Enhancing the Nutritional and Health-Related Properties of Taralli Through the Use of Pleurotus eryngii: Focus on Antioxidant and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Dough Preparation
2.3. Chemical and Nutritional Analyses of Taralli Samples
2.3.1. Proximate Composition of Samples
2.3.2. Sample Extract Preparation
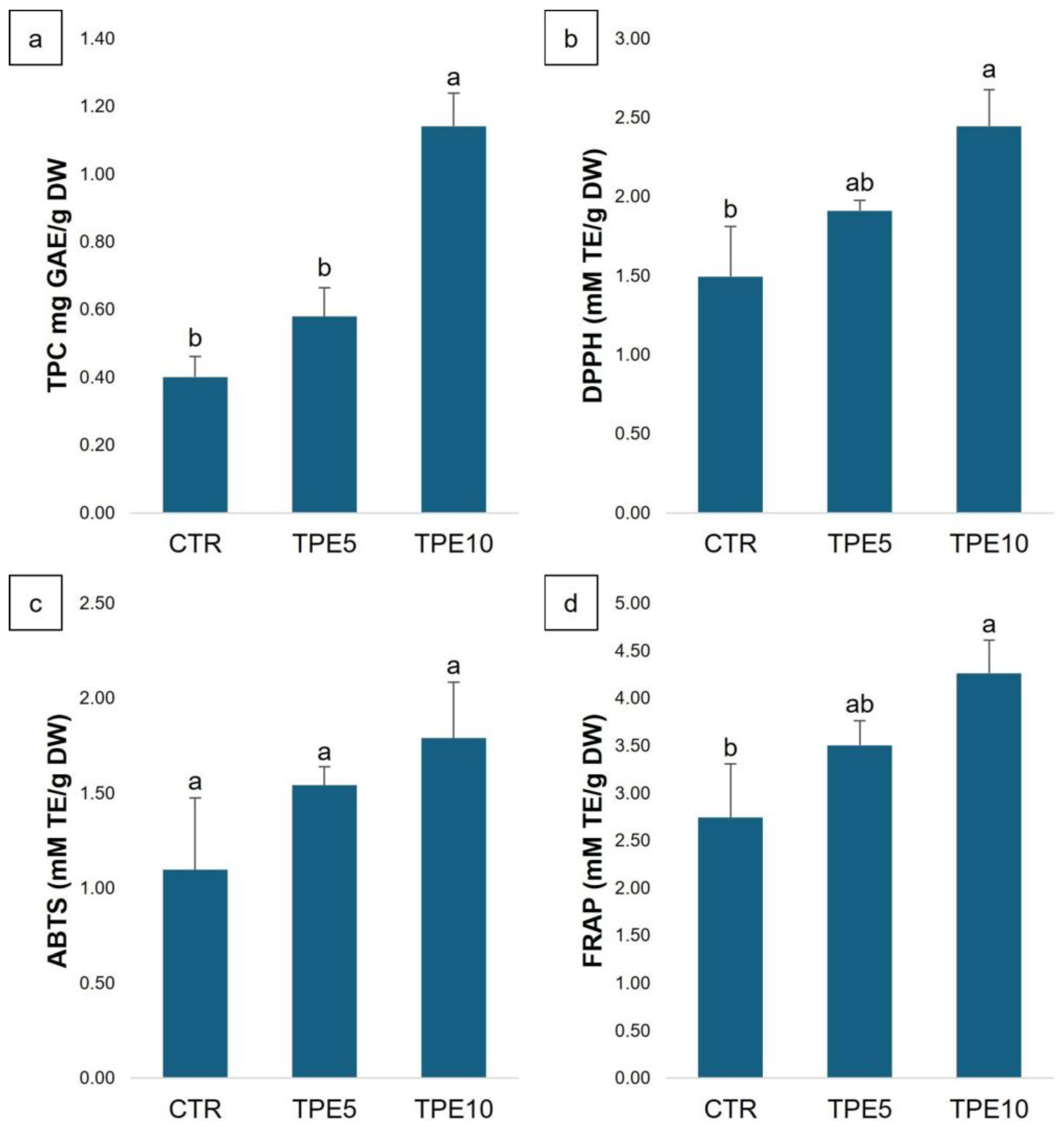
2.3.3. Total Phenol Content and Antioxidant Activity
2.3.4. Determination of β-Glucan Content
2.4. In Vitro Starch Hydrolysis and Predicted Glycaemic Index
2.5. In Vitro Gastrointestinal Digestion of Taralli
2.6. Colour and Texture Analyses
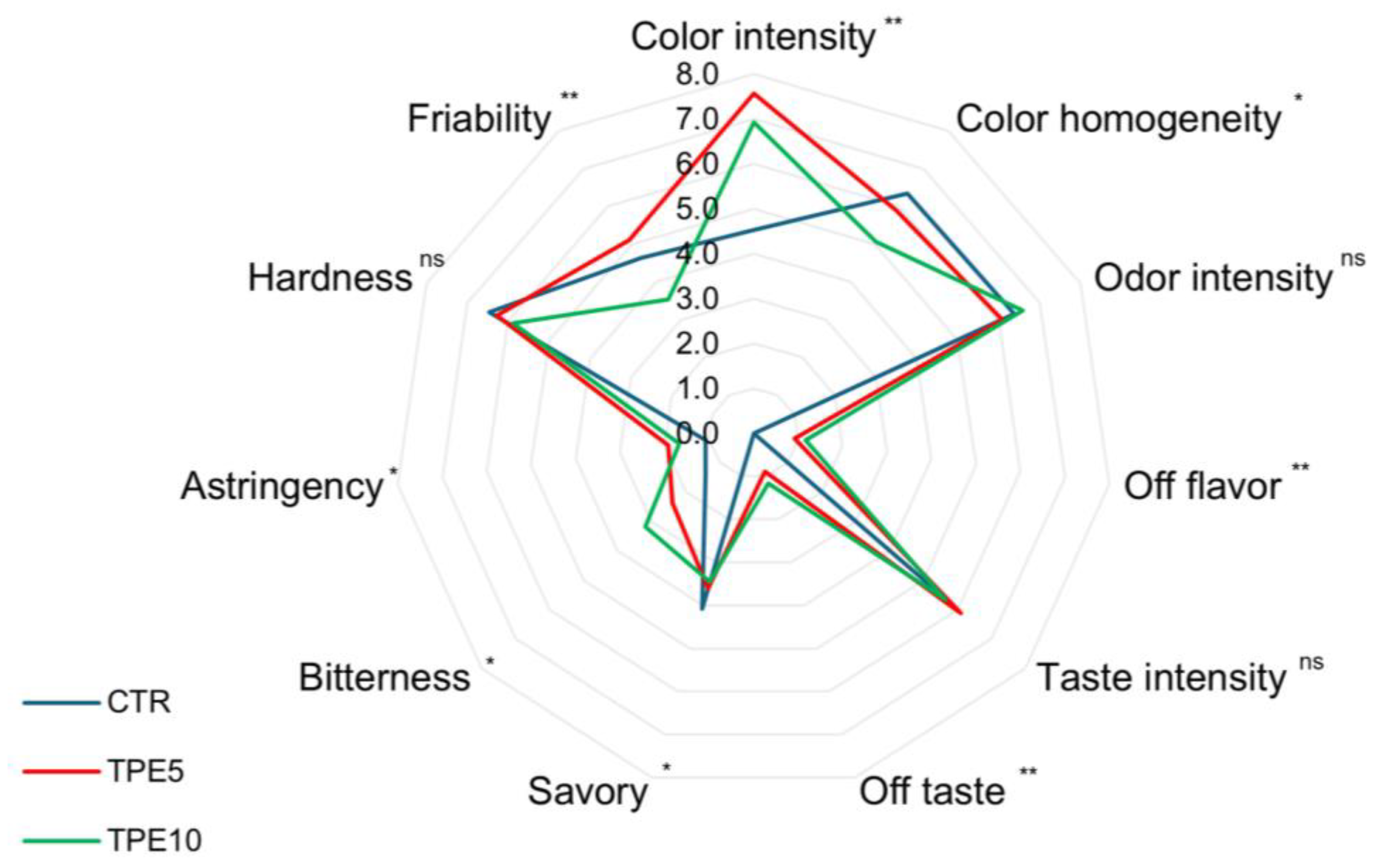
2.7. Sensory Evaluation
2.8. In Vitro Assays of Digested Samples on Cell Cultures
2.8.1. Cell Culture and Treatments
2.8.2. Antibodies
2.8.3. Calcein-AM Cell Viability Assay
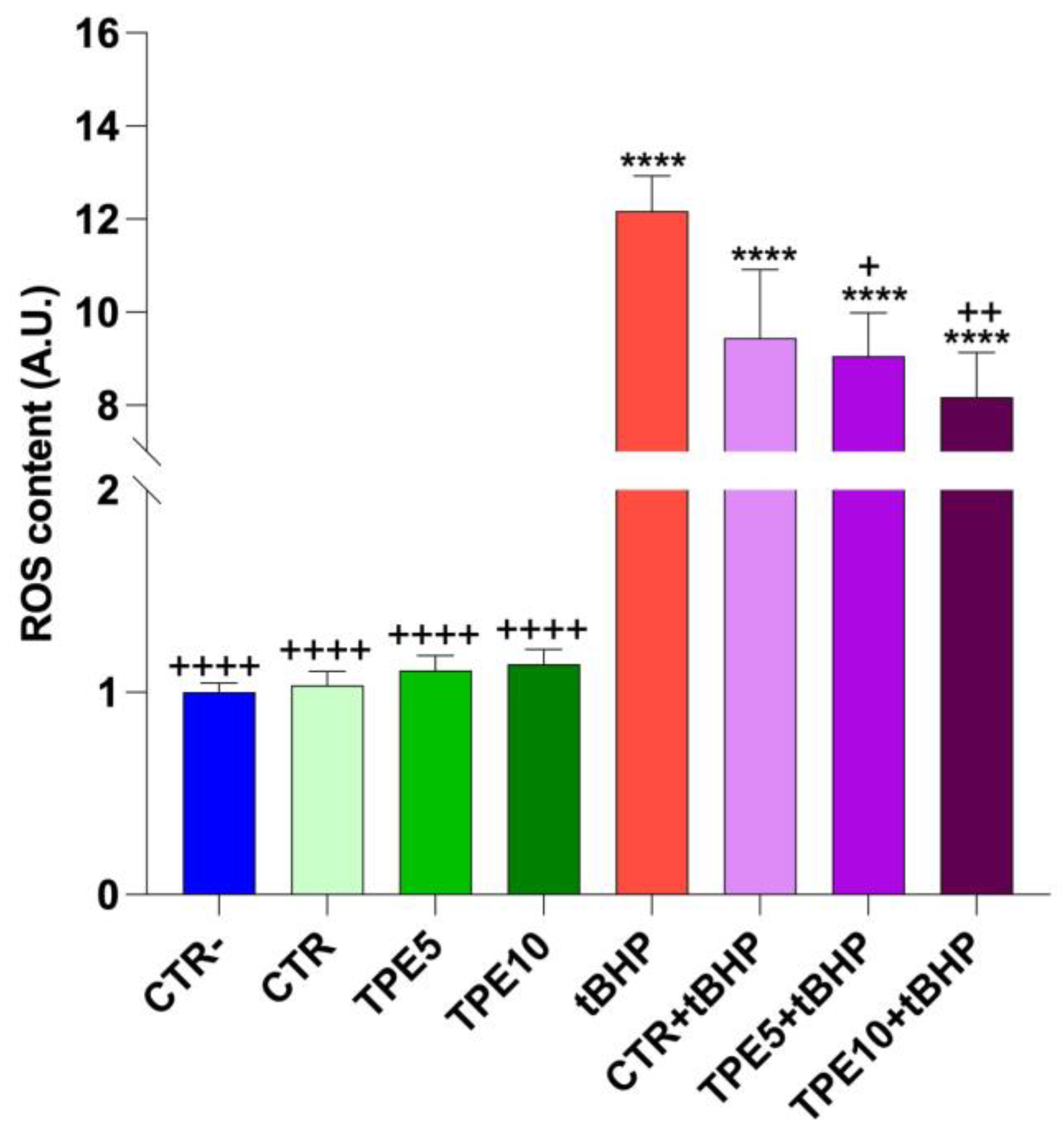
2.8.4. ROS Detection
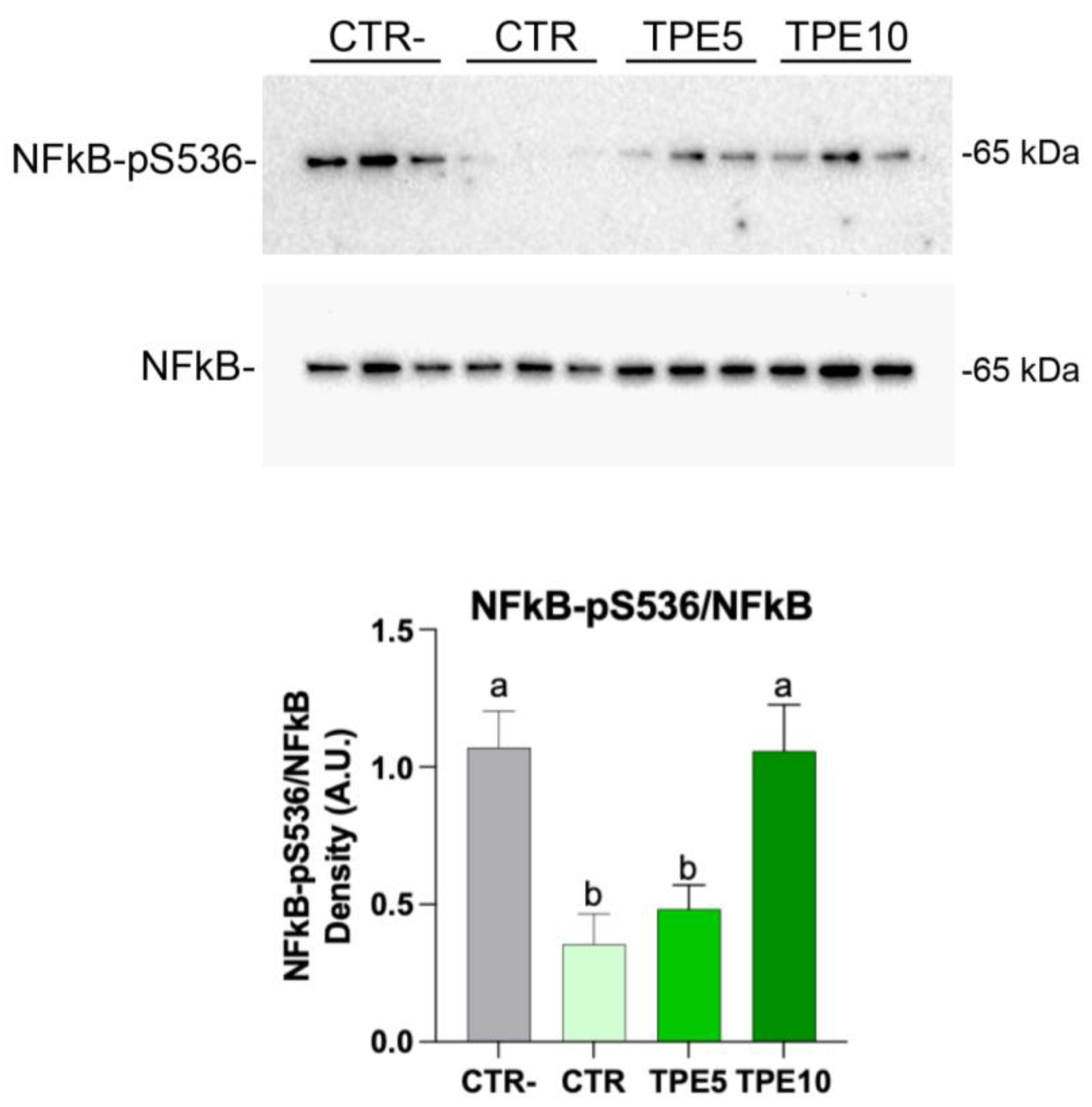
2.8.5. Cell Lysates and Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Chemical and Nutritional Characterization of Taralli Samples
3.1.1. Nutritional Composition
3.1.2. Phenolic Compounds and Antioxidant Activity
3.2. In Vitro Starch Hydrolysis and Predicted Glycemic Index
3.3. Texture and Color Profile of Taralli Samples
3.4. Sensory Analysis
3.5. In Vitro Characterization of Samples on Cell Cultures
3.5.1. Cell Viability and ROS Detection
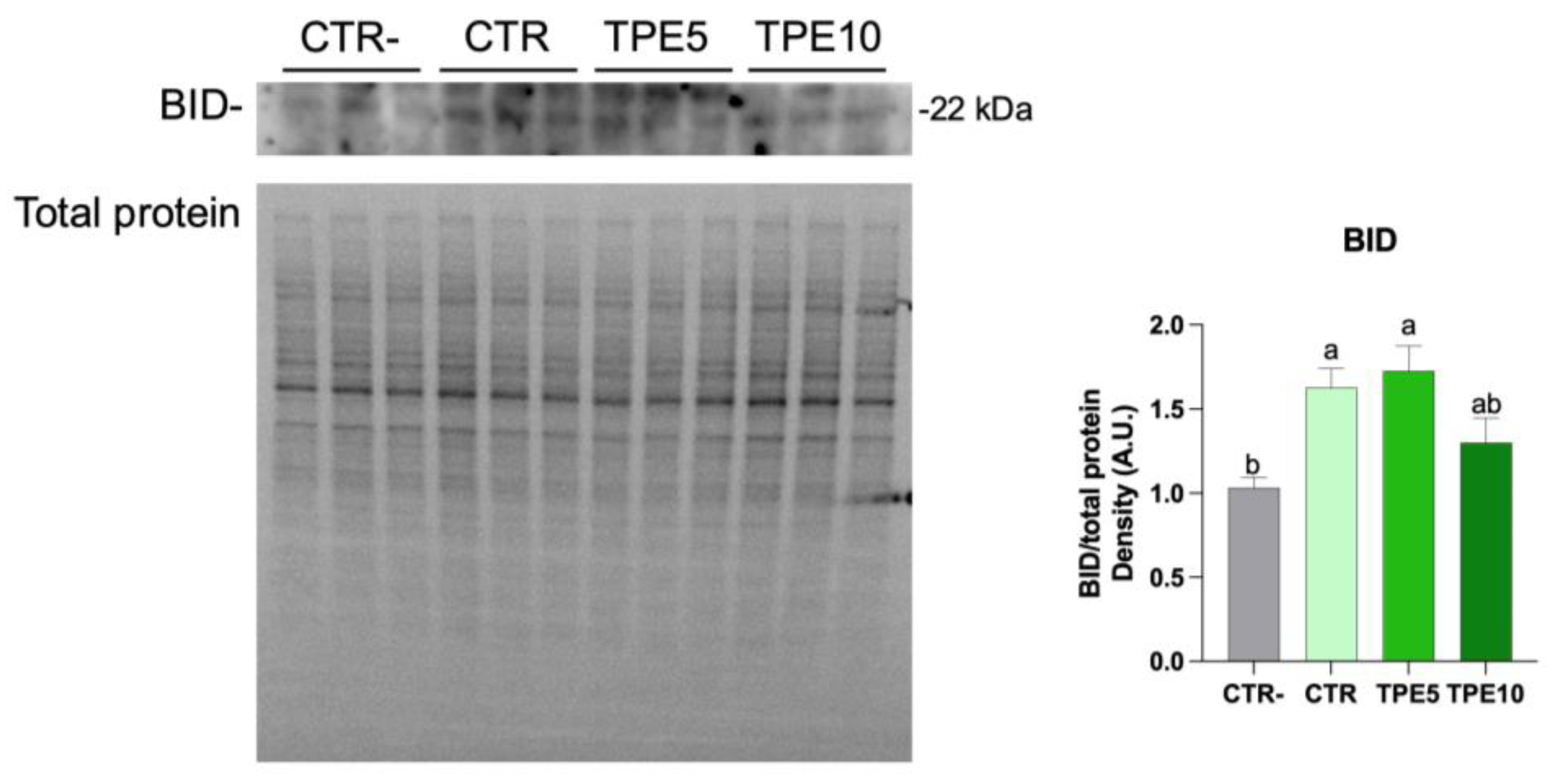
3.5.2. Western Blotting Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PeP | Pleurotus eryngii powder |
| ROS | Reactive oxygen species |
| TPA | textural profile analysis |
| TPC | total phenol content |
| GAE | Gallic acid equivalents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric reducing antioxidant power |
| TE | Trolox equivalents |
| HI | hydrolysis index |
| pGI | predicted glycaemic index |
| GP | gastric phase |
| DP | duodenal phase |
| BI | brown index |
| NFkB | nuclear factor kappa B |
| NFkB-pS536 | phospho-nuclear factor kappa B at serine 536 |
| BID | BH3 Interacting Domain Death Agonist |
| tBHP | tert-butyl hydroperoxide |
| SD | standard deviation |
| ISO | International Organization for Standardization |
| GI | glycemic index |
| TNF-α | Tumor necrosis factor-α |
| IL-1 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| Bcl-2 | B cell CLL/lymphoma-2 |
References
- Keservani, R.K.; Ahire, E.D. Applications of Functional Foods in Disease Prevention, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Brennan, M.; Brennan, C.; Qin, Y.; Cheng, G.; Liu, Y. Physical, Chemical, Sensorial Properties and in Vitro Digestibility of Wheat Bread Enriched with Yunnan Commercial and Wild Edible Mushrooms. LWT 2022, 169, 113923. [Google Scholar] [CrossRef]
