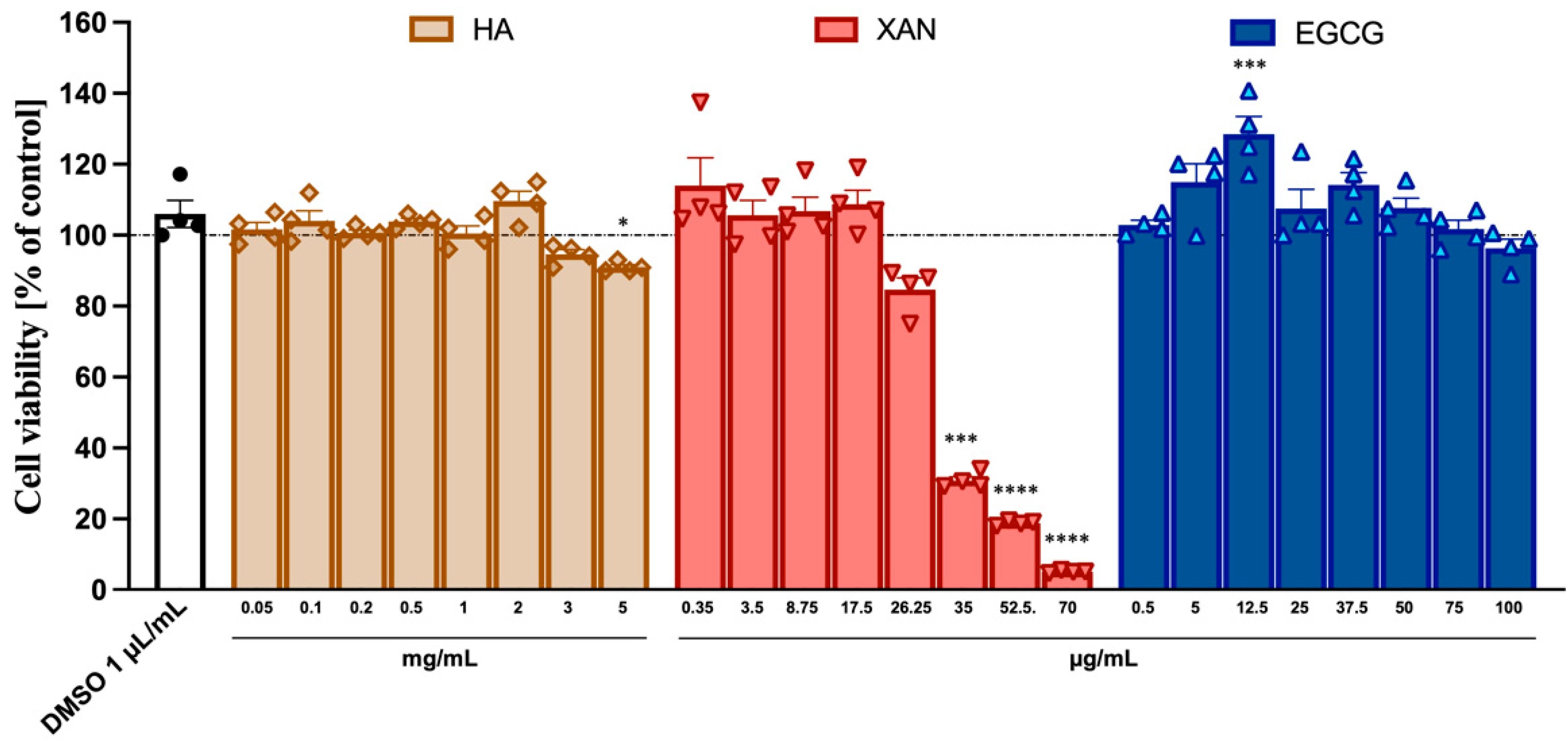
3.1. Cytotoxicity of HA, XAN, and EGCG to SW982
3.2. Effect of HA in Combination with EGCG and/or XAN on IL-1 β-Induced ROS Production and NO Release in SW982 Cells
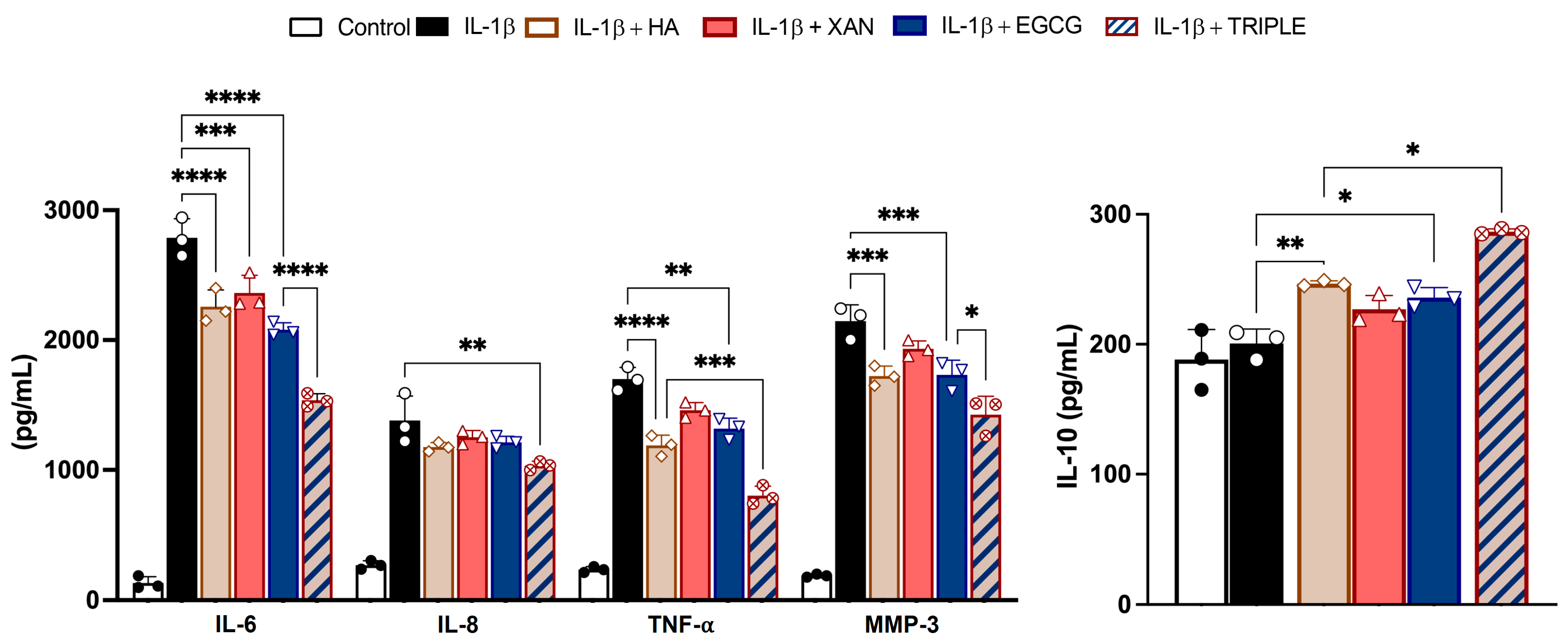
3.3. Effect of HA in Combination with XAN Plus EGCG on Cytokine and Metal Protease Release
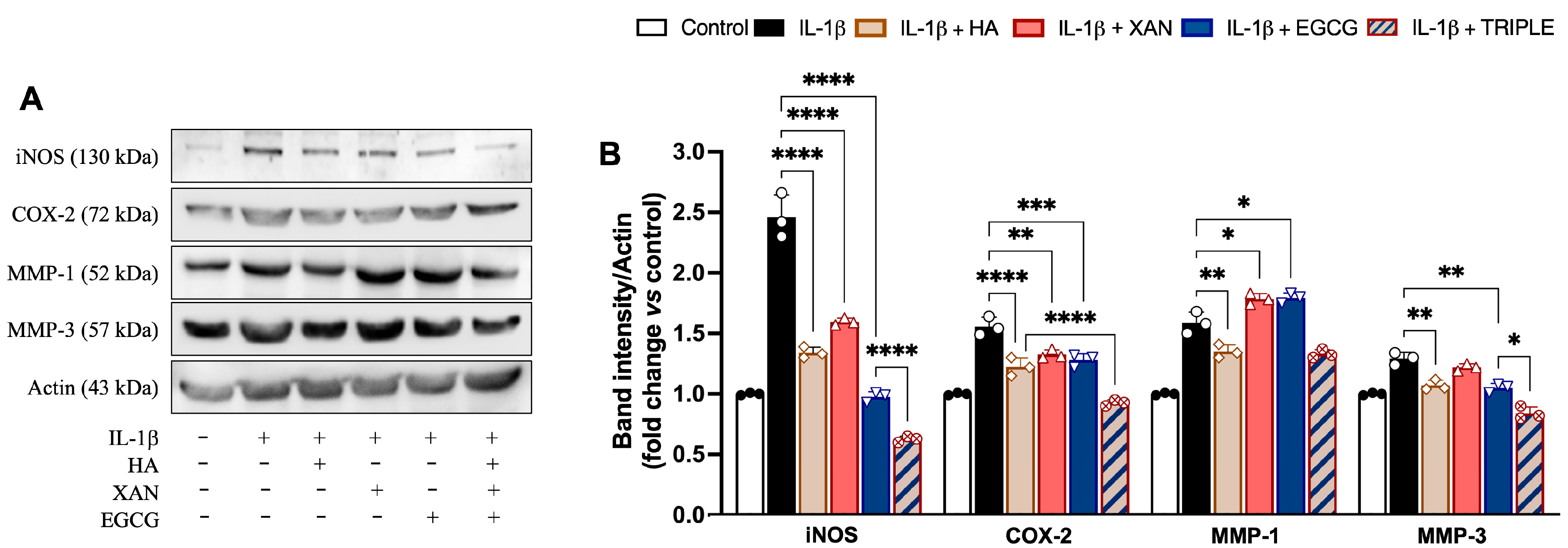
3.4. Effect of HA in Combination with XAN Plus EGCG on Expression of Pro-Inflammatory Enzymes
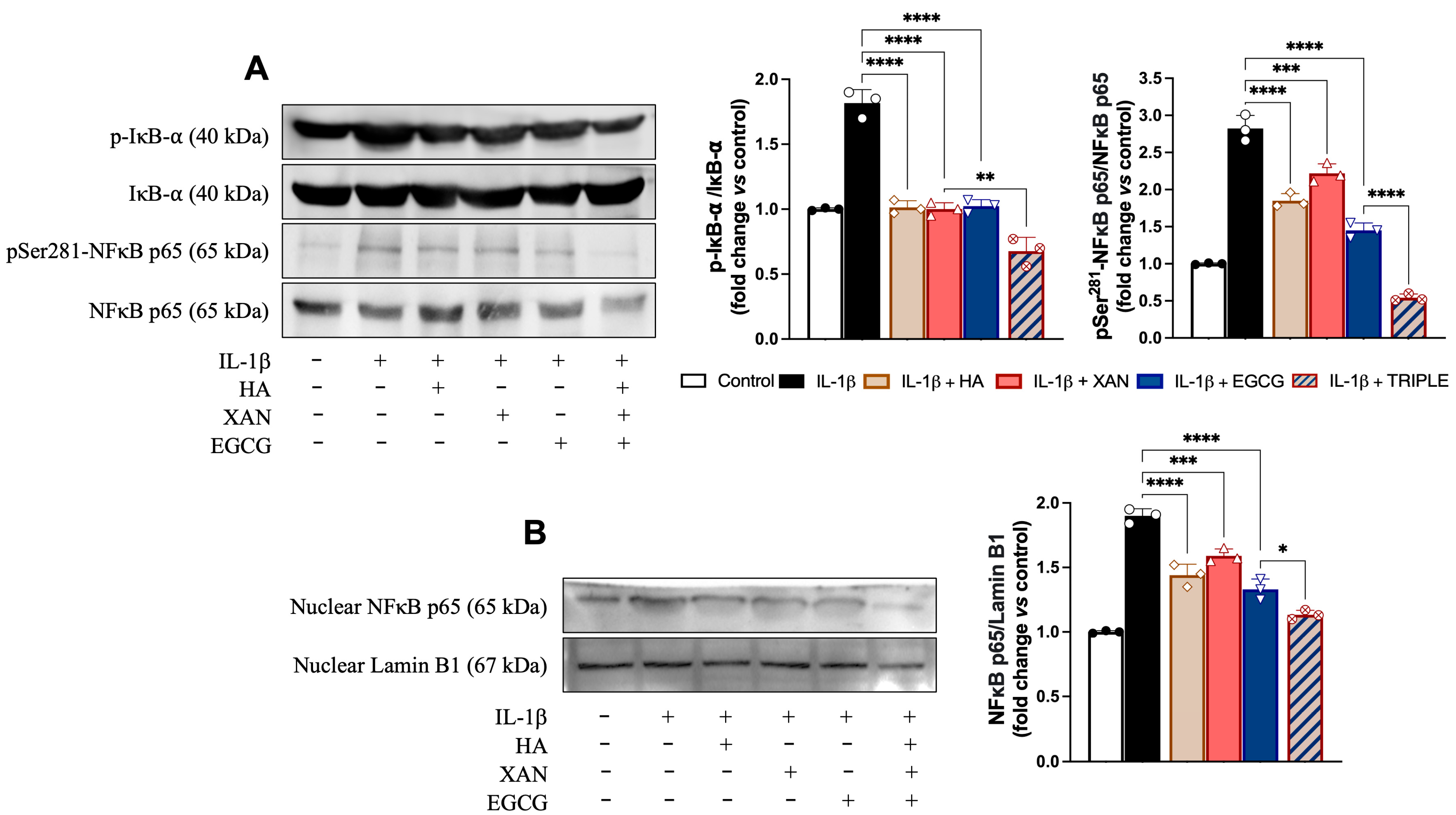
3.5. Effect of HA in Combination with XAN Plus EGCG on Activation of Redox-Dependent Transcription Factor NF-κB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | hyaluronic acid |
| XAN | Xanthohumol |
| EGCG | Epigallocatechin-3-O-Gallate |
| IL-1β | interleukin-1beta |
| ROS | reactive oxygen species |
| NO | nitric oxide |
| TRIPLE | combination of HA, XAN, and EGCG |
| COX-2 | cyclooxygenase-2 |
| iNOS | nitric oxide synthetase |
| MMPs | matrix metalloproteases |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DCFDA | dichloro-dihydro-fluorescein diacetate |
| CI | combination index |
| fa | effect fraction |
| DRI | dose reduction index |
| TTBS | Tween20/Tris-buffered saline |
| SD | standard deviation |
| ANOVA | one-way analysis of variance |
References
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A.R.; Sheehy, E.J.; Jay, G.D.; Scott, W.M.; O’Brien, F.J.; Schmidt, T.A. The role of synovial fluid constituents in the lubrication of collagen-glycosaminoglycan scaffolds for cartilage repair. J. Mech. Behav. Biomed. Mater. 2021, 118, 104445. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.M.; Capra, J.; Rilla, K.; Lehenkari, P.; Oikari, S.; Kääriäinen, T.; Joukainen, A.; Kröger, H.; Paakkonen, T.; Matilainen, J.; et al. Characterization of hyaluronan-coated extracellular vesicles in synovial fluid of patients with osteoarthritis and rheumatoid arthritis. BMC Musculoskelet. Disord. 