Combined Herbal Eye Drops Exhibit Neuroprotective and Intraocular Pressure-Reducing Effects in a Glaucoma Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Rat Model of Glaucoma
2.3. Topical Administration of Botanicals
2.4. Measurement of IOP
2.5. Electroretinography (ERG)
2.6. Optical Coherence Tomography (OCT)
2.7. Analysis of Ganglion Cell Changes on Whole-Mount Retinal Preparations
2.8. Isolectin-B4 Staining on Retinal Vessels
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Properties of Herbal Eye Drops
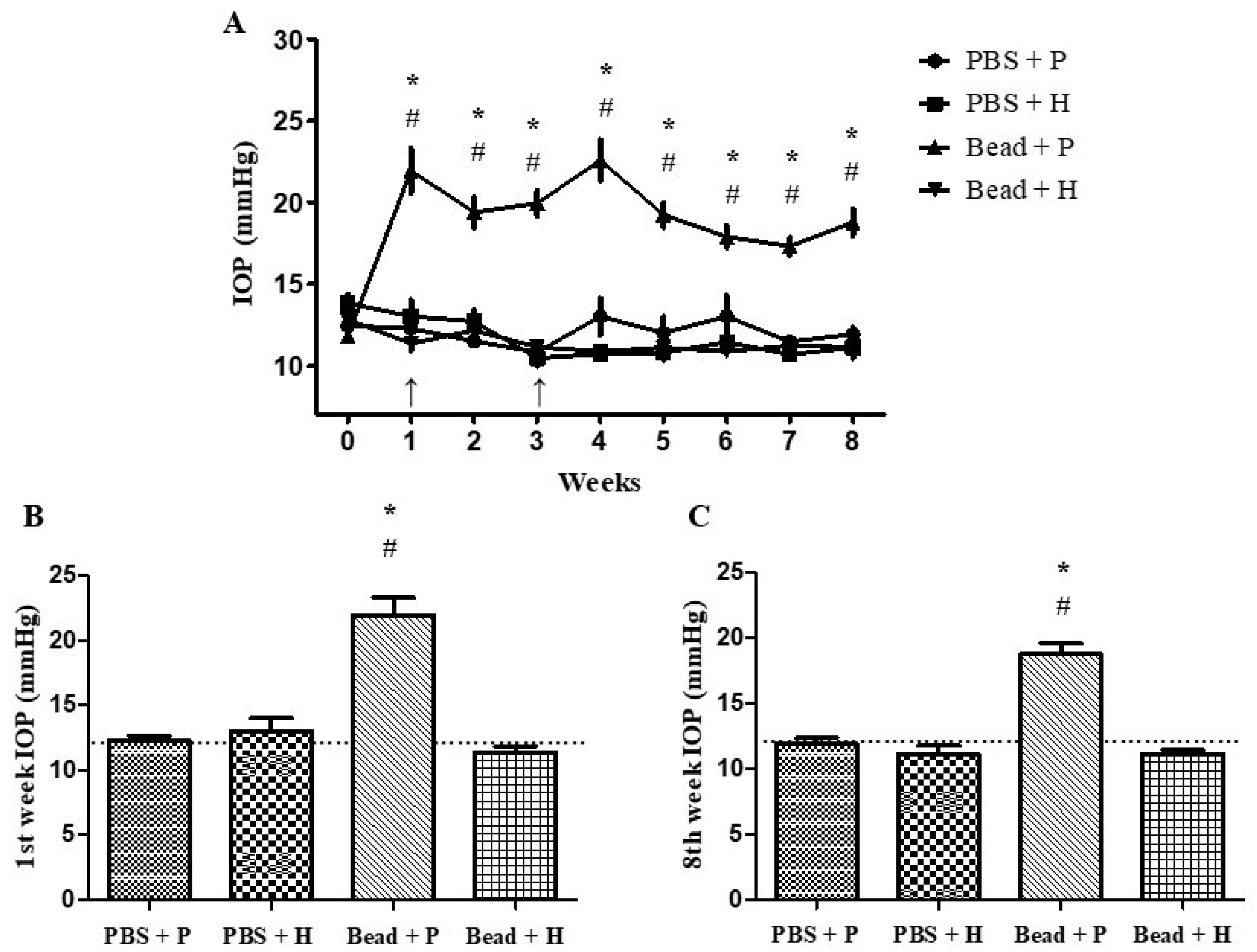
3.2. Effects of Combined Herbal Eye Drops on IOP
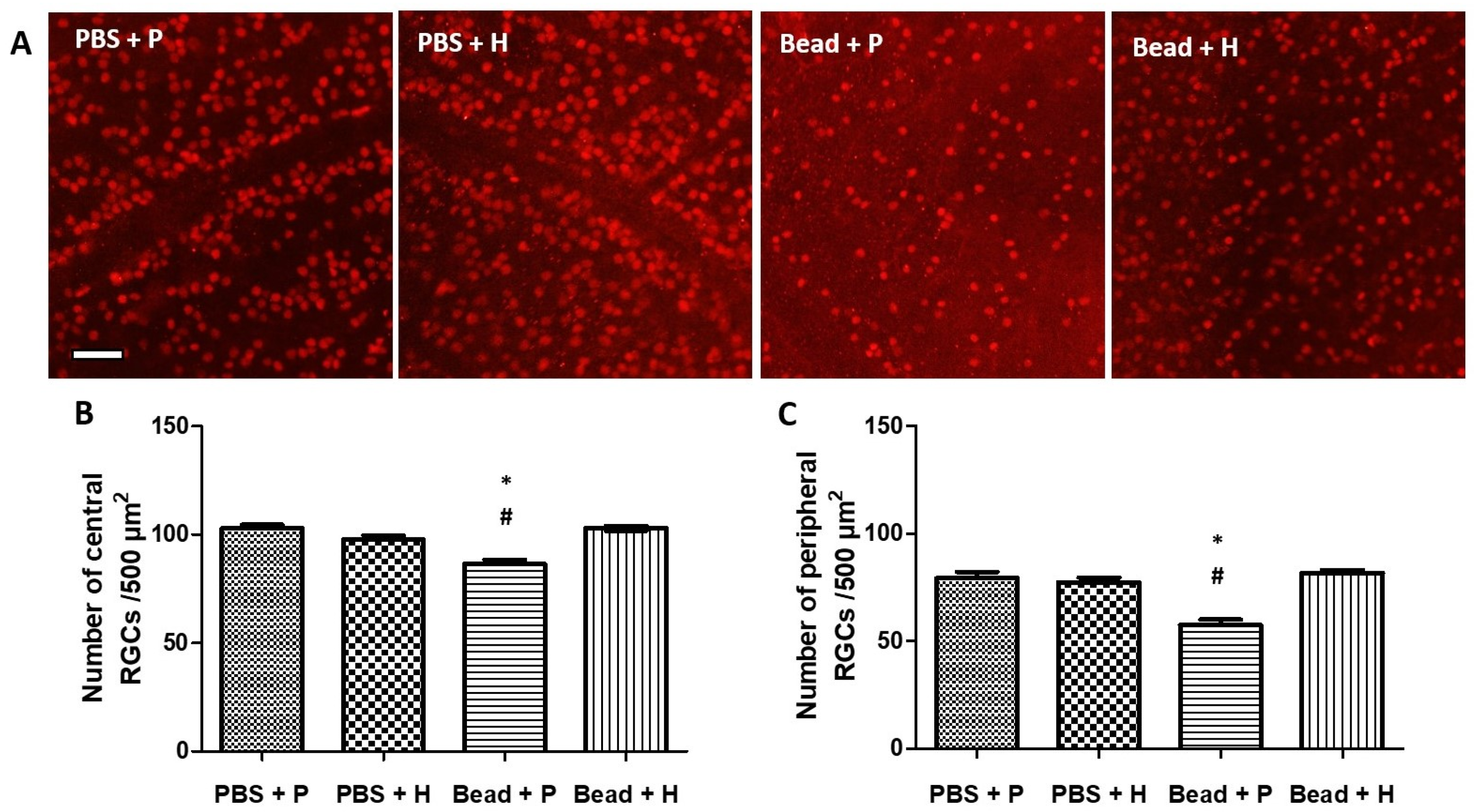
3.3. Effects of Herbal Eye Drop Treatment on Retinal Ganglion Cells (RGCs) and Their Immunohistochemical (Brn3a WholeMount) Expression
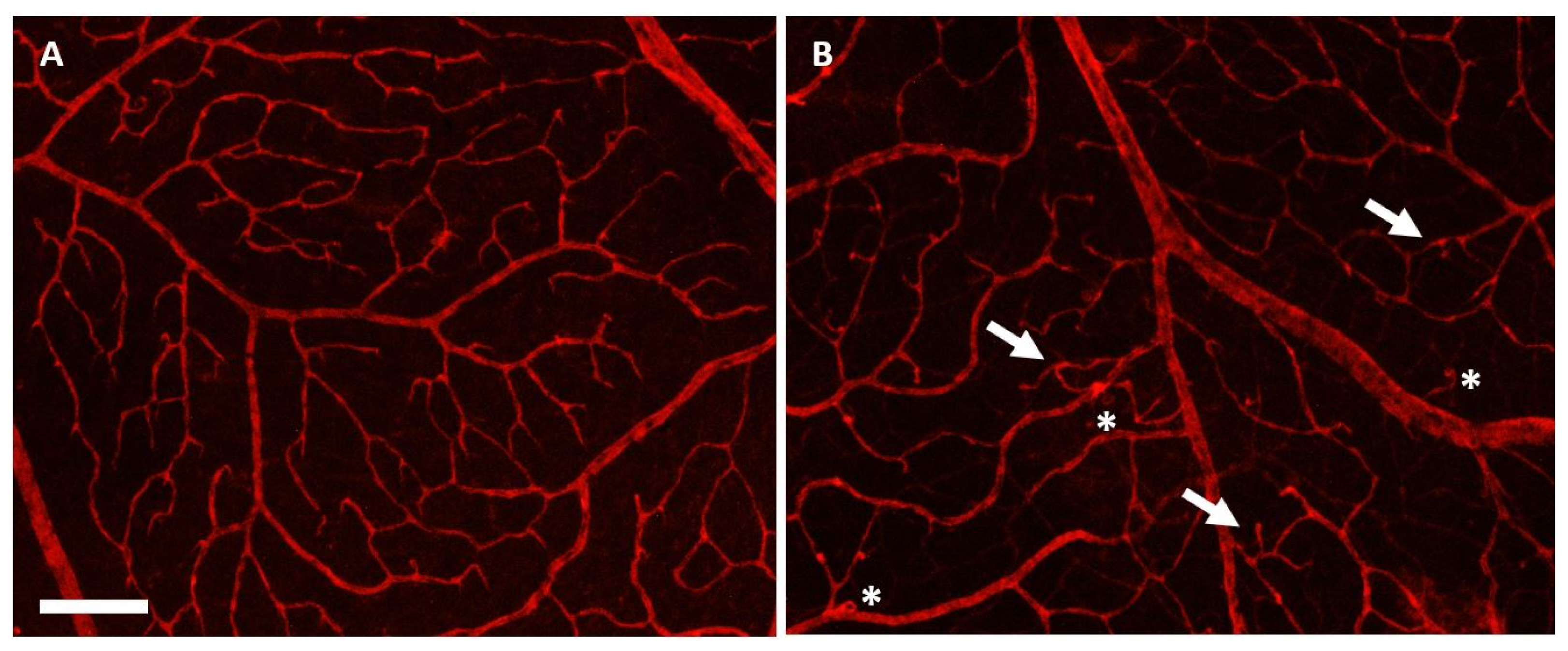
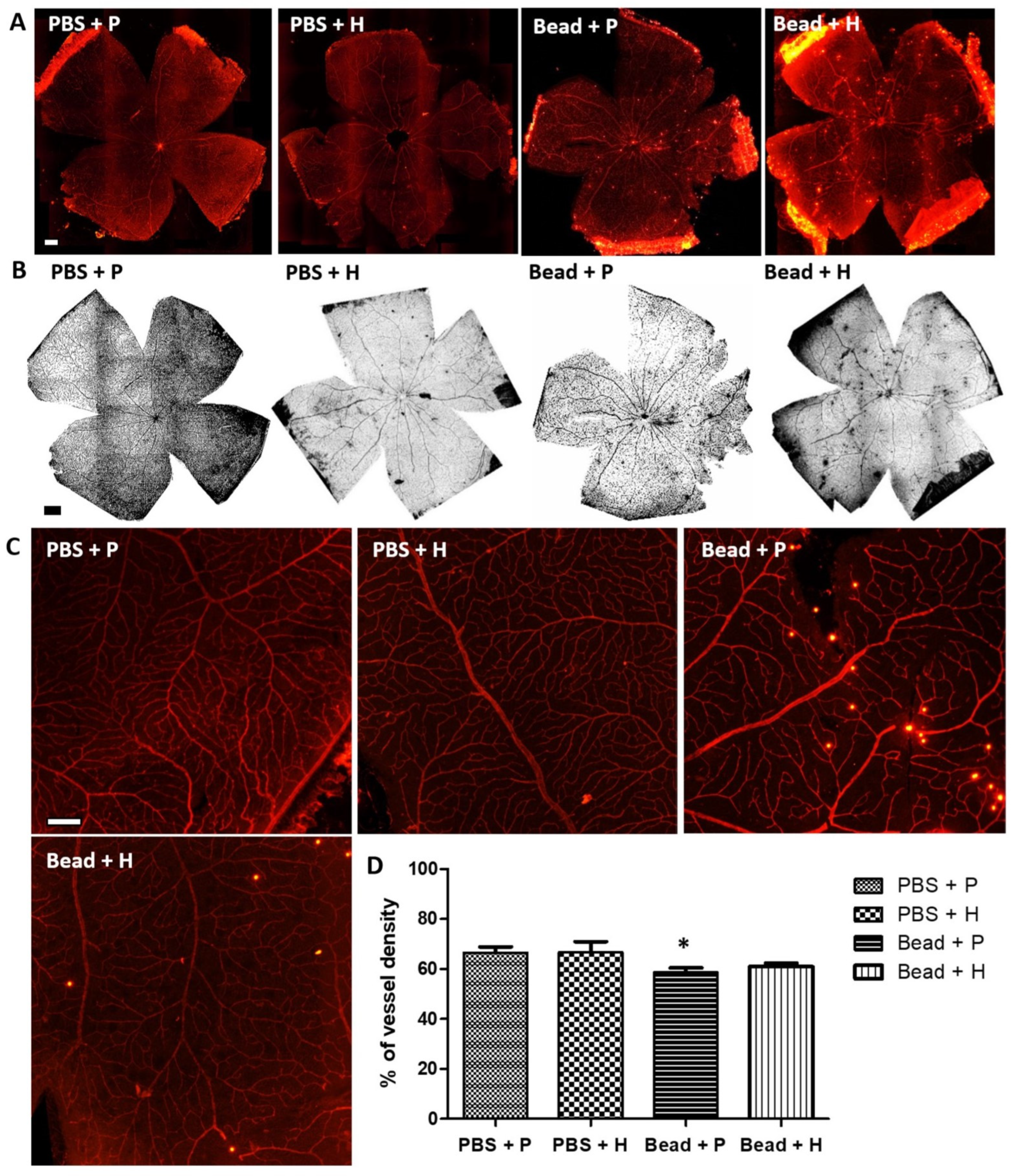
3.4. Effects of Herbal Eye Drops Treatment on Retinal Vessels (Isolectin-B4 Expression)
3.5. Retinoprotective Effects of Herbal Eye Drop Treatment on Morphological Changes
3.6. Effect of Herbal Eye Drops on Visual Responses
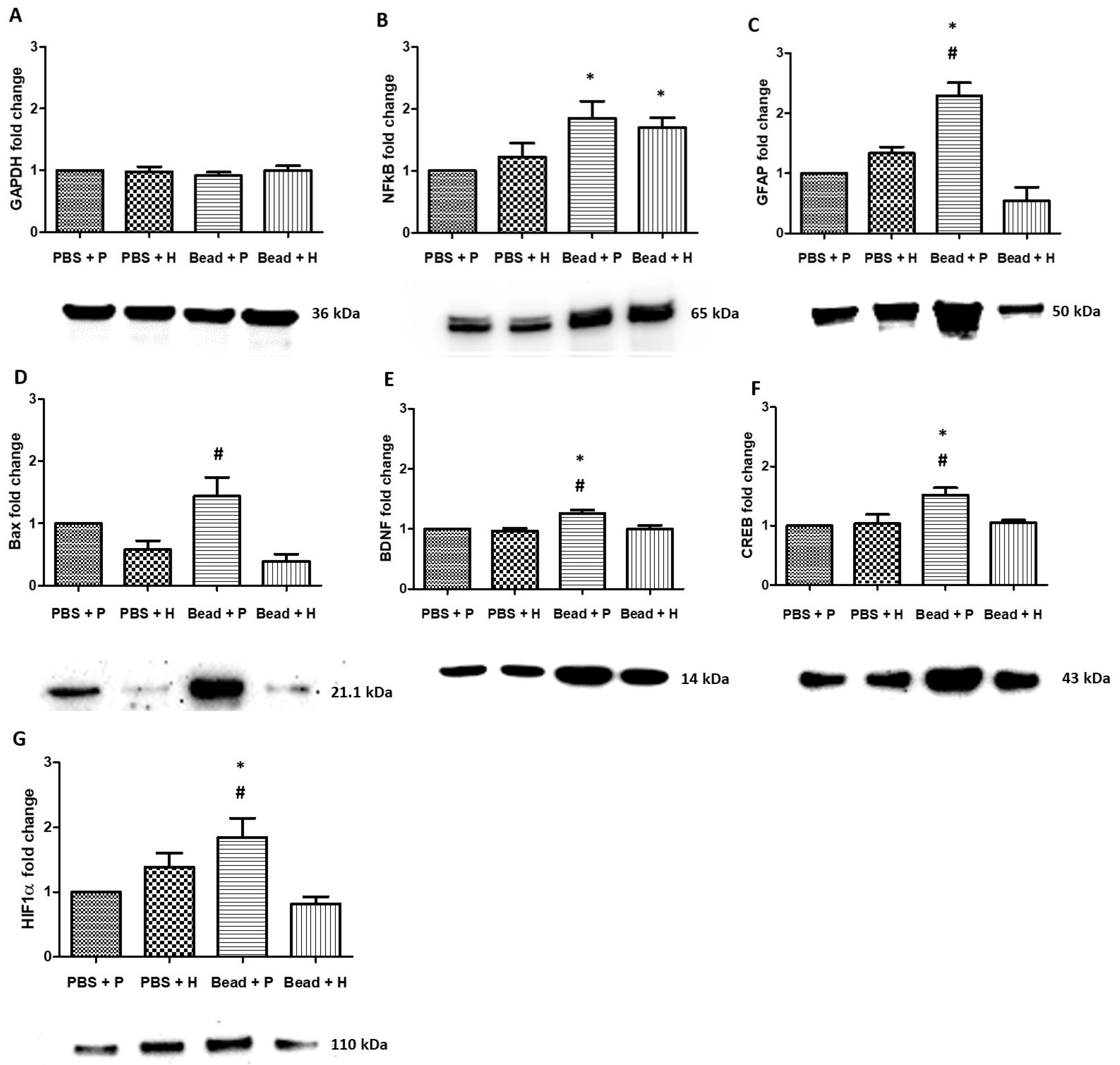
3.7. Western Blot Protein Analysis
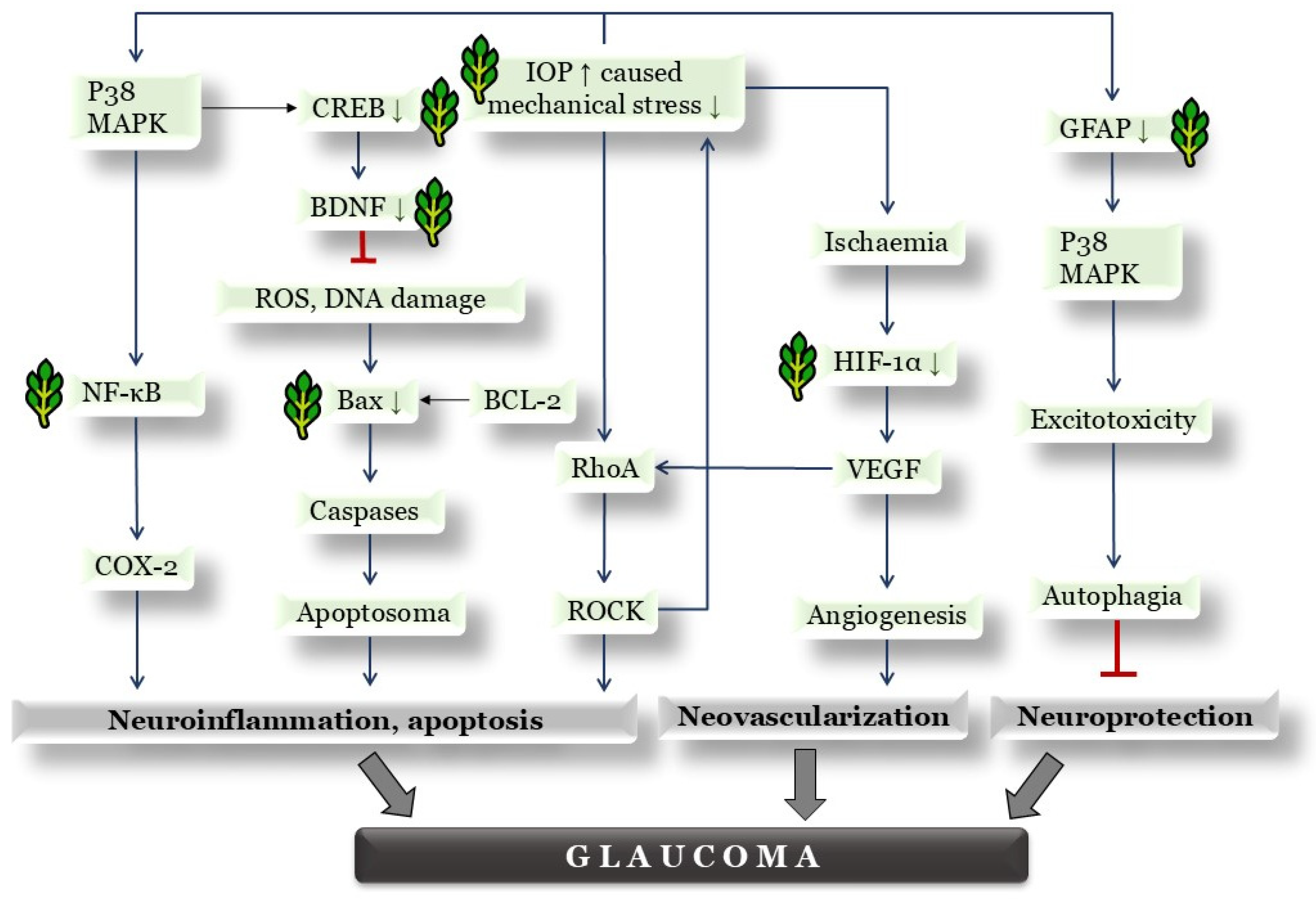
4. Discussion
4.1. Effects of Combined Herbal Eye Drops on IOP
4.2. Effects of Herbal Eye Drop Treatment on Histological Changes of the Retina
4.3. Effects of Herbal Eye Drop Treatment on Retinal Ganglion Cells (RGCs) and Their Immunohistochemical (Brn3a Whole-Mount) Expression
4.4. Effect of Herbal Eye Drops on Visual Responses
4.5. Properties of Herbal Eye Drops by Western Blot Protein Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| AMD | age-related macular degeneration |
| BCL-2 | B cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| Brn3a | brain-specific homeobox/POU domain protein 3A |
| COX-2 | cyclooxigenase-2 |
| DNA | deoxyribonucleic acid |
| EDTA | ethylenediaminetetraacetic acid |
| ERG | electroretinography |
| FoNo | Formulae normales (standard formulations in the Pharmacopoeia Hungarica) |
| GABA | γ-aminobutyric acid |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GCL | ganglion cell layer |
| GFAP | glial fibrillary acidic protein |
| Hif1α | hypoxia-inducible factor 1-alpha |
| HPLC | high-performance liquid chromatography |
| IL | interleukin |
| ILM | inner limiting membrane |
| INL | internal nuclear layer |
| IOP | intraocular pressure |
| IPL | internal plexiform layer |
| IS | (photoreceptor) internal segment |
| ND | no data |
| NFκB | nuclear factor kappa B |
| OCT | optical coherence tomography |
| OLM | outer limiting membrane |
| ONL | outer nuclear layer |
| OPL | outer plexiform layer |
| OS | (photoreceptor) outer segment |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PBS | phosphate buffer saline |
| PBST | phosphate buffer saline with Tween™ detergent |
| POAG | primary open-angle glaucoma |
| p38-MAPK | p38-mitogen-activated protein kinases |
| RGC | retinal ganglion cell |
| RhoA | Ras homolog family member A |