- Sławińska, A.; Sołowiej, B.G.; Radzki, W.; Fornal, E. Wheat Bread Supplemented with Agaricus Bisporus Powder: Effect on Bioactive Substances Content and Technological Quality. Foods 2022, 11, 3786. [Google Scholar] [CrossRef] [PubMed]
- Cirlincione, F.; Venturella, G.; Gargano, M.L.; Ferraro, V.; Gaglio, R.; Francesca, N.; Rizzo, B.A.; Russo, G.; Moschetti, G.; Settanni, L.; et al. Functional Bread Supplemented with Pleurotus eryngii Powder: A Potential New Food for Human Health. Int. J. Gastron. Food Sci. 2022, 27, 100449. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Oludemi, T.; Fernandes, Â.; Ferreira, I.C.F.R.; Barros, L. Chapter 5: Bioactive Properties of Mushrooms with Potential Health Benefits. In Edible Fungi: Chemical Composition, Nutrition and Health Effects; Stojković, D., Barros, L., Eds.; The Royal Society of Chemistry: London, UK, 2022; pp. 161–231. [Google Scholar]
- Gargano, M.L.; Balenzano, G.; Venturella, G.; Cavalluzzi, M.M.; Rotondo, N.P.; Lentini, G.; Cirlincione, F.; Mirabile, G.; Zapora, E.; Wołkowycki, M.; et al. Nutritional Contents and Antimicrobial Activity of the Culinary-Medicinal Mushroom Leccinum Scabrum. Mycology 2025, 16, 402–412. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional Foods Based on Extracts or Compounds Derived from Mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Barbieri, S.; Bendini, A.; Balestra, F.; Palagano, R.; Rocculi, P.; Gallina Toschi, T. Sensory and Instrumental Study of Taralli, a Typical Italian Bakery Product. Eur. Food Res. Technol. 2018, 244, 73–82. [Google Scholar] [CrossRef]
- Pagani, M.A.; Lucisano, M.; Mariotti, M. Traditional Italian Products from Wheat and Other Starchy Flours. In Handbook of Food Products Manufacturing. Principles, Bakery, Beverages, Cereals, Cheese, Confectionery, Fats, Fruits, and Functional Foods; Hui, Y.H., Ed.; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2007; pp. 327–388. [Google Scholar]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant activity of polysaccharides from the edible mushroom Pleurotus eryngii. Molecules 2023, 28, 2176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, J.; Fu, Q.; Fu, X.; Shu, T.; Bi, Y.; Song, B. Antitumor Activity of a Polysaccharide from Pleurotus Eryngii on Mice Bearing Renal Cancer. Carbohydr. Polym. 2013, 95, 615–620. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Muinde Kimatu, B.; Yang, W. Polyphenols-Rich Extract from Pleurotus eryngii with Growth Inhibitory of HCT116 Colon Cancer Cells and Anti-Inflammatory Function in RAW264.7 Cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef]
