Abstract
Rhododendron tomentosum is a widespread evergreen shrub used in folk medicine due to the high biological activity of its secondary metabolites, including lignans, that has not been sufficiently studied, and overcoming this problem requires advanced analytical techniques. This study proposes a two-stage analytical strategy for non-targeted screening and identification of lignans in plant extracts that involves the detection of specific lignan-related structures by 2D NMR and the establishment of corresponding fragment ions for further mass spectrometry analysis (HPLC-ESI-MS/MS). The polyphenolic fraction of R. tomentosum stem extract was the object of the study. Eight secoisolariciresinol-type lignans (xylosides, glucosides, and rhamnoside), including one previously unknown compound (5-methoxysecoisolariciresinol 9-xyloside), were identified for the first time. The structures of the five compounds were additionally confirmed by preparative HPLC isolation and NMR studies. All of the obtained compounds had antioxidant activity (FRAP and DPPH) similar to that of ascorbic acid. The proposed analytical strategy can be considered an efficient tool for rapid and reliable group screening and identification of lignan derivatives in plant extracts. Its application in the study of R. tomentosum extracts has revealed a number of lignan glycosides that may contribute to the medicinal properties of the plant.
1. Introduction
Marsh rosemary, also known as Rhododendron tomentosum or Ledum palustre L., is a small, evergreen shrub that is widely distributed throughout Central and Northern Europe, as well as Northern Asia and North America [1]. It is commonly used in traditional folk medicine to treat bacterial and viral infections, digestive disorders, lung, skin, and internal diseases, as well as rheumatic conditions [2,3,4]. Hypouricemic properties of R. tomentosum were also noted [5]. Its extracts possess pronounced insecticidal, acaricidal, and/or insect repellent properties [6,7].
In the literature, studies of R. tomentosum chemical composition have focused mainly on the characterization of isoprenoid compounds of essential oils. Moreover, oxygenated sesquiterpenes (ledol, palustrol, and cyclocolorenone isomers), bicyclic monoterpenoids (ascaridole and others), and monoterpene hydrocarbons (p-cymene, myrcene, and limonene) have also been detected [8,9,10,11,12,13,14,15,16]. Other substances in R. tomentosum extracts include pentacyclic triterpenoids (uvaol, uvaol acetate, ursolic acid, ursolic acid acetate, lupeol, α-amyrin, taraxerol, and others) [17,18] and low-molecular-weight polyphenols, most of which are flavonoids (quercetin, hyperoside, catechin, epicatechin, and others) and coumarins (scopoletin, esculetin, fraxetin, fraxidin, and others) [17,19,20].
Many species of the genus Rhododendron (R. mariae, R. minutiflorum, R. alutaceum, and others) contain bioactive lignans of various structures in stems and leaves [21]. However, their presence in R. tomentosum has not been sufficiently investigated. To date, only one study has reported the detection of lignan medioresinol in the supercritical CO2 extract of its leaves and stems [19]. However, the authors used low-resolution HPLC-MS/MS and available literature data for identifying the detected compounds.
Lignans are low-molecular-weight polyphenols of plant origin that contain two phenylpropane units in their structure. They possess a wide range of biologically active properties (hepatoprotective, antioxidant, antitumor, and others), and their glycosylated derivatives are of great interest owing to their increased bioavailability [22].
Currently, gas chromatography (GC) and high-performance liquid chromatography (HPLC), combined with mass spectrometry (MS), are mainly used for the highly sensitive determination of lignans in plant biomass. GC–MS is a less popular technique because of the low volatility of lignans and the need for labor- and time-consuming derivatization procedures [23,24]. HPLC-MS (MS/MS) is characterized by its high sensitivity and selectivity when analyzing lignans and has been successfully employed in several studies. Thus, this technique was implemented in the quantification of lariciresinol, matairesinol, pinoresinol, and secoisolariciresinol in foods [25] and for the determination of a wide variety of lignans in cereals, oilseeds, and nuts [26], including flax seeds [27]. However, most of the available publications on HPLC–MS analysis of plant extracts address the targeted determination of specific lignans, while the non-targeted screening of unknown compounds of this class in extremely complex matrices is challenging and still not fully resolved.
