Replicative Senescence in Mesenchymal Stem Cells: An In Vitro Study on Mitochondrial Dynamics and Metabolic Alterations
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Human bMSCs
2.2. Western Blot
2.3. Confocal Microscopy
2.4. Real-Time PCR (RT-PCR)
2.5. Protein Array
2.6. MitoSOX
2.7. Reduced/Oxidized Glutathione
2.8. Mitochondrial Membrane Potential (ΔΨm)
2.9. Fatty Acid Oxidation (FAO) Analysis
2.10. ATP Quantification
2.11. Triglyceride (TG) Quantification
2.12. Lipid Droplet Staining
2.13. Intracellular Lactate Quantification
2.14. Statistical Analysis
3. Results
3.1. Human bMSCs Exhibit Scenescence Markers at Passages 11 and 16
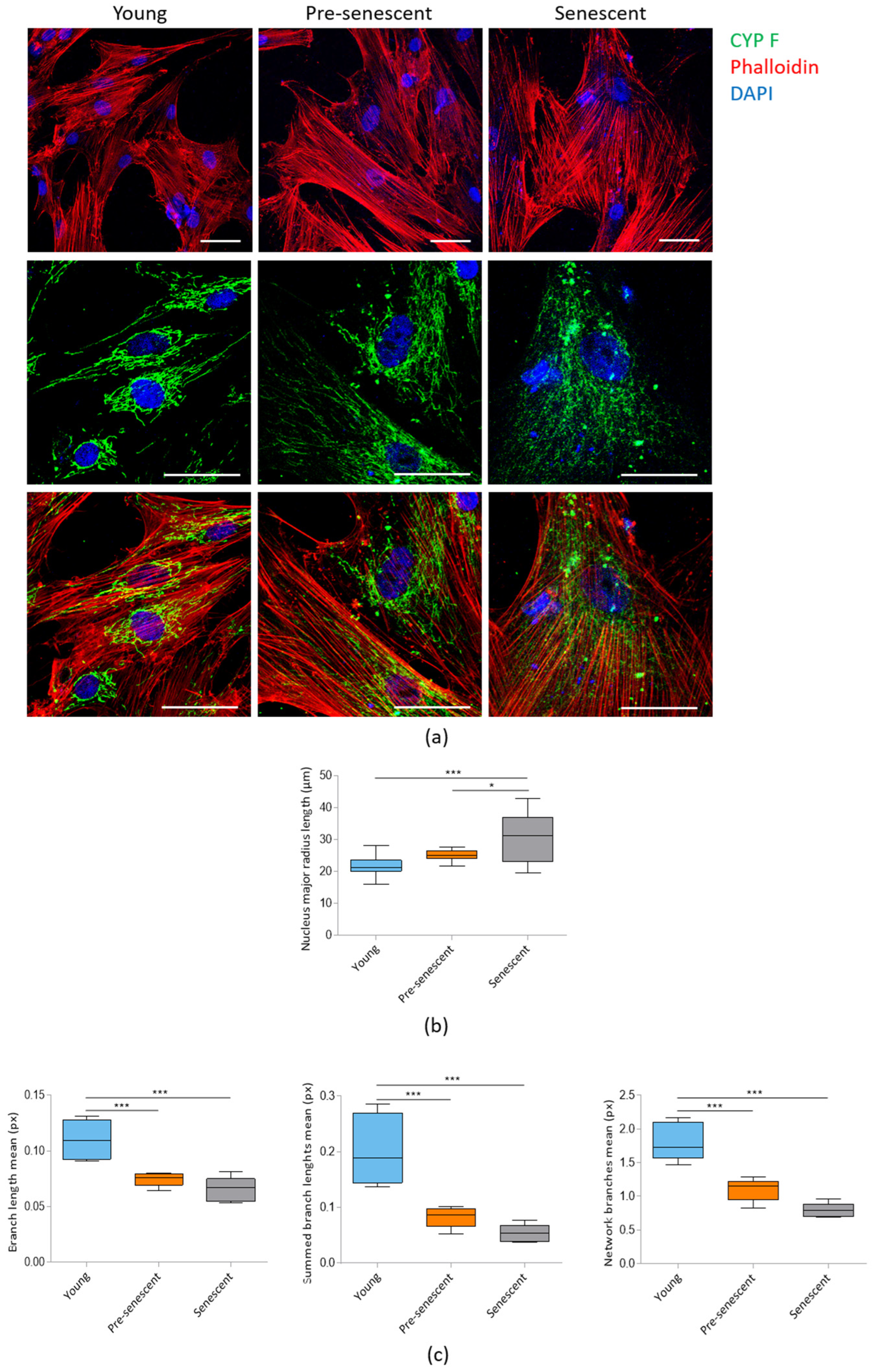
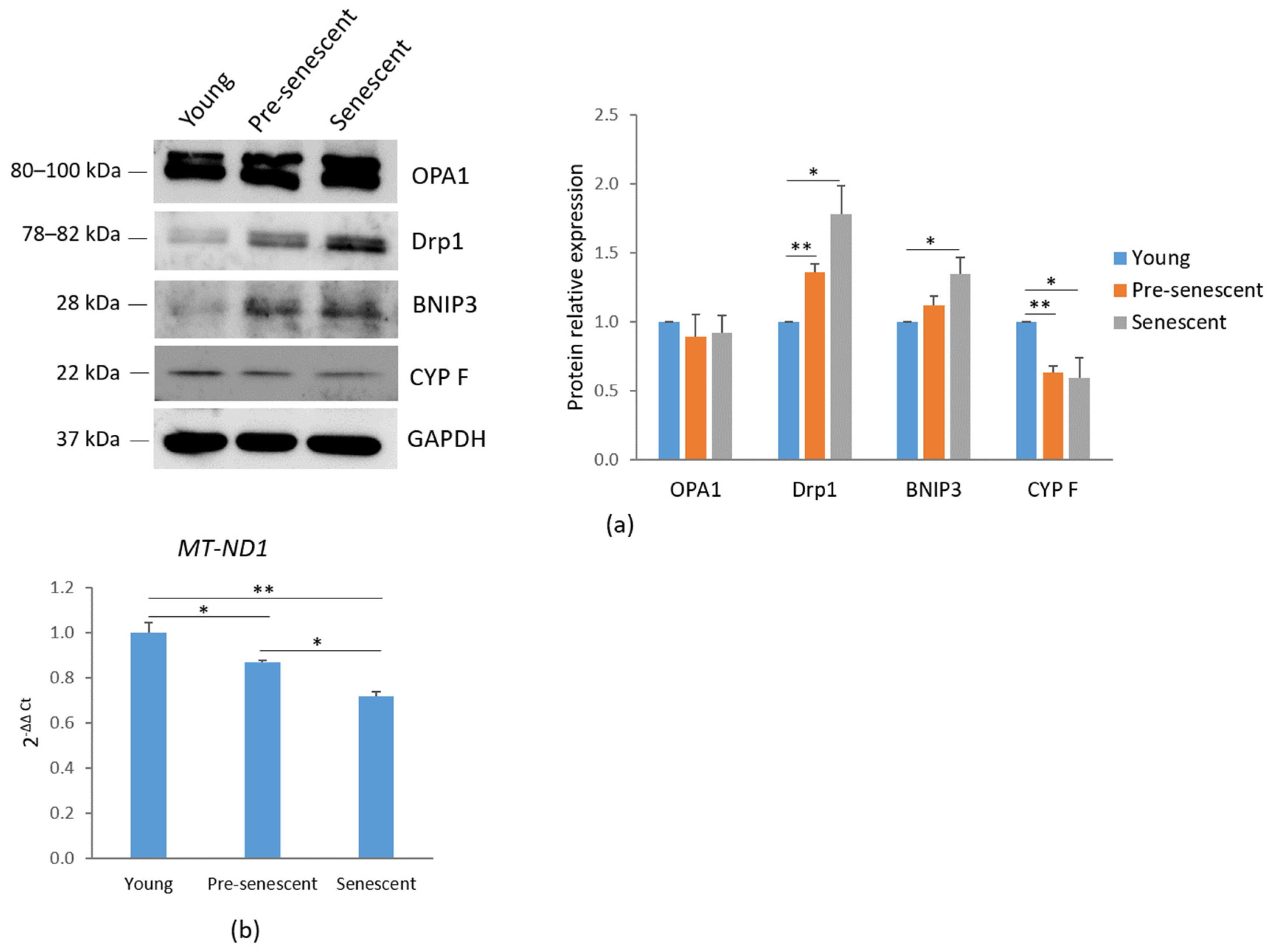
3.2. Pre-Senescent and Senescent bMSCs Exhibit Morphological and Mitochondrial Alterations
3.3. Pre-Senescent and Senescent bMSCs Show a Reduction in Mitochondrial Membrane Potential, an Increase in ROS Production and a Decrease in GSH/GSSG Ratio
3.4. Fatty Acid Oxidation (FAO) Is Reduced and Intracellular Neutral Lipids Are Increased in Pre-Senescent and Senescent bMSCs
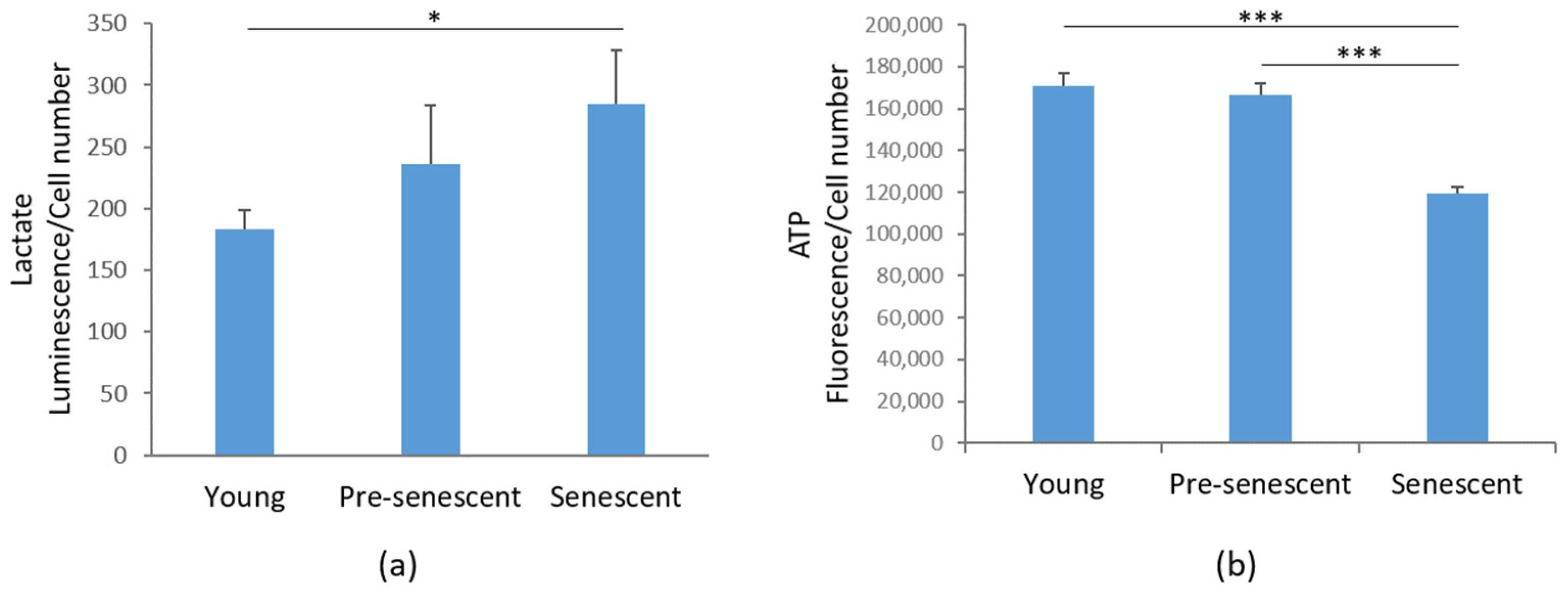
3.5. Lactate Production Is Increased, While ATP Levels Are Reduced in Senescent bMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bMSCs | Bone Marrow Mesenchymal Stem Cells |
| ROS | Reactive Oxygen Species |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovine Serum |
| SA-β-gal | Senescence-Associated β-galactosidase |
| PBS | Phosphate-Buffered Saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| OPA1 | Optic Atrophy 1 |
| BSA | Bovine Serum Albumin |
| Drp1 | Dynamin-Related Protein 1 |
| BNIP3 | BCL2 Interacting Protein 3 |
| CYP F | Cyclophilin F |