2021, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Huffer, A.; Mao, M.; Ballard, K.; Ozdemir, T. Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function. Biomimetics 2024, 9, 499. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A novel hyaluronic acid matrix ingredient with regenerative, anti-aging and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 4774. [Google Scholar] [CrossRef]
- Johnson, P.; Arif, A.A.; Lee-Sayer, S.S.M.; Dong, Y. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front. Immunol. 2018, 9, 2787. [Google Scholar] [CrossRef]
- Parnigoni, A.; Viola, M.; Karousou, E.; Vigetti, D.; Giaroni, C.; Rovera, S.; Passi, A. Hyaluronan in pathophysiology of vascular diseases: Specific roles in smooth muscle cells, endothelial cells, and macrophages. Am. J. Physiol. Cell Physiol. 2022, 323, C505–C519. [Google Scholar] [CrossRef]
- Hashizume, M.; Mihara, M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem. Biophys. Res. Commun. 2010, 403, 184–189. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent advances in understanding the role of cartilage lubrication in osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.; Gill, H.; Singh, N. Long-term safety of biologic and targeted synthetic disease modifying drugs in rheumatology. Curr. Opin. Rheumatol. 2024, 36, 113–119. [Google Scholar] [CrossRef]
- Gierut, A.; Perlman, H.; Pope, R.M. Innate immunity and rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2010, 36, 271–296. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Cowman, M.K. Hyaluronan and hyaluronan fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59. [Google Scholar] [CrossRef]