As shown earlier, one of the most promising approaches for the detection and identification of secondary plant metabolites involves the combination of 2D NMR and HPLC-HRMS [28,29,30] since the former allows the specific structural fragments in complex mixtures to be revealed, and the latter provides molecular-level analysis capabilities in the detection of parent compounds. This combination has previously been successfully used to analyze lignans and other substances in the knotwood of various coniferous trees [31].
In this study, we propose to further develop this methodology by applying a novel two-stage analytical strategy based on 2D NMR and HPLC-HRMS for the non-targeted screening and identification of lignans in an extract of R. tomentosum. It is based on the idea that NMR data can be used for the prediction of corresponding fragment ions in tandem mass spectra for further high-resolution mass spectrometry analysis, which greatly simplifies HPLC-HRMS data mining and interpretation.
2. Materials and Methods
2.1. Chemicals
Methylene chloride (for HPLC, Khimmed, Moscow, Russia) and methanol (HPLC grade, Khimmed, Moscow, Russia) were used in plant biomass extraction. HPLC gradient-grade acetonitrile (Cryochrom, St. Petersburg, Russia), ACS reagent-grade (≥96%) formic acid (Sigma–Aldrich, St. Louis, MO, USA), and type I water obtained from a Milli-Q system (Millipore, Molsheim, France) were used in the preparation of mobile-phase preparative HPLC. For the NMR studies, deuterated dimethyl sulfoxide (DMSO-d6, ≥99.8%, Merck, Darmstadt, Germany) was used as the solvent. DPPH (2,2-diphenyl-1-picrylhydrazyl radical, Sigma–Aldrich, Steinheim, Germany), TPTZ (2,4,6-tripyridyl-S-triazine, 99%, Sisco Research Laboratories, Mumbai, India), iron(II) sulfate (FeSO4, chem. pure, Veton, Moscow, Russia), iron(III) chloride (FeCl3, chem. pure, Khimmed, Moscow, Russia), and hydrochloric acid (HCl, chem. pure, Neva-Reaktiv, St. Petersburg, Russia) were used in the antioxidant activity determination procedures. Secoisolariciresinol (98%), isolated from the compression wood of coniferous species [32], and ascorbic acid (puriss. p.a., ≥99.0%), obtained from Sigma–Aldrich (Steinheim, Germany), were used as standards of known antioxidants in the FRAP and DPPH assays.
2.2. Plant Material and Preparation of Extract
R. tomentosum plant materials were collected in the Primorsky district of the Arkhangelsk region (Russia) in July 2023. Voucher specimen no. NARFU2023-RhT was deposited in the herbarium of the Northern (Arctic) Federal University. Secondary metabolites were extracted with a methanol–dichloromethane mixture (1:1, v/v) from the finely ground and carefully averaged stems of R. tomentosum (10 g dry weight), using a solvent/sample ratio of 10 mL g−1 in three 30 min stages under sonication (35 kHz) in an ultrasonic bath (Sapphire, Moscow, Russia). The obtained extract was fractionated by column chromatography using a Polygoprep 60–50 C18 octadecyl silica column (Macherey-Nagel, Duren, Germany) and four different solvents in order of decreasing polarity [33]. As a result, four fractions of the extract were obtained: E1 (water), E2 (aqueous methanol mixture, 1:1, v/v), E3 (methanol), and E4 (methanol–dichloromethane mixture, 1:1, v/v). The full separation procedure was presented in detail in previous work [30]. The total extract yield was 5% (calculated based on dry matter), with 1% being the aqueous methanol extract (E2) with the highest total polyphenol content (325 ± 17 mg eq. GA/g) among the four extracts obtained.