| RNFL | retinal nerve fiber layer |
| RPE | retinal pigment epithelium |
| ROCK | Rho associated coiled coil forming kinase |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl-sulfate polyacrylamide gel electrophoresis |
| SEM | standard error of mean |
| SD-OCT | spectral domain optical coherence tomography |
| TBS | tris-buffered saline |
| TGFβ | transforming growth factor beta |
| TNFα | tumor necrosis factor alpha |
| TRT | total retinal thickness |
| TrkB | tropomyosin receptor kinase B |
| VEGF | vascular endothelial growth factor |
References
- Artero-Castro, A.; Rodriguez-Jimenez, F.J.; Jendelova, P.; VanderWall, K.B.; Meyer, J.S.; Erceg, S. Glaucoma as a Neurodegenerative Disease Caused by Intrinsic Vulnerability Factors. Prog. Neurobiol. 2020, 193, 101817. [Google Scholar] [CrossRef] [PubMed]
- Ige, M.; Liu, J. Focus: Plant-Based Medicine and Pharmacology: Herbal Medicines in Glaucoma Treatment. Yale J. Biol. Med. 2020, 93, 347. [Google Scholar]
- Kovács-Valasek, A.; Rák, T.; Pöstyéni, E.; Csutak, A.; Gábriel, R. Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them. Int. J. Mol. Sci. 2023, 24, 8728. [Google Scholar] [CrossRef]
- Kapetanakis, V.V.; Chan, M.P.Y.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global Variations and Time Trends in the Prevalence of Primary Open Angle Glaucoma (POAG): A Systematic Review and Meta-Analysis. Br. J. Ophthalmol. 2016, 100, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Li, F.; Chen, H.; Wang, Y.; Zhu, Y.; Yang, X.; Zhu, J.; Wu, F.; Ouyang, H.; Ge, J.; et al. Caspase-8 Promotes NLRP1/NLRP3 Inflammasome Activation and IL-1β Production in Acute Glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 11181–11186. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic Potential of Curcumin in Eye Diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cela, D.; Brignole-Baudouin, F.; Labbé, A.; Baudouin, C. The Trabecular Meshwork in Glaucoma: An Inflammatory Trabeculopathy? J. Fr. Ophtalmol. 2021, 44, e497–e517. [Google Scholar] [CrossRef]
- Rák, T.; Csutak, A. Complementary Practices in Pharmacy and Their Relation to Glaucoma—Classification, Definitions, and Limitations. Sci. Pharm. 2024, 92, 16. [Google Scholar] [CrossRef]
- Wan, M.J.; Daniel, S.; Kassam, F.; Mutti, G.; Butty, Z.; Kasner, O.; Trope, G.E.; Buys, Y.M. Survey of Complementary and Alternative Medicine Use in Glaucoma Patients. J. Glaucoma 2012, 21, 79–82. [Google Scholar] [CrossRef]
- Bower, T.N.; Muhsen, S.; Overbury, O.; Birt, C.; Kasner, O. Canadian Ophthalmologists’ Opinions Concerning Complementary and Alternative Medicine (CAM) Use in Glaucoma. J. Glaucoma 2014, 23, 430–434. [Google Scholar] [CrossRef]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus Officinalis L. on Memory Performance, Anxiety, Depression, and Sleep Quality in University Students: A Randomized Clinical Trial. Complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.A.; Neves, J.A.; Oliveira, R.d.C.M. Pharmacological and Biotechnological Advances with Rosmarinus