- Ghosh, S.; Hoselton, S.A.; Dorsam, G.P.; Schuh, J.M. Hyaluronan fragments as mediators of inflammation in allergic pulmonary disease. Immunobiology 2015, 220, 575–588. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The Role of IL-1β in the Bone Loss during Rheumatic Diseases. Mediat. Inflamm. 2015, 2015, 782382. [Google Scholar] [CrossRef]
- Ouyang, Z.; Dong, L.; Yao, F.; Wang, K.; Chen, Y.; Li, S.; Zhou, R.; Zhao, Y.; Hu, W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int. J. Mol. Sci. 2023, 24, 9841. [Google Scholar] [CrossRef]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: A review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Paoletta, M.; Moretti, A.; Liguori, S.; Iolascon, G. The perspectives of intra-articular therapy in the management of osteoarthritis. Expert Opin. Drug Deliv. 2020, 17, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Patel, R.; Vannabouathong, C.; Sales, B.; Rabinovich, A.; McCormack, R.; Belzile, E.L.; Bhandari, M. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1974–1983. [Google Scholar] [CrossRef]
- Miller, L.E.; Fredericson, M.; Altman, R.D. Hyaluronic Acid Injections or Oral Nonsteroidal Anti-inflammatory Drugs for Knee Osteoarthritis: Systematic Review and Meta-analysis of Randomized Trials. Orthop. J. Sports Med. 2020, 8, 2325967119897909. [Google Scholar] [CrossRef]
- Toropitsyn, E.; Pravda, M.; Rebenda, D.; Ščigalková, I.; Vrbka, M.; Velebný, V. A composite device for viscosupplementation treatment resistant to degradation by reactive oxygen species and hyaluronidase. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2595–2611. [Google Scholar] [CrossRef]
- Kamel, S.I.; Rosas, H.G.; Gorbachova, T. Local and Systemic Side Effects of Corticosteroid Injections for Musculoskeletal Indications. AJR Am. J. Roentgenol. 2024, 222, e2330458. [Google Scholar] [CrossRef]