2.3. NMR Spectroscopy
One-dimensional 1H and two-dimensional (2D) 1H-13C HSQC, 1H-13C HMBC, and 1H-1H TOCSY NMR spectra of the polyphenolic fraction (F2) or isolated individual compounds were acquired in DMSO-d6 at 298 K on an AVANCE III 600 spectrometer (Bruker, Ettlingen, Germany) with an operating frequency for protons of 600 MHz. Pulse sequences from the standard Bruker library were implemented. Topspin 3.2 software from Bruker (Ettlingen, Germany) was used to acquire and process the experimental data. The cross-peak assignment for identifying specific lignan structures was performed using ACD/Structure Elucidator expert system software version 2019 from ACD/Labs (Toronto, ON, Canada). Examples of 2D NMR lignan compositional analyses are presented in Figures S3 and S4 (Supplementary Materials).
2.4. Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS)
Analysis of the extracted samples was performed using LC-HRMS. The analysis was carried out using an HPLC-HRMS system, which consisted of an LC-30 liquid chromatograph from Shimadzu (Kyoto, Japan) equipped with a UV–Vis diode array spectrophotometric detector and an Orbitrap ID-X high-resolution mass spectrometer from Thermo Scientific (Waltham, MA, USA), which included linear and orbital ion trap mass analyzers. An OptaMax NG ion source for the mass spectrometer was equipped with a heated electrospray ionization (HESI) probe. The conditions of chromatographic separation and mass spectrometric detection were presented in detail in previous work [30]. The identification procedure involved a targeted search for specific product ions in MS/MS chromatograms (dd-MS2) corresponding to the fragments discovered by 2D NMR. The detected signals were attributed to the peaks in the total ion current (TIC) MS1 chromatogram; thus, the deprotonated molecules of the parent metabolites belonging to the target family were found. Their identification was carried out via accurate mass-based elemental compositions, MS/MS fragmentation patterns, and on-line library searches (Pubchem, Lotus, and Chemspider).
2.5. Preparative Chromatography and Fraction Purity Assessment
Preparative high-performance liquid chromatography separation was carried out on an LC-20 Prominence preparative HPLC system from Shimadzu (Kyoto, Japan). The components of the chromatograph, semi-preparative column, mobile phase composition, and column temperature were described in previous work [29]. The mobile phase flow rate was 22.0 mL/min, and the detection wavelength was 280 nm. The gradient was programmed as follows: 0 min—20% B; 15 min—20% B; 17.5 min—100% B, held for 7.5 min. The total separation time was 25 min.
The extract (30.0 mg) was dissolved in 2 mL of aqueous methanol (50%) and injected to the semi-preparative column. The fractions corresponding to the main chromatographic peaks (Figure S5) were collected in glass vials and then evaporated under vacuum in a rotary evaporator until dryness. The purity of the obtained fractions was assessed via additional chromatographic analysis on the same HPLC system using a Nucleodur C18 Gravity analytical column from Macherey-Nagel (Duren, Germany), 5 μm, 250 × 4.6 mm. The percentage of the target compound was determined by comparing the peak area of the target compound in the chromatogram with the total area of all peaks present in the chromatogram.
The characteristics of the obtained fractions (F1–F4) were as follows. F1: light beige powder; yield: 3.3 (0.37)% from extract (raw materials (RM)); purity 93%; UV (MeOH): λ max 228, 280 nm. F2: light beige powder; yield: 2.6 (0.21)% from extract (RM); purity 93%; UV (MeOH): λ max 228, 280 nm. F3: light beige powder; yield: 0.24 (0.02)% from extract (RM); purity 98%; UV (MeOH): λ max 228, 280 nm. F4 (two compounds): light beige powder; yield: 0.39 (0.032)% from extract (RM); purity 98%; UV (MeOH): λ max 228, 280 nm. The 1H NMR (600 MHz, DMSO-d6) and 13C NMR (150 MHz, DMSO-d6) data for all of the obtained fractions are presented in Tables S1 and S2.