Officinalis L. Expert Opin. Ther. Pat. 2018, 28, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.C.; Moreira, C.P.D.S.; Castro, B.F.M.; Cotta, O.A.L.; Silva, L.M.; Fulgêncio, G.D.O.; Silva-Cunha, A.; Fialho, S.L. Rosmarinic Acid Intravitreal Implants: A New Therapeutic Approach for Ocular Neovascularization. Planta Med. 2020, 86, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.D.L.M.; Chahud, F.; Ramalho, L.N.; Modulo, C.M.; Vieira, L.C.; Reinach, P.S.; Rodrigues, M.D.L.V.; Cunha, A.S.; Paula, J.S. Rosmarinic Acid Suppresses Subconjunctival Neovascularization in Experimental Glaucoma Surgery. Curr. Eye Res. 2014, 40, 1134–1140. [Google Scholar] [CrossRef]
- Lindheimer, J.B.; Loy, B.D.; O’Connor, P.J. Short-Term Effects of Black Pepper (Piper Nigrum) and Rosemary (Rosmarinus Officinalis and Rosmarinus Eriocalyx) on Sustained Attention and on Energy and Fatigue Mood States in Young Adults with Low Energy. J. Med. Food 2013, 16, 765–771. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Refined Exposure Assessment of Extracts of Rosemary (E 392) from Its Use as Food Additive. EFSA J. 2018, 16, e05373. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Agrawal, S.S.; Srivastava, S.; Saxena, R. Oculohypotensive Effects of Foeniculum Vulgare in Experimental Models of Glaucoma. Indian J. Physiol. Pharmacol. 2008, 52, 77–83. [Google Scholar]
- Dongare, V.; Kulkarni, C.; Kondawar, M.; Magdum, C.; Haldavnekar, V.; Arvindekar, A. Inhibition of Aldose Reductase and Anti-Cataract Action of Trans-Anethole Isolated from Foeniculum Vulgare Mill. Fruits. Food Chem. 2012, 132, 385–390. [Google Scholar] [CrossRef]
- Imran, A.; Xiao, L.; Ahmad, W.; Anwar, H.; Rasul, A.; Imran, M.; Aziz, N.; Razzaq, A.; Arshad, M.U.; Shabbir, A.; et al. Foeniculum Vulgare (Fennel) Promotes Functional Recovery and Ameliorates Oxidative Stress Following a Lesion to the Sciatic Nerve in Mouse Model. J. Food Biochem. 2019, 43, e12983. [Google Scholar] [CrossRef]
- Antunes Viegas, D.; Palmeira-De-Oliveira, A.; Salgueiro, L.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Helichrysum Italicum: From Traditional Use to Scientific Data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of Phytochemicals from Helichrysum Italicum: An Analysis of Different Isolation Techniques and Biological Activity of Prepared Extracts. Phytochemistry 2017, 138, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.d.C.; Giner, R.M.; Máñez, S.; Tournier, H.; Schinella, G.; Ríos, J.-L. Anti-Inflammatory and Antioxidant Properties of Helichrysum Italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum Vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Wasnik, A.; Mayekar, C.; Nangude, M.; Yadav, S.; Thange, A. Unveilling The Therapeutic Potential of Rosmarinus Officinalis (Rosemary)—A Review on Pharmacological Spectrum. Int. J. Pharm. Sci. 2025, 3, 80–93. [Google Scholar] [CrossRef]