| PLIN2 | Lipid droplet protein perilipin 2 |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| BSA | Bovine Serum Albumin |
| CDKN1A | Cyclin Dependent Kinase Inhibitor 1A |
| MT-ND1 | Mitochondrially encoded NADH Dehydrogenase-1 |
| GSH | Glutathione |
| GSSG | Glutathione Disulfide |
| FAO | Fatty Acid Oxidation |
| TG | Triglyceride |
| X-Gal | 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside |
| SASP | Senescence-Associated Secretory Phenotype |
| IL | Interleukin |
| MIF | Macrophage Migration Inhibitory Factor |
References
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part. A J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Danilina, T.I.; Goryunov, K.V.; Pevzner, I.B.; Zorova, L.D.; Popkov, V.A.; Chernikov, V.P.; Plotnikov, E.Y.; Sukhikh, G.T.; et al. Age-related changes in bone-marrow mesenchymal stem cells. Cells 2021, 10, 1273. [Google Scholar] [CrossRef]
- Marote, A.; Santos, D.; Mendes-Pinheiro, B.; Serre-Miranda, C.; Anjo, S.I.; Vieira, J.; Ferreira-Antunes, F.; Correia, J.S.; Borges-Pereira, C.; Pinho, A.G.; et al. Cellular aging secretes: A comparison of bone-marrow-derived and induced mesenchymal stem cells and their secretome over long-term culture. Stem Cell Rev. Rep. 2023, 19, 248–263. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef]
- Hartmann, C.; Herling, L.; Hartmann, A.; Köckritz, V.; Fuellen, G.; Walter, M.; Hermann, A. Systematic estimation of biological age of in vitro cell culture systems by an age-associated marker panel. Front. Aging 2023, 4, 1129107. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.-Q.; Liu, W.; Zhang, W.J.; Hong, T.-H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.