- Han, Y.; Tang, X.; Zhang, Y.; Hu, X.; Ren, L.J. The Current Status of Biotechnological Production and the Application of a Novel Antioxidant Ergothioneine. Crit. Rev. Biotechnol. 2021, 41, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A.; et al. Immunomodulating Activity of Pleurotus eryngii Mushrooms Following Their In Vitro Fermentation by Human Fecal Microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef]
- Cirlincione, F.; Pirrone, A.; Gugino, I.M.; Todaro, A.; Naselli, V.; Francesca, N.; Alfonzo, A.; Mirabile, G.; Ferraro, V.; Balenzano, G.; et al. Technological and Organoleptic Parameters of Craft Beer Fortified with Powder of the Culinary–Medicinal Mushroom Pleurotus eryngii. J. Fungi 2023, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- AOAC 999.11-2006; Lead, Cadmium, Copper, Iron, and Zinc in Food. AOAC International: Rockville, MD, USA, 2006. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=1000 (accessed on 25 November 2024).
- Zhao, X.; Zhang, S.S.; Zhang, X.K.; He, F.; Duan, C.Q. An Effective Method for the Semi-Preparative Isolation of High-Purity Anthocyanin Monomers from Grape Pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Allegretta, I.; Pasqualone, A.; Caponio, F. Grape Skin and Seed Flours as Functional Ingredients of Pizza: Potential and Drawbacks Related to Nutritional, Physicochemical and Sensory Attributes. LWT 2023, 175, 114494. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Verardo, V.; Guerra-Hernández, E.J.; García-Villanova Ruiz, B.; Gómez-Caravaca, A.M. Response Surface Methodology for the Optimization of Flavan-3-Ols Extraction from Avocado By-Products via Sonotrode Ultrasound-Assisted Extraction. Antioxidants 2023, 12, 1409. [Google Scholar] [CrossRef]
- McCleary, B.V.; Charnock, S.J.; Rossiter, P.C.; O’Shea, M.F.; Power, A.M.; Lloyd, R.M. Measurement of Carbohydrates in Grain, Feed and Food. J. Sci. Food Agric. 2006, 86, 1648–1661. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Stępniewska, S.; Salamon, A.; Cacak-Pietrzak, G.; Piecyk, M.; Kowalska, H. The Impact of Oyster Mushrooms (Pleurotus ostreatus) on the Baking Quality of Rye Flour and Nutrition Composition and Antioxidant Potential of Rye Bread. Foods 2025, 14, 199. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef]
- Caponio, G.R.; Difonzo, G.; de Gennaro, G.; Calasso, M.; De Angelis, M.; Pasqualone, A. Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected? Front. Nutr. 2022, 9, 830932. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Canale, M.; Sanfilippo, R.; Strano, M.C.; Amenta, M.; Allegra, M.; Proetto, I.; Papa, M.; Palmeri, R.; Todaro, A.; Spina, A. Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage. Foods 2023, 12, 3419. [Google Scholar] [CrossRef]
- Vurro, F.; Summo, C.; Squeo, G.; Caponio, F.; Pasqualone, A. The Use of Durum Wheat Oil in the Preparation of Focaccia: Effects on the Oxidative Stability and Physical and Sensorial Properties. Foods 2022, 11, 2679. [Google Scholar] [CrossRef]