- Bourhia, M.; Laasri, F.E.; Aourik, H.; Boukhris, A.; Ullah, R.; Bari, A.; Ali, S.S.; El Mzibri, M.; Benbacer, L.; Gmouh, S. Antioxidant and Antiproliferative Activities of Bioactive Compounds Contained in Rosmarinus Officinalis Used in the Mediterranean Diet. Evid. Based. Complement. Alternat. Med. 2019, 2019, 7623830. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Pióro-Jabrucka, E.; Przybył, J.L.; Gniewosz, M.; Kraśniewska, K.; Szyndel, M.S.; Costa, R.; Bączek, K.B. Antioxidant and Antibacterial Activity of Helichrysum Italicum (Roth) G. Don. from Central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, S.D.; Androutsopoulou, C.; Hahalis, P.; Kotsalou, C.; Vantarakis, A.; Lamari, F.N. Rosemary Extract and Essential Oil as Drink Ingredients: An Evaluation of Their Chemical Composition, Genotoxicity, Antimicrobial, Antiviral, and Antioxidant Properties. Foods 2021, 10, 3143. [Google Scholar] [CrossRef]
- Ersoy, Ş.K. Isolation of Rosmarinic Acid from Rosemary Extract Utilizing a Molecularly Imprinted Polymer. Food Anal. Methods 2025, 18, 1–12. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ascrizzi, R. In Vitro Anticollagenase and Antielastase Activities of Essential Oil of Helichrysum Italicum Subsp. Italicum (Roth) G. Don. J. Med. Food 2019, 22, 1041–1046. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential Oil Composition and Larvicidal Activity of Six Mediterranean Aromatic Plants against the Mosquito Aedes Albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Mahboubi, M. Antimicrobial Activity of Rosemary, Fennel and Galbanum Essential Oils against Clinical Isolates of Staphylococcus Aureus. Biharean Biol. 2011, 5, 4–7. [Google Scholar]
- Ben Abdesslem, S.; Boulares, M.; Elbaz, M.; Ben Moussa, O.; St-Gelais, A.; Hassouna, M.; Aider, M. Chemical Composition and Biological Activities of Fennel (Foeniculum Vulgare Mill.) Essential Oils and Ethanolic Extracts of Conventional and Organic Seeds. J. Food Process. Preserv. 2021, 45, e15034. [Google Scholar] [CrossRef]
- Satyal, P.; Jones, T.H.; Lopez, E.M.; McFeeters, R.L.; Ali, N.A.A.; Mansi, I.; Al-Kaf, A.G.; Setzer, W.N. Chemotypic Characterization and Biological Activity of Rosmarinus Officinalis. Foods 2017, 6, 20. [Google Scholar] [CrossRef]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, D.H.J.; Maria, A.E.; Mohamed Mihoub, Z. Chemical Constituents of Helichrysum Italicum (Roth) G. Don Essential Oil and Their Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria, Filamentous Fungi and Candida Albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus Officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Futur. Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, C.E.; Kavaz, D.; Adekunle, Y.A.; Olawade, D.B. Phytochemical Assessment, Elemental Composition, and Biological Kinetics of Foeniculum Vulgare Mill. Stalks. Pharmacol. Res.-Mod. Chin. Med. 2024, 11, 100453. [Google Scholar] [CrossRef]
- Cávar Zeljković, S.; Šolić, M.E.; Maksimović, M. Volatiles of Helichrysum Italicum (Roth) G. Don from Croatia. Nat. Prod. Res. 2015, 29, 1874–1877. [Google Scholar] [CrossRef]