- Russell, S.J.; Sala, R.; Conaghan, P.G.; Habib, G.; Vo, Q.; Manning, R.; Kivitz, A.; Davis, Y.; Lufkin, J.; Johnson, J.R.; et al. Triamcinolone acetonide extended-release in patients with osteoarthritis and type 2 diabetes: A randomized, phase 2 study. Rheumatology 2018, 57, 2235–2241. [Google Scholar] [CrossRef]
- Zhu, W.; Tang, H.; Cao, L.; Guo, C.; Ma, D.; Li, J.; Zhang, J. Epigallocatechin-3-O-gallate ameliorates oxidative stress-induced chondrocyte dysfunction and exerts chondroprotective effects via the Keap1/Nrf2/ARE signaling pathway. Chem. Biol. Drug Des. 2022, 100, 108–120. [Google Scholar] [CrossRef]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Cheng, T.L.; Yang, C.D.; Chang, C.F.; Ho, C.J.; Chuang, S.C.; Li, J.-Y.; Huang, S.-H.; Lin, Y.-S.; Shen, H.-Y.; et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharmacother. 2021, 137, 111238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, R.; Zheng, T.; Chen, Z.; Ji, G.; Peng, F.; Wang, W. Xanthohumol Attenuated Inflammation and ECM Degradation by Mediating HO-1/C/EBPβ Pathway in Osteoarthritis Chondrocytes. Front. Pharmacol. 2021, 12, 680585. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, T.; Shuqing, Z.; Yu, L.; Chen, S.; Lu, H.; Zhu, H.; Min, X.; Li, X.; Liu, L. Xanthohumol relieves arthritis pain in mice by suppressing mitochondrial-mediated inflammation. Mol. Pain 2023, 19, 17448069231204051. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yokoyama, T.; Akatsu, H.; Tukiyama, T.; Tokiwa, T. Phenotypic characterization of a human synovial sarcoma cell line, SW982, and its response to dexamethasone. Vitr. Cell Dev. Biol. Anim. 2003, 39, 337–339. [Google Scholar] [CrossRef]