- Vurro, F.; Miani, M.G.; Summo, C.; Caponio, F.; Pasqualone, A. Effect of Durum Wheat Oil on the Physico-Chemical and Sensory Features of Biscuits. Foods 2022, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO Sensory Analysis; International Organization for Standardization: Geneve, Switzerland, 2016. [Google Scholar]
- Caponio, G.R.; Annunziato, A.; Vacca, M.; Difonzo, G.; Celano, G.; Minervini, F.; Ranieri, M.; Valenti, G.; Tamma, G.; De Angelis, M. Nutritional, Antioxidant and Biological Activity Characterization of Orange Peel Flour to Produce Nutraceutical Gluten-Free Muffins. Food Funct. 2024, 15, 8459–8476. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Ranieri, M.; Di Mise, A.; Centrone, M.; Sveito, M.; Valenti, G. Glutathionylation of the Aquaporin-2 Water Channel a novel post-translational modification modulated by the oxidative stress. J. Biol. Chem. 2014, 289, 27807–27813. [Google Scholar] [CrossRef]
- Owheruo, J.O.; Edo, G.I.; Oluwajuyitan, D.T.; Faturoti, A.O.; Martins, I.E.; Akpoghelie, P.O.; Agbo, J.J. Quality Evaluation of Value-Added Nutritious Biscuit with High Antidiabetic Properties from Blends of Wheat Flour and Oyster Mushroom. Food Chem. Adv. 2023, 3, 100375. [Google Scholar] [CrossRef]
- Sławińska, A.; Jabłońska-Ryś, E.; Gustaw, W. Physico-Chemical, Sensory, and Nutritional Properties of Shortbread Cookies Enriched with Agaricus Bisporus and Pleurotus Ostreatus Powders. Appl. Sci. 2024, 14, 1938. [Google Scholar] [CrossRef]
- Biao, Y.; Chen, X.; Wang, S.; Chen, G.; Mcclements, D.J.; Zhao, L. Impact of Mushroom (Pleurotus eryngii) Flour upon Quality Attributes of Wheat Dough and Functional Cookies-Baked Products. Food Sci. Nutr. 2020, 8, 361–370. [Google Scholar] [CrossRef]
- Farooq, M.; Rakha, A.; Hassan, J.U.; Solangi, I.A.; Shakoor, A.; Bakhtiar, M.; Khan, M.N.; Khan, S.; Ahmad, I.; Ahmed, S.; et al. Physicochemical and Nutritional Characterization of Mushroom Powder Enriched Muffins. J. Innov. Sci. 2021, 7, 110–120. [Google Scholar] [CrossRef]
- Losoya-Sifuentes, C.; Simões, L.S.; Cruz, M.; Rodriguez-Jasso, R.M.; Loredo-Treviño, A.; Teixeira, J.A.; Nobre, C.; Belmares, R. Development and Characterization of Pleurotus ostreatus Mushroom—Wheat Bread. Starch-Stärke 2022, 74, 2100126. [Google Scholar] [CrossRef]
- Losoya-Sifuentes, C.; Cruz, M.; del Refugio Rocha-Pizaña, M.; Loredo-Treviño, A.; Belmares, R. Edible Mushrooms: A Nutrient-Rich Ingredient for Healthier Food Products–A Review. Curr. Nutr. Rep. 2025, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of the European Union of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, 404, 9–25.