- Sertkaya, E.; Kaya, K.; Soylu, S. Chemical Compositions and Insecticidal Activities of the Essential Oils from Several Medicinal Plants against the Cotton Whitefly, Bemisia Tabaci. Asian J. Chem. 2010, 22, 2982–2990. [Google Scholar]
- Mesfin, M.; Asres, K.; Shibeshi, W. Evaluation of Anxiolytic Activity of the Essential Oil of the Aerial Part of Foeniculum Vulgare Miller in Mice. BMC Complement. Altern. Med. 2014, 14, 310. [Google Scholar] [CrossRef]
- Mancini, E.; De Martino, L.; Marandino, A.; Scognamiglio, M.R.; De Feo, V. Chemical Composition and Possible in Vitro Phytotoxic Activity of Helichrsyum Italicum (Roth) Don Ssp. Italicum. Molecules 2011, 16, 7725–7735. [Google Scholar] [CrossRef]
- Han, X.; Beaumont, C.; Stevens, N. Chemical Composition Analysis and in Vitro Biological Activities of Ten Essential Oils in Human Skin Cells. Biochim. Open 2017, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Helichrysum Italicum (Roth) G. Don Fil. (Asteraceae) from Montenegro. Nat. Prod. Res. 2020, 34, 445–448. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare (EDQM). European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023; ISBN 978-92-871-9059-4. [Google Scholar]
- Szabo, E.; Patko, E.; Vaczy, A.; Molitor, D.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Retinoprotective Effects of PACAP Eye Drops in Microbead-Induced Glaucoma Model in Rats. Int. J. Mol. Sci. 2021, 22, 8825. [Google Scholar] [CrossRef]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The Microbead Occlusion Model: A Paradigm for Induced Ocular Hypertension in Rats and Mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Sáez Martínez, V.; Martínez de Cestafe, N.; Alonso, J.M.; Olavarrieta, C.; Ucelay López de Heredia, M.; Benito Cid, S.; Pérez González, R. Effectiveness Evaluation of Hyaluronic Acid-Based Commercial Eye Drops to Treat Ophthalmic Dry Eye Disease. Carbohydr. Polym. Technol. Appl. 2024, 8, 100577. [Google Scholar] [CrossRef]
- Georgiou, A.L.; Guo, L.; Francesca Cordeiro, M.; Salt, T.E. Electroretinogram and Visual-Evoked Potential Assessment of Retinal and Central Visual Function in a Rat Ocular Hypertension Model of Glaucoma. Curr. Eye Res. 2014, 39, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.U.; Danias, J.; Brodie, S.; Chen, B.; Shen, F.; Podos, S.M.; Mittag, T.W.; Bayer, A.U.; Maag, K.P. Electroretinographic Abnormalities in a Rat Glaucoma Model with Chronic Elevated Intraocular Pressure. Exp. Eye Res. 2001, 72, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, N.; Khalili, Y. Al Neuroanatomy, Retina; StatPearls: Tampa, FL, USA, 2023. [Google Scholar]
- Razboršek, M.I. Stability Studies on Trans-Rosmarinic Acid and GC–MS Analysis of Its Degradation Product. J. Pharm. Biomed. Anal. 2011, 55, 1010–1016. [Google Scholar] [CrossRef]
- Choy, Y.-J.; Choi, J.-H. Comparison of Intraocular Pressure Values of Normotensive and Glaucomatous Rats Using Two Types of Tonometers. Korean J. Vis. Sci. 2018, 20, 589–596. [Google Scholar] [CrossRef]