-X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Martin, I.; Galipeau, J.; Kessler, C.; Le Blanc, K.; Dazzi, F. Challenges for mesenchymal stromal cell therapies. Sci. Transl. Med. 2019, 11, e2189. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Locatelli, L.; Castiglioni, S.; Maier, J.A.M. The dynamic adaptation of primary human endothelial cells to simulated microgravity. FASEB J. 2019, 33, 5957–5966. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Hao, J.-L.; Li, L.; Ruan, Y.-T.; An, X.-N.; Huang, Q.-L.; Dong, X.-M.; Gao, P. VSTM2L protects prostate cancer cells against ferroptosis via inhibiting VDAC1 oligomerization and maintaining mitochondria homeostasis. Nat. Commun. 2025, 16, 1160. [Google Scholar] [CrossRef]
- Castiglioni, S.; Locatelli, L.; Cazzaniga, A.; Orecchio, F.M.; Santaniello, T.; Piazzoni, C.; Bureau, L.; Borghi, F.; Milani, P.; Maier, J.A. Cluster-assembled zirconia substrates accelerate the osteogenic differentiation of bone marrow mesenchymal stem cells. Nanomaterials 2023, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, M.; Bartolini, M.; Maier, J.A.; Castiglioni, S. Low extracellular magnesium induces phenotypic and metabolic alterations in C2C12-derived myotubes. Sci. Rep. 2023, 13, 19425. [Google Scholar] [CrossRef]
- von der Malsburg, A.; Sapp, G.M.; Zuccaro, K.E.; von Appen, A.; Moss, F.R., 3rd; Kalia, R.; Bennett, J.A.; Abriata, L.A.; Dal Peraro, M.; van der Laan, M.; et al. Structural mechanism of mitochondrial membrane remodelling by human OPA1. Nature 2023, 620, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zerihun, M.; Sukumaran, S.; Qvit, N. The Drp1-mediated mitochondrial fission protein interactome as an emerging core player in mitochondrial dynamics and cardiovascular disease therapy. Int. J. Mol. Sci. 2023, 24, 5785. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, Q.; Sun, H.; Liu, L.; Wang, C.; Li, Z.; Xu, Y.; Wang, L.; Zhang, L.; Zhang, H.; et al. BNIP3 (BCL2 interacting protein 3) regulates pluripotency by modulating mitochondrial homeostasis via mitophagy. Cell Death Dis. 2022, 13, 334. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Choi, C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS ONE 2011, 6, e23211. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q. Senescent mesenchymal stem cells: Disease mechanism and treatment strategy. Curr. Mol. Biol. Rep. 2020, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol. Cell Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Ke, H.; Peng, S.; Hu, C. Secretion of IL-6 and IL-8 in the senescence of bone marrow mesenchymal stem cells is regulated by autophagy via FoxO3a. Exp. Gerontol. 2023, 172, 112062. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell 2019, 176, 1083–1097.e18. [Google Scholar] [CrossRef]
- Manohar, S.; Neurohr, G.E. Too big not to fail: Emerging evidence for size-induced senescence. FEBS J. 2024, 291, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.A.; Quinsay, M.N.; Orogo, A.M.; Giang, K.; Rikka, S.; Gustafsson, Å.B. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 2012, 287, 19094–19104. [Google Scholar] [CrossRef] [PubMed]
- Landes, T.; Emorine, L.J.; Courilleau, D.; Rojo, M.; Belenguer, P.; Arnauné-Pelloquin, L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010, 11, 459–465. [Google Scholar] [CrossRef]