- Singla, A.; Gupta, O.P.; Sagwal, V.; Kumar, A.; Patwa, N.; Mohan, N.; Ankush; Kumar, D.; Vir, O.; Singh, J.; et al. Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients 2024, 16, 900. [Google Scholar] [CrossRef] [PubMed]
- Calasso, M.; Lisi, A.; Ressa, A.; Caponio, G.R.; Difonzo, G.; Minervini, F.; Gargano, M.L.; Vacca, M.; Angelis, M. De Incorporating Fresh Durum Wheat Semolina Pasta Fortified with Cardoncello (Pleurotus eryngii) Mushroom Powder as a Mediterranean Diet Staple. Antioxidants 2025, 14, 284. [Google Scholar] [CrossRef]
- Oloke, J.K.; Adebayo, E.A. Effectiveness of Immunotherapies from Oyster Mushroom (Pleurotus species) in the Management of Immunocompromised Patients. Int. J. Immunol. 2015, 3, 8–20. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, P.; Deng, C.; Xu, L.; Yu, M.; Yang, W.; Zhao, R.; Li, B. Effects of Pleurotus eryngii (Mushroom) Powder and Soluble Polysaccharide Addition on the Rheological and Microstructural Properties of Dough. Food Sci. Nutr. 2019, 7, 2113–2122. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. β-Glucans: A Potential Source for Maintaining Gut Microbiota and the Immune System. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Ali, M.I.K.; Abdel-Salam, F.F. Nutritional and Quality Characteristics of Some Foods Fortified with Dried Mushroom Powder as a Source of Vitamin D. Int. J. Food Sci. 2022, 2022, 2792084. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, M.; Ahmadi, F.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Comprehensive Review on Phytochemical Profiling in Mushrooms: Occurrence, Biological Activities, Applications and Future Prospective. Food Rev. Int. 2024, 40, 924–951. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Blicharz-Kania, A.; Bosacka, A.; Pecyna, A.; Ivanišová, E.; Kozłowicz, K.; Kovačiková, E. Impact of Incorporating Dried Chaga Mushroom (Inonotus obliquus) into Gluten-Free Bread on Its Antioxidant and Sensory Characteristics. Molecules 2024, 29, 3801. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- ISO. ISO Food Products; International Organization for Standardization: Geneve, Switzerland, 2010. [Google Scholar]
- Dhillon, G.K.; Mahajan, M.; Kour, A.; Kaur, K. Incorporation of Oyster Mushroom Powder and Pearl Millet Flour Improves the Nutritional Quality and Glycemic Response of Indian Flatbread. Int. J. Food Eng. 2024, 20, 63–72. [Google Scholar] [CrossRef]
- Di Cairano, M.; Condelli, N.; Caruso, M.C.; Marti, A.; Cela, N.; Galgano, F. Functional Properties and Predicted Glycemic Index of Gluten Free Cereal, Pseudocereal and Legume Flours. LWT 2020, 133, 109860. [Google Scholar] [CrossRef]
- Kamil, K.M.; Rohana, A.J.; Mohamed, W.M.I.W.; Ishak, W.R.W. Effect of Incorporating Dietary Fiber Sources in Bakery Products on Glycemic Index and Starch Digestibility Response: A Review. Nutrire 2023, 48, 36. [Google Scholar] [CrossRef]