- Kannan, G.; Paul, B.M.; Thangaraj, P. Stimulation, regulation, and inflammaging interventions of natural compounds on nuclear factor kappa B (NF-κB) pathway: A comprehensive review. Inflammopharmacology 2025, 33, 145–162. [Google Scholar] [CrossRef]
- Di Prima, G.; Belfiore, E.; Migliore, M.; Scarpaci, A.G.; Angellotti, G.; Restivo, I.; Allegra, M.; Arizza, V.; De Caro, V. Green Extraction of Polyphenols from Waste Bentonite to Produce Functional Antioxidant Excipients for Cosmetic and Pharmaceutical Purposes: A Waste-to-Market Approach. Antioxidants 2022, 11, 2493. [Google Scholar] [CrossRef]
- Restivo, I.; Tesoriere, L.; Frazzitta, A.; Livrea, M.A.; Attanzio, A.; Allegra, M. Anti-Proliferative Activity of A Hydrophilic Extract of Manna from Fraxinus angustifolia Vahl through Mitochondrial Pathway-Mediated Apoptosis and Cell Cycle Arrest in Human Colon Cancer Cells. Molecules 2020, 25, 5055. [Google Scholar] [CrossRef]
- Restivo, I.; Basilicata, M.G.; Giardina, I.C.; Massaro, A.; Pepe, G.; Salviati, E.; Pecoraro, C.; Carbone, D.; Cascioferro, S.; Parrino, B.; et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants 2023, 12, 1621. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; D’Anneo, A.; Frazzitta, A.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Restivo, I. The Phytochemical Indicaxanthin Synergistically Enhances Cisplatin-Induced Apoptosis in HeLa Cells via Oxidative Stress-Dependent p53/p21^waf1 Axis. Biomolecules 2020, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Restivo, I.; Attanzio, A.; Tesoriere, L.; Allegra, M.; Garcia-Llatas, G.; Cilla, A. A Mixture of Dietary Plant Sterols at Nutritional Relevant Serum Concentration Inhibits Extrinsic Pathway of Eryptosis Induced by Cigarette Smoke Extract. Int. J. Mol. Sci. 2023, 24, 1264. [Google Scholar] [CrossRef]