- van Oterendorp, C.; Lagrèze, W.A.; Gutekunst, E.M.; Sgouris, S.; Maier, P.; Biermann, J. Enrichment of Retinal Ganglion Cells in Rat Retinal Lysate by Excimer Laser Ablation of the Outer Retina. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2061–2067. [Google Scholar] [CrossRef]
- Fileta, J.B.; Huang, W.; Kwon, G.P.; Filippopoulos, T.; Ben, Y.; Dobberfuhl, A.; Grosskreutz, C.L. Efficient Estimation of Retinal Ganglion Cell Number: A Stereological Approach. J. Neurosci. Methods 2008, 170, 1–8. [Google Scholar] [CrossRef]
- Danias, J.; Shen, F.; Goldblum, D.; Chen, B.; Ramos-Esteban, J.; Podos, S.M.; Mittag, T. Cytoarchitecture of the Retinal Ganglion Cells in the Rat. Investig. Ophthalmol. Vis. Sci. 2002, 43, 587–594. [Google Scholar]
- Curcio, C.A.; Allen, K.A. Topography of Ganglion Cells in Human Retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a Marker of Retinal Ganglion Cells: Qualitative and Quantitative Time Course Studies in NaÏve and Optic Nerve–Injured Retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Markand, S.; Al-Shabrawey, M.; Mayo, J.N.; Reynolds, J.; Bearden, S.E.; Ganapathy, V.; Smith, S.B. Alterations of Retinal Vasculature in Cystathionine–β-Synthase Heterozygous Mice: A Model of Mild to Moderate Hyperhomocysteinemia. Am. J. Pathol. 2014, 184, 2573. [Google Scholar] [CrossRef]
- Pesce, N.A.; Canovai, A.; Plastino, F.; Lardner, E.; Kvanta, A.; Cammalleri, M.; André, H.; Dal Monte, M. An Imbalance in Autophagy Contributes to Retinal Damage in a Rat Model of Oxygen-Induced Retinopathy. J. Cell. Mol. Med. 2021, 25, 10480–10493. [Google Scholar] [CrossRef] [PubMed]
- Patko, E.; Szabo, E.; Vaczy, A.; Molitor, D.; Tari, E.; Li, L.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Protective Effects of Pituitary Adenylate-Cyclase-Activating Polypeptide on Retinal Vasculature and Molecular Responses in a Rat Model of Moderate Glaucoma. Int. J. Mol. Sci. 2023, 24, 13256. [Google Scholar] [CrossRef]
- Nie, X.; Li, C.; Hu, S.; Xue, F.; Kang, Y.J.; Zhang, W. An Appropriate Loading Control for Western Blot Analysis in Animal Models of Myocardial Ischemic Infarction. Biochem. Biophys. Rep. 2017, 12, 108. [Google Scholar] [CrossRef]
- Jonas, J.B.; Jonas, R.A.; Jonas, S.B.; Panda-Jonas, S. Ciliary Body Size in Chronic Angle-Closure Glaucoma. Sci. Rep. 2023, 13, 16914. [Google Scholar] [CrossRef]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105, 1–169. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Maci, S.; Quintela, F.; Fonseca, B.A.; Cammalleri, M.; Dal Monte, M. Efficacy of a Spearmint (Mentha Spicata L.) Extract as Nutritional Support in a Rat Model of Hypertensive Glaucoma. Transl. Vis. Sci. Technol. 2023, 12, 6. [Google Scholar] [CrossRef]