- Zhuang, H.; Chen, Z.; Feng, T.; Yang, Y.; Zhang, J.; Liu, G.; Li, Z.; Ye, R. Characterization of Lentinus edodes β-Glucan Influencing the in Vitro Starch Digestibility of Wheat Starch Gel. Food Chem. 2017, 224, 294–301. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Dietary Glycemic Index, Glycemic Load and All-Cause and Cause-Specific Mortality: A Meta-Analysis of Prospective Cohort Studies. Clin. Nutr. 2023, 42, 1827–1838. [Google Scholar] [CrossRef]
- Rathore, H.; Sehwag, S.; Prasad, S.; Sharma, S. Technological, Nutritional, Functional and Sensorial Attributes of the Cookies Fortified with Calocybe Indica Mushroom. J. Food Meas. Charact. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Gaglio, R.; Guarcello, R.; Venturella, G.; Palazzolo, E.; Francesca, N.; Moschetti, G.; Settanni, L.; Saporita, P.; Gargano, M.L. Microbiological, Chemical and Sensory Aspects of Bread Supplemented with Different Percentages of the Culinary Mushroom Pleurotus eryngii in Powder Form. Int. J. Food Sci. Technol. 2019, 54, 1197–1205. [Google Scholar] [CrossRef]
- Chen, C.; Han, Y.; Li, S.; Wang, R.; Tao, C. Nutritional, Antioxidant, and Quality Characteristics of Novel Cookies Enriched with Mushroom (Cordyceps militaris) Flour. CyTA–J. Food 2021, 19, 137–145. [Google Scholar] [CrossRef]
- Shams, R.; Singh, J.; Dash, K.K.; Dar, A.H.; Pandiselvam, R. Utilization of Button Mushroom (Agaricus bisporus) Powder to Improve the Physiochemical and Functional Properties of Cookies. Sus. Food Technol. 2023, 1, 306–318. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.A.; Hui, X.; Wang, R.; Peressini, D.; Bai, W.; Cheng, P.; Brennan, C.S. Utilisation of Dried Shiitake, Black Ear and Silver Ear Mushrooms into Sorghum Biscuits Manipulates the Predictive Glycaemic Response in Relation to Variations in Biscuit Physical Characteristics. Int. J. Food Sci. Technol. 2022, 57, 2715–2728. [Google Scholar] [CrossRef]
- Miranda, D.V.; Rojas, M.L.; Pagador, S.; Lescano, L.; Sanchez-Gonzalez, J.; Linares, G. Gluten-Free Snacks Based on Brown Rice and Amaranth Flour with Incorporation of Cactus Pear Peel Powder: Physical, Nutritional, and Sensorial Properties. Int. J. Food Sci. 2018, 2018, 7120327. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, F.; de Oliveira Santana, W.; Fontana, R.C.; Camassola, M. Use of Pleurotus albidus Mycoprotein Flour to Produce Cookies: Evaluation of Nutritional Enrichment and Biological Activity. Innov. Food Sci. Emerg. Technol. 2021, 68, 102642. [Google Scholar] [CrossRef]