- Lee, F.; Bae, K.H.; Ng, S.; Yamashita, A.; Kurisawa, M. Hyaluronic acid–green tea catechin conjugates as a potential therapeutic agent for rheumatoid arthritis. RSC Adv. 2021, 11, 14285–14294. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Liu, J.; Jiao, H.; Liu, B. Anti-inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl. Mater. Interfaces 2018, 10, 31138–31149. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, C.; Shao, A.; Wu, L.; Hu, H.; Zhang, T. Emerging interleukin-1 receptor-associated kinase 4 (IRAK4) inhibitors or degraders as therapeutic agents for autoimmune diseases and cancer. Acta Pharm. Sin. B 2024, 14, 5091–5105. [Google Scholar] [CrossRef]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef]
- Shimizu, M.; Yasuda, T.; Nakagawa, T.; Yamashita, E.; Hiramitsu, T.; Nakamura, T.; Julovi, S.M. Hyaluronan inhibits matrix metalloproteinase-1 production by rheumatoid synovial fibroblasts stimulated by proinflammatory cytokines. J. Rheumatol. 2003, 30, 1164–1172. [Google Scholar]
- Hiramitsu, T.; Yasuda, T.; Ito, H.; Shimizu, M.; Julovi, S.M.; Kakinuma, T.; Akiyoshi, M.; Yoshida, M.; Nakamura, T. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1β-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-κB and p38. Rheumatology 2006, 45, 824–832. [Google Scholar] [CrossRef]
- Lee, C.H.; Chiang, C.F.; Kuo, F.C.; Su, S.C.; Huang, C.L.; Liu, J.S.; Lu, C.-H.; Hsieh, C.-H.; Wang, C.-C.; Lee, C.-H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cervantes, G.I.; Ortega, D.R.; Blanco Ayala, T.; Pérez de la Cruz, V.; Esquivel, D.F.G.; Salazar, A.; Pineda, B. Redox and Anti-Inflammatory Properties from Hop Components in Beer-Related to Neuroprotection. Nutrients 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.R.; Miranda, C.L.; Hobbs, D.J.; Proteau, R.R.; Stevens, J.F. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: Structure–activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2). Planta Med. 2010, 76, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Kunnumakkara, A.B.; Ahn, K.S.; Anand, P.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood 2009, 113, 2003–2013. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Won, H.R.; Lee, P.; Oh, S.R.; Kim, Y.M. Epigallocatechin-3-gallate suppresses the expression of TNF-α-induced MMP-1 via MAPK/ERK signaling pathways in human dermal fibroblasts. Biol. Pharm. Bull. 2021, 44, 18–24. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natarajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrix metalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar]
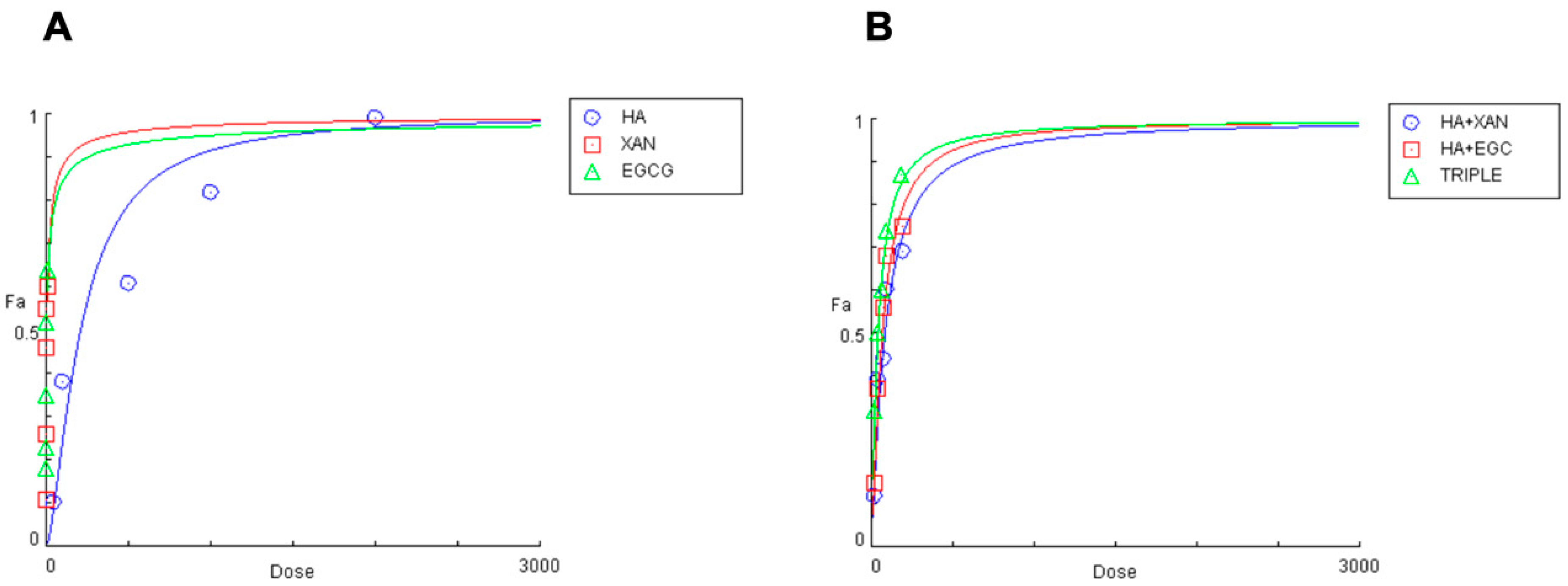
| Compound | IC50 | CI Values | DRI Value | DRI Value | DRI Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combo with XAN | Combo with EGCG | Combo with XAN Plus EGCG | |||||||||||
| mg/mL | fα0.25 | fα0.5 | fα0.75 | fα0.25 | fα0.5 | fα0.75 | fα0.25 | fα0.5 | fα0.75 | fα0.25 | fα0.5 | fα0.75 | |
| HA | 208.66 | 3.27 | 2.62 | 2.11 | 3.77 | 3.24 | 2.78 | 4.34 | 5.56 | 7.12 | |||
| XAN | 6.17 | ||||||||||||
| EGCG | 4.59 | ||||||||||||
| HA:XAN | 81.89 | 1.00 | 0.77 | 0.69 | |||||||||
| (33:1) | |||||||||||||
| HA:EGCG | 65.80 | 1.21 | 0.62 | 0.46 | |||||||||
| (50:1) | |||||||||||||
| HA:XAN:EGCG | 39.40 | 0.88 | 0.52 | 0.39 | |||||||||
| TRIPLE (50:1.5:1) | |||||||||||||
| Compound | IC50 | CI Values | DRI Value | DRI Value | DRI Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combo with XAN | Combo with EGCG | Combo with XAN Plus EGCG | |||||||||||
| mg/mL | fα0.25 | fα0.5 | fα0.75 | fα0.25 | fα0.5 | fα 0.75 | fα0.25 | fα0.5 | fα0.75 | fα0.25 | fα0.5 | fα0.75 | |