2.6. In Vitro Analysis of Antioxidant Activity
The antioxidant potential was determined via two commonly used methods: the radical scavenging assay (DPPH) and the ferric reducing antioxidant power (FRAP) assay. The FRAP assay was performed according to the method of Benzie and Strain [34] with minor modifications. The FRAP reagent was prepared fresh daily from acetate buffer with pH 3.6, 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3 × 6H2O in relative proportions of 10:1:1 (v/v), respectively, and was warmed to 37 °C prior to use. Next, 10 µL of the sample with a concentration of 50 mg/L was mixed with 90 µL of water and 200 µL of FRAP reagent. The absorbance was determined at 593 nm in a 96-well plate on a FlexA-200HT Microplate Reader from Hangzhou Allsheng Instruments Co. (Hangzhou, China) after incubation at 37 °C for 10 min. The results are expressed in mmol Fe2+ per gram of sample. The calibration was carried out immediately before the analysis using FeSO4 × 7H2O solutions in water (0–20 µM).
The DPPH assay was carried out according to the known procedure [35] with minor modifications. The DPPH reagent (0.3 mM) was freshly prepared daily, and accurately weighed samples (~6 mg) were dissolved in 50 mL of ethanol and incubated for 1 h in the dark prior to use. Next, 50 µL of the sample with a concentration of 20–100 mg/L was mixed with ethanol (50 µL) and 0.3 mM DPPH reagent (100 µL). The absorbance was measured at 517 nm after incubation at room temperature for 30 min in the same way as in FRAP assay. Antioxidant activity (IC50) was calculated from three parallel measurements.
All of the assays were performed in triplicate. The results of the parallel measurements were analyzed using the descriptive statistics tool of OriginPro 2019b software (OriginLab Corp., Northampton, MA, USA) at a significance level of p < 0.05. Data are expressed as mean ± standard deviation.
3. Results and Discussion
3.1. Targeted Screening of Lignans by 2D NMR (Stage 1)
The 2D HSQC NMR spectrum of the polyphenolic fraction (E2) is shown in Figure S1. For the targeted search for lignans, the region of δC/δH 30–100/1.5–5.0 ppm, in which the main signals characteristic of lignans are concentrated [31,32], was studied. The combination of HSQC data with correlations from the HMBC and TOCSY spectra made it possible to establish the presence of two types of lignans (Figure S2) possessing aryltetralin and dibenzylbutane structures with 9(9′) oxygen atoms [36,37]. The most intense cross-peaks, observed at δC/δH 105.6/6.35, 106.9/.6.56, 40.7/4.26, 44.3/1.91, and 38.5/1.50 ppm, were assigned to 5,5′-dimethoxyisolariciresinol, a representative of the aryltetralin family (Figure S3). The second group of signals (δC/δH 106.0/6.35, 39.4/2.02, 42.1/1.84, and 33.9/2.57 ppm) indicated the presence of secoisolariciresinol structures, related to dibenzylbutane-type lignans (Figure S4). However, the correlations observed in the HMBC spectrum (δC/δH 106/6.35 ppm) revealed that the aromatic component of the identified structures possessed two methoxyl groups as substituents in the 3- and 5-positions of the benzene ring. At the same time, the presence of lignans with one aromatic methoxyl group could not be excluded. In addition, the observed correlations supported the conclusion that the aliphatic hydroxyl groups at position 9 (or 9′) were bonded with a carbohydrate fragment (H(Glu) → C(9) 68.6/4.11 ppm).