- Mokrzycki Cardinal Stefan, W.; Tatol, M. Color Difference Delta E-A Survey Colour Difference ∆E-A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and Identification of Pigments from Oyster Mushrooms with Black, Yellow and Pink Caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef]
- Ulziijargal, E.; Yang, J.H.; Lin, L.Y.; Chen, C.P.; Mau, J.L. Quality of Bread Supplemented with Mushroom Mycelia. Food Chem. 2013, 138, 70–76. [Google Scholar] [CrossRef]
- Moshtaghian, H.; Parchami, M.; Rousta, K.; Lennartsson, P.R. Application of Oyster Mushroom Cultivation Residue as an Upcycled Ingredient for Developing Bread. Appl. Sci. 2022, 12, 11067. [Google Scholar] [CrossRef]
- Cannas, M.; Conte, P.; Urgeghe, P.P.; Piga, A.; Alañón, M.E.; Del Caro, A. Artichoke By-Products: Promising Ingredients for Breadstick Fortification. LWT 2024, 202, 116307. [Google Scholar] [CrossRef]
- Khan, A.; Gani, A.; Masoodi, F.; Khanday, F. Antioxidant, Antiproliferative, Immunomodulatory, Antimicrobial and Functional Properties of Wild Mushroom (Coprinus atramentarius) β-Glucan Extract As. Can. J. Clin. Nutr. 2020, 8, 107–134. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sreeja, P.S.; Yang, X. The Antioxidant Properties of Mushroom Polysaccharides Can Potentially Mitigate Oxidative Stress, Beta-Cell Dysfunction and Insulin Resistance. Front. Pharmacol. 2022, 13, 874474. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The Antioxidant Potential of Different Edible and Medicinal Mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-ΚB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Wright, J.G.; Christman, J.W. The Role of Nuclear Factor Kappa B in the Pathogenesis of Pulmonary Diseases: Implications for Therapy. Am. J. Resp. Med. 2003, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-KB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- Perkins, N.D.; Gilmore, T.D. Good Cop, Bad Cop: The Different Faces of NF-ΚB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Vaskivuo, T.E.; Stenbäck, F.; Tapanainen, J.S. Apoptosis and Apoptosis-Related Factors Bcl-2, Bax, Tumor Necrosis Factor-α, and NF-ΚB in Human Endometrial Hyperplasia and Carcinoma. Cancer 2002, 95, 1463–1471. [Google Scholar] [CrossRef]
- Eid, M.A.; Lewis, R.W.; Abdel-Mageed, A.B.; Kumar, M.V. Reduced Response of Prostate Cancer Cells to TRAIL Is Modulated by NFkappaB-Mediated Inhibition of Caspases and Bid Activation. Int. J. Oncol. 2002, 21, 111–117. [Google Scholar] [CrossRef] [PubMed]

| CTR | TPE5 | TPE10 | |
|---|---|---|---|
| Energy (kcal) | 467.02 ± 0.15 a | 466.63 ± 0.90 a | 464.45 ± 0.55 b |
| Moisture | 3.58 ± 0.20 a | 3.27 ± 0.03 ab | 3.18 ± 0.15 b |
| Protein | 9.04 ± 0.05 a | 8.94 ± 0.09 a | 8.78 ± 0.37 a |
| Lipid | 20.2 ± 0.09 b | 20.99 ± 0.09 a | 21.07 ± 0.01 a |
| Total carbohydrate | 64.6 ± 0.02 a | 63.75 ± 0.04 b | 63.88 ± 0.24 b |