| HA | 222.64 | 2.11 | 3.08 | 4.51 | 2.31 | 2.87 | 3.54 | 3.81 | 5.77 | 8.75 | |||
| XAN | 5.08 | ||||||||||||
| EGCG | 3.40 | ||||||||||||
| HA:XAN | 74.28 | 1.04 | 0.75 | 0.54 | |||||||||
| (33:1) | |||||||||||||
| HA:EGCG | 79.44 | 1.31 | 0.85 | 0.57 | |||||||||
| (50:1) | |||||||||||||
| HA:XAN:EGCG | 40.51 | 1.01 | 0.62 | 0.39 | |||||||||
| TRIPLE (50:1.5:1) | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, F.; Massaro, A.; Mauro, M.; Allegra, M.; Arizza, V.; Tesoriere, L.; Restivo, I. Cooperative Interaction of Hyaluronic Acid with Epigallocatechin-3-O-gallate and Xanthohumol in Targeting the NF-κB Signaling Pathway in a Cellular Model of Rheumatoid Arthritis. Antioxidants 2025, 14, 713. https://doi.org/10.3390/antiox14060713
Longo F, Massaro A, Mauro M, Allegra M, Arizza V, Tesoriere L, Restivo I. Cooperative Interaction of Hyaluronic Acid with Epigallocatechin-3-O-gallate and Xanthohumol in Targeting the NF-κB Signaling Pathway in a Cellular Model of Rheumatoid Arthritis. Antioxidants. 2025; 14(6):713. https://doi.org/10.3390/antiox14060713
Chicago/Turabian StyleLongo, Francesco, Alessandro Massaro, Manuela Mauro, Mario Allegra, Vincenzo Arizza, Luisa Tesoriere, and Ignazio Restivo. 2025. "Cooperative Interaction of Hyaluronic Acid with Epigallocatechin-3-O-gallate and Xanthohumol in Targeting the NF-κB Signaling Pathway in a Cellular Model of Rheumatoid Arthritis" Antioxidants 14, no. 6: 713. https://doi.org/10.3390/antiox14060713
APA StyleLongo, F., Massaro, A., Mauro, M., Allegra, M., Arizza, V., Tesoriere, L., & Restivo, I. (2025). Cooperative Interaction of Hyaluronic Acid with Epigallocatechin-3-O-gallate and Xanthohumol in Targeting the NF-κB Signaling Pathway in a Cellular Model of Rheumatoid Arthritis. Antioxidants, 14(6), 713. https://doi.org/10.3390/antiox14060713