3.2. Screening of Lignans by HPLC-ESI-HRMS (Stage 2)
The group screening of lignans in polyphenolic fraction E2 by HPLC-ESI-HRMS was carried out by searching for specific product ions in the tandem mass spectra. The latter were selected on the basis of the 2D NMR data and represented four aryltetralin and dibenzylbutane aglycone structures bearing one or two aromatic methoxyl groups (Table 1, Figure 1). This allowed for the detection of at least eight glycosylated lignans belonging mainly to glucosides and xylosides (Table 1). The structural formulas of all of the tentatively identified (on the basis of accurate mass and tandem mass spectra) compounds are shown in Figure 2.

Table 1.
Tentative identification of lignans in the polyphenolic fraction of the extract by HPLC-ESI(–)-MS/MS.
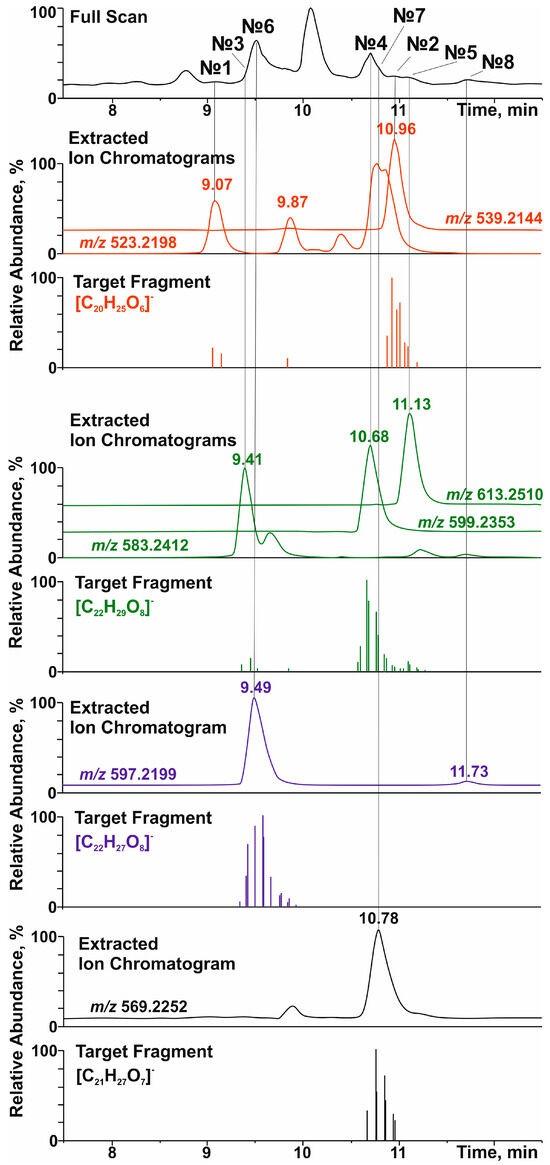
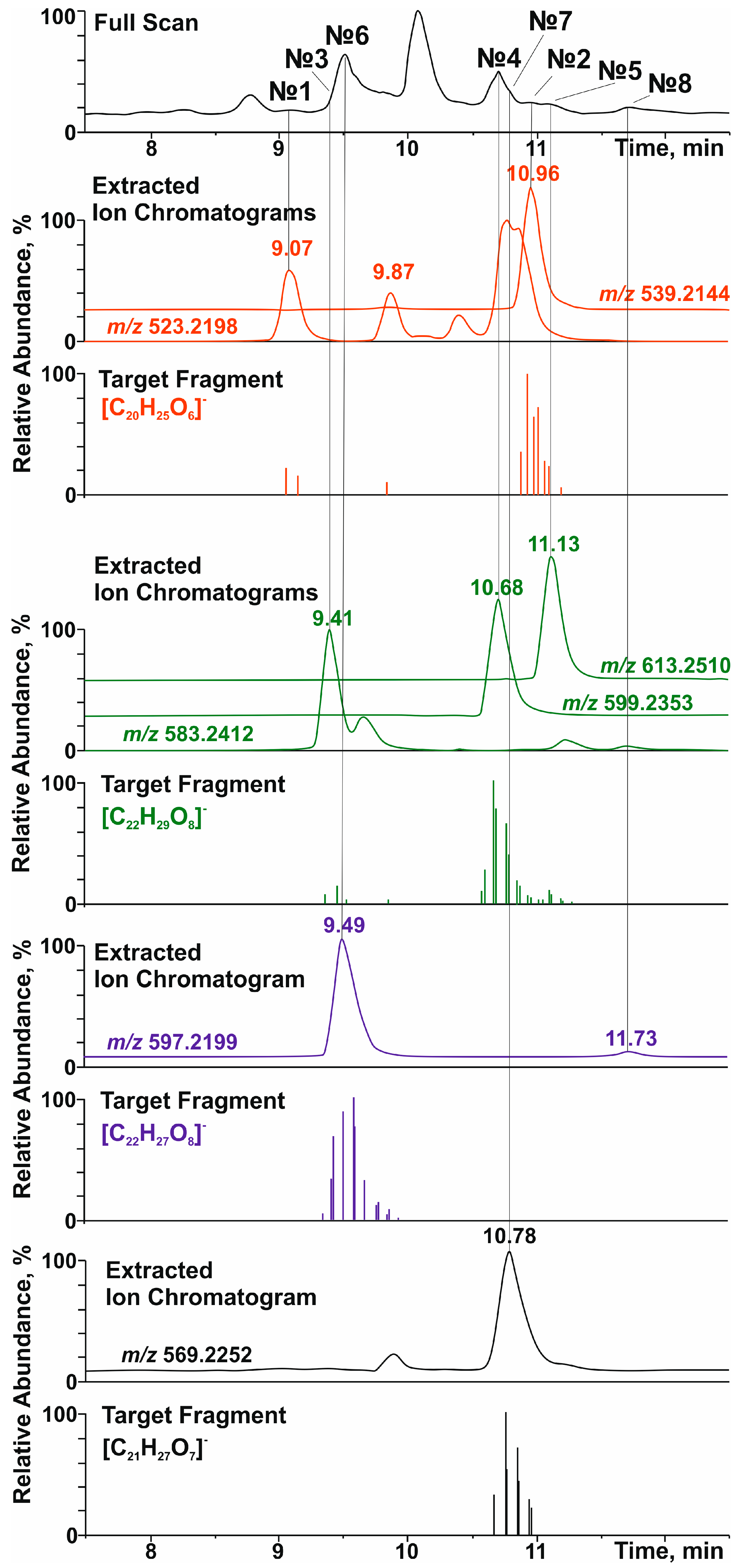
Figure 1.
Total ion current (full scan), accurate mass extracted, and selected product-ion (target fragment) chromatograms of lignans in the polyphenolic fraction of the extract of R. tomentosum stems.
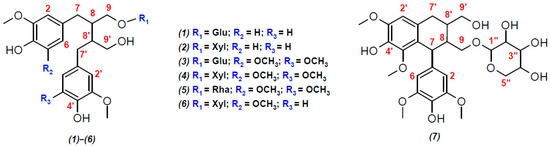
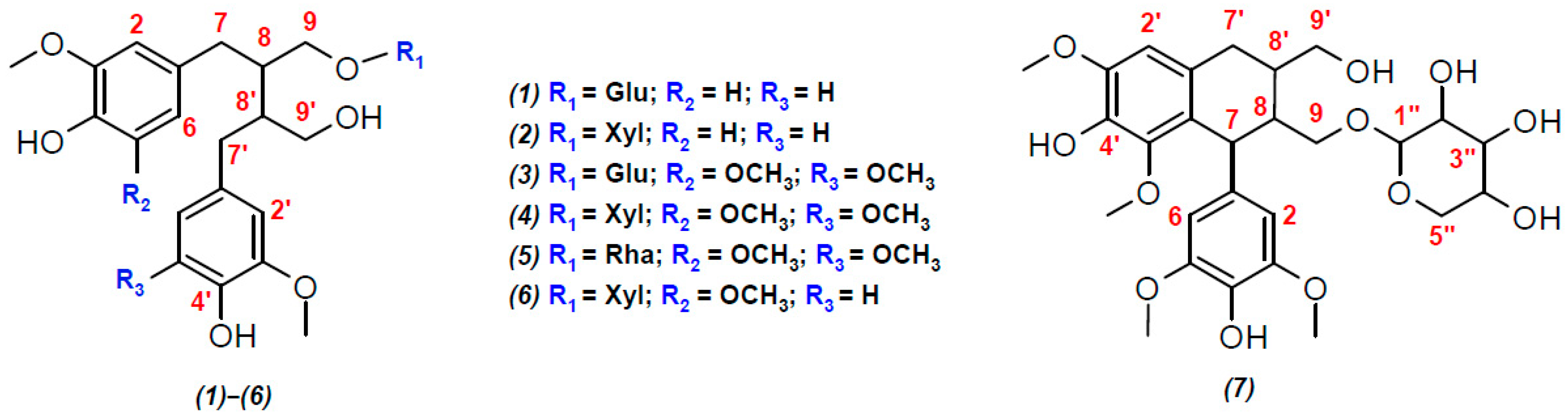
Figure 2.