- Sato, K.; Nishiguchi, K.M.; Maruyama, K.; Moritoh, S.; Fujita, K.; Ikuta, Y.; Kasai, H.; Nakazawa, T. Topical Ocular Dexamethasone Decreases Intraocular Pressure and Body Weight in Rats. J. Negat. Results Biomed. 2016, 15, 5. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Gao, K.; Zhang, X. Combined Subconjunctival Injection of Dexamethasone for the Management of Acute Primary Angle Closure: A Randomised Controlled Trial. Br. J. Ophthalmol. 2020, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hámor, A.; Markó, R.; Rák, T.; Csutak, A. Effect of Steroid Therapy on Intraocular Pressure. [A Szteroidterápia Hatása Az Intraocularis Nyomásra]. Orv. Hetil. 2022, 163, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Jonas, J.B. Decreased Photoreceptor Count in Human Eyes with Secondary Angle-Closure Glaucoma. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2532–2536. [Google Scholar]
- Guo, L.; Normando, E.M.; Nizari, S.; Lara, D.; Francesca Cordeiro, M. Tracking Longitudinal Retinal Changes in Experimental Ocular Hypertension Using the CSLO and Spectral Domain-OCT. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6504. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.V.; He, Z.; Vingrys, A.J.; Nguyen, C.T.O.; Wong, V.H.Y.; Fortune, B. Using the Electroretinogram to Understand How Intraocular Pressure Elevation Affects the Rat Retina. J. Ophthalmol. 2013, 2013, 15. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579. [Google Scholar] [CrossRef]
- Rák, T.; Kovács-Valasek, A.; Pöstyéni, E.; Csutak, A.; Gábriel, R. Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy. Cells 2023, 12, 2699. [Google Scholar] [CrossRef]
- Kumar, S.; Modgil, S.; Bammidi, S.; Minhas, G.; Shri, R.; Kaushik, S.; Singh, V.; Anand, A. Allium Cepa Exerts Neuroprotective Effect on Retinal Ganglion Cells of Pterygopalatine Artery (PPA) Ligated Mice. J. Ayurveda Integr. Med. 2020, 11, 489. [Google Scholar] [CrossRef]
- Yoo, H.S.; Shanmugalingam, U.; Smith, P.D. Harnessing Astrocytes and Müller Glial Cells in the Retina for Survival and Regeneration of Retinal Ganglion Cells. Cells 2021, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, H.; Wang, Z.; Guan, W.; Kang, X.; Tai, X.; Sun, Y. Silibinin Declines Blue Light-Induced Apoptosis and Inflammation through MEK/ERK/CREB of Retinal Ganglion. Cells 2019, 47, 4059–4065. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. CAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, C.K.; Mawrin, C.; Knop, C.O.; Behrens–Baumann, W. Assessment of MAPK–CREB Signaling in Acute and Chronic Optic Nerve Damage. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2076. [Google Scholar]
- Singh, N.K.; Kotla, S.; Kumar, R.; Rao, G.N. Cyclic AMP Response Element Binding Protein Mediates Pathological Retinal Neovascularization via Modulating DLL4-NOTCH1 Signaling. EBioMedicine 2015, 2, 1767–1784. [Google Scholar] [CrossRef]



| Chemical Compounds | Rosmarinus officinalis | Foeniculum vulgare | Helichrysum italicum | References a |
|---|---|---|---|---|
| Aqueous extract | ||||
| Apigenin | 0.01–0.2% | 0.1–12.5% | ND | [23,24] |
| Carnosic acid b | 7.5–17.3% | ND | ND | [24,25] |
| Carnosol | 0.5–3.0% | ND | 0.1–0.2% | [24,25] |
| Chlorogenic acid b | 0.5–2.0% | 0.11–6.8% | 3.38% | [23,24,25,26] |
| Luteolin | 0.1–0.5% | 0.1–0.3% | ND | [24] |