- Vizioli, M.G.; Liu, T.; Miller, K.N.; Robertson, N.A.; Gilroy, K.; Lagnado, A.B.; Perez-Garcia, A.; Kiourtis, C.; Dasgupta, N.; Lei, X.; et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020, 34, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.D.; Weilandt, D.R.; Liang, L.; MacArthur, M.R.; Jaiswal, N.; Ong, O.; Mann, C.G.; Chu, Q.; Hunter, C.J.; Ryseck, R.-P.; et al. Lactate homeostasis is maintained through regulation of glycolysis and lipolysis. Cell Metab. 2025, 37, 758–771. [Google Scholar] [CrossRef]
- Clémot, M.; Sênos Demarco, R.; Jones, D.L. Lipid mediated regulation of adult stem cell behavior. Front. Cell Dev. Biol. 2020, 8, 115. [Google Scholar] [CrossRef]
- Obaseki, E.; Adebayo, D.; Bandyopadhyay, S.; Hariri, H. Lipid droplets and fatty acid-induced lipotoxicity: In a nutshell. FEBS Lett. 2024, 598, 1207–1214. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casorati, B.; Zafferri, I.; Castiglioni, S.; Maier, J.A. Replicative Senescence in Mesenchymal Stem Cells: An In Vitro Study on Mitochondrial Dynamics and Metabolic Alterations. Antioxidants 2025, 14, 446. https://doi.org/10.3390/antiox14040446
Casorati B, Zafferri I, Castiglioni S, Maier JA. Replicative Senescence in Mesenchymal Stem Cells: An In Vitro Study on Mitochondrial Dynamics and Metabolic Alterations. Antioxidants. 2025; 14(4):446. https://doi.org/10.3390/antiox14040446
Chicago/Turabian StyleCasorati, Beatrice, Isabella Zafferri, Sara Castiglioni, and Jeanette A. Maier. 2025. "Replicative Senescence in Mesenchymal Stem Cells: An In Vitro Study on Mitochondrial Dynamics and Metabolic Alterations" Antioxidants 14, no. 4: 446. https://doi.org/10.3390/antiox14040446
APA StyleCasorati, B., Zafferri, I., Castiglioni, S., & Maier, J. A. (2025). Replicative Senescence in Mesenchymal Stem Cells: An In Vitro Study on Mitochondrial Dynamics and Metabolic Alterations. Antioxidants, 14(4), 446. https://doi.org/10.3390/antiox14040446