| Dietary fiber | 4.67 ± 0.01 c | 6.52 ± 0.02 b | 7.91 ± 0.04 a |
| β-glucans | 0.15 ± 0.01 c | 1.61 ± 0.11 b | 3.03 ± 0.21 a |
| Ash | 2.58 ± 0.05 b | 3.05 ± 0.14 a | 3.09 ± 0.02 a |
| CTR | TPE5 | TPE10 | |
|---|---|---|---|
| Hardness (N/mm2) | 30.22 ± 0.67 a | 21.26 ± 1.09 b | 31.59 ± 0.99 a |
| Fracturability (mm) | 0.13 ± 0.02 a | 0.04 ± 0.03 b | 0.14 ± 0.01 a |
| L* (D65) | 52.03 ± 0.53 a | 48.41 ± 0.11 b | 40.68 ± 0.80 c |
| a* (D65) | 13.78 ± 0.46 b | 14.52 ± 0.08 ab | 14.61 ± 0.31 a |
| b* (D65) | 35.64 ± 0.26 a | 33.68 ± 0.14 b | 30.03 ± 1.08 a |
| ΔE | - | 4.19 | 11.95 |
| BI | 47.97 ± 0.44c | 51.60 ± 0.09 b | 59.32 ± 0.65 a |
| CTR- | CTR+ | CTR | TPE5 | TPE10 | |
|---|---|---|---|---|---|
| 1:400 | 1.00 ± 0.03 a | 0.42 ± 0.03 c | 1.01 ± 0.05 a | 0.92 ± 0.06 b | 0.96 ± 0.07 ab |
| 1:200 | 1.00 ± 0.02 a | 0.42 ± 0.03 c | 0.99 ± 0.04 a | 0.92 ± 0.04 b | 0.96 ± 0.07 ab |
| 1:150 | 1.00 ± 0.03 a | 0.42 ± 0.03 c | 1.01 ± 0.03 a | 0.88 ± 0.12 b | 0.99 ± 0.03 a |
| 1:100 | 1.00 ± 0.03 a | 0.42 ± 0.03 c | 0.97 ± 0.06 a | 0.87 ± 0.11 b | 0.98 ± 0.06 a |
| 1:50 | 1.00 ± 0.02 a | 0.42 ± 0.03 d | 0.98 ± 0.07 ab | 0.92 ± 0.06 bc | 0.90 ± 0.06 c |
| 1:25 | 1.00 ± 0.03 a | 0.42 ± 0.03 c | 0.82 ± 0.12 b | 0.83 ± 0.03 b | 0.86 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponio, G.R.; Difonzo, G.; Troilo, M.; Marcotuli, I.; Gadaleta, A.; Tamma, G.; Gargano, M.L.; Cirlincione, F. Enhancing the Nutritional and Health-Related Properties of Taralli Through the Use of Pleurotus eryngii: Focus on Antioxidant and Anti-Inflammatory Properties. Antioxidants 2025, 14, 550. https://doi.org/10.3390/antiox14050550
Caponio GR, Difonzo G, Troilo M, Marcotuli I, Gadaleta A, Tamma G, Gargano ML, Cirlincione F. Enhancing the Nutritional and Health-Related Properties of Taralli Through the Use of Pleurotus eryngii: Focus on Antioxidant and Anti-Inflammatory Properties. Antioxidants. 2025; 14(5):550. https://doi.org/10.3390/antiox14050550
Chicago/Turabian StyleCaponio, Giusy Rita, Graziana Difonzo, Marica Troilo, Ilaria Marcotuli, Agata Gadaleta, Grazia Tamma, Maria Letizia Gargano, and Fortunato Cirlincione. 2025. "Enhancing the Nutritional and Health-Related Properties of Taralli Through the Use of Pleurotus eryngii: Focus on Antioxidant and Anti-Inflammatory Properties" Antioxidants 14, no. 5: 550. https://doi.org/10.3390/antiox14050550
APA StyleCaponio, G. R., Difonzo, G., Troilo, M., Marcotuli, I., Gadaleta, A., Tamma, G., Gargano, M. L., & Cirlincione, F. (2025). Enhancing the Nutritional and Health-Related Properties of Taralli Through the Use of Pleurotus eryngii: Focus on Antioxidant and Anti-Inflammatory Properties. Antioxidants, 14(5), 550. https://doi.org/10.3390/antiox14050550