| Rosmanol | 0.5–2.0% | ND | ND | [24] |
| Rosmarinic acid b | 10.0–84.0% | 14.9–18.0% | 4.54% | [23,24,26,27,28] |
| Rutoside | ND | 0.01–0.3% | 0.0–0.19% | [24,25] |
| Oil extract | ||||
| Geraniol | 0.5–1.8% | ND | 3.0–6.80% | [26,29,30,31,32,33] |
| Limonene b | 1.5–5.0% | 2.41–11.45% | 2.17–6.07% | [24,34,35,36] |
| Linalool | 1.41–6.2.0% | ND | 2.8–4.7% | [24,27,29,30,32,33,34,37,38,39,40,41,42] |
| Trans-anethole b | 0.1–3.45% | 54.26–88.28% | ND | [23,38] |
| Week | PBS + P | PBS + H | Bead + P | Bead + H |
|---|---|---|---|---|
| 0. | 0 | 11.13 | −5.13 | 2.96 |
| 1. | 0 | 6.12 | 78.83 * | −7.32 |
| 2. | 0 | 10.60 | 68.65 * | 5.83 |
| 3. | 0 | −3.43 | 83.93 * | 2.89 |
| 4. | 0 | −17.62 | 73.58 * | −16.49 |
| 5. | 0 | −10.07 | 60.13 * | −7.49 |
| 6. | 0 | −12.09 | 37.50 * | −16.49 |
| 7. | 0 | −6.88 | 51.06 * | −2.14 |
| 8. | 0 | −6.85 | 57.41 * | −6.63 |
| PBS + P | PBS + H | Bead + P | Bead + H | |
|---|---|---|---|---|
| Central | 0 | −4.991 | −16.078 | −0.034 |
| Peripheric | 0 | −2.590 | −27.441 | 2.853 |
| Total Retinal Thickness (TRT) (%) | ||||
| PBS + P | PBS + H | Bead + P | Bead + H | |
| Week 1 | 0 | −2.12 | −4.99 | −0.92 |
| Week 4 | 0 | −1.07 | −8.18 | −3.39 |
| Week 8 | 0 | 1.14 | −8.05 | −5.17 |
| RNFL-INL thickness (%) | ||||
| PBS + P | PBS + H | Bead + P | Bead + H | |
| Week 1 | 0 | −3.19 | 0.20 | 1.67 |
| Week 4 | 0 | 0.33 | 0.82 | 3.52 |
| Week 8 | 0 | 0.19 | 0.83 | 0.31 |
| OPL-ONL thickness (%) | ||||
| PBS + P | PBS + H | Bead + P | Bead + H | |
| Week 1 | 0 | 1.85 | −6.49 | −5.97 |
| Week 4 | 0 | 1.35 | −9.66 | −4.40 |
| week 8 | 0 | 2.78 | −14.98 | −11.82 |
| IS-RPE thickness (%) | ||||
| PBS + P | PBS + H | Bead + P | Bead + H | |
| week 1 | 0 | −0.04 | −4.61 | −0.35 |
| week 4 | 0 | 0.06 | −8.01 | −3.80 |
| week 8 | 0 | 0.73 | −6.03 | −3.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak, T.; Patko, E.; Szabo, E.; Vaczy, A.; Molitor, D.; Reglodi, D.; Csutak, A.; Atlasz, T. Combined Herbal Eye Drops Exhibit Neuroprotective and Intraocular Pressure-Reducing Effects in a Glaucoma Rat Model. Antioxidants 2025, 14, 549. https://doi.org/10.3390/antiox14050549
Rak T, Patko E, Szabo E, Vaczy A, Molitor D, Reglodi D, Csutak A, Atlasz T. Combined Herbal Eye Drops Exhibit Neuroprotective and Intraocular Pressure-Reducing Effects in a Glaucoma Rat Model. Antioxidants. 2025; 14(5):549. https://doi.org/10.3390/antiox14050549
Chicago/Turabian StyleRak, Tibor, Evelin Patko, Edina Szabo, Alexandra Vaczy, Dorottya Molitor, Dora Reglodi, Adrienne Csutak, and Tamas Atlasz. 2025. "Combined Herbal Eye Drops Exhibit Neuroprotective and Intraocular Pressure-Reducing Effects in a Glaucoma Rat Model" Antioxidants 14, no. 5: 549. https://doi.org/10.3390/antiox14050549
APA StyleRak, T., Patko, E., Szabo, E., Vaczy, A., Molitor, D., Reglodi, D., Csutak, A., & Atlasz, T. (2025). Combined Herbal Eye Drops Exhibit Neuroprotective and Intraocular Pressure-Reducing Effects in a Glaucoma Rat Model. Antioxidants, 14(5), 549. https://doi.org/10.3390/antiox14050549