Structural formulas of lignans, presumably identified in the polyphenolic fraction of the extract from the stems of R. tomentosum by HPLC-ESI(–)-MS/MS: (1) secoisolariciresinol glucoside; (2) secoisolariciresinol xyloside; (3) 5,5′-dimethoxy-secoisolariciresinol glucoside; (4) ssioriside; (5) 5,5′-dimethoxy-secoisolariciresinol rhamnoside; (6) methoxysecoisolariciresinol xyloside; and (7) lyoniside. Explanation for different colors: red—the atom numbers; blue—substituents.
Among aryltetralin-type lignans, only one compound with a retention time (tR) of 9.5 min was identified. The observation of a signal at m/z 419.1726 ([C22H27O8]−) in its tandem mass spectrum (Figure 1 and Figure S6) indicated the presence of 5,5′-dimethoxyisolariciresinol structural fragments. In an ion source, this metabolite formed an adduct with formic acid ([M-H+FA]−) with m/z 597.2199. This was confirmed by the cleavage of a neutral fragment with a mass of 46 Da (HCOOH) during collisionally activated dissociation (Figure S6). The loss of another neutral fragment with a mass of 132 Da (C5H8O4) indicated that 5,5′-dimethoxyisolariciresinol was present in R. tomentosum as a pentose conjugate derivative, presumably lyoniside (7, Table 1).
Importantly, in addition to lyoniside, another compound (8) with the same elemental composition and retention time of 11.7 min was detected in the extracted ion (m/z 597.2199, [M-H+FA]−) chromatogram (Figure 1). Despite the similar loss of formic acid and pentose moiety (Figure S7), the fragmentation of the aglycone structure differed significantly from that of 5,5′-dimethoxyisolariciresinol. In addition, the different chromatographic retention time indicated clear differences in the structure of the aglycone (other than the aryltetraline-type lignan). However, it was not possible to accurately determine its structure by HRMS.
Dibenzylbutanes with 9(9′) oxygen atoms were the second type of lignans, the group search for which was carried out on the basis of characteristic fragments in the tandem mass spectra. In this case, the product ions with m/z 361.1666 ([C20H25O6]−), 391.1774 ([C21H27O7]−), and 421.1880 ([C22H29O8]−) were used in the screening procedure. They corresponded to the deprotonated molecules of secoisolariciresinol, methoxysecoisolariciresinol, and dimethoxysecoisolariciresinol, respectively. Two detected compounds with retention times of 9.07 and 10.96 min and product ion [C20H25O6]− were present in the studied polyphenolic fraction (Figure 1). The first chromatographic peak was attributed to the parent analyte with m/z 523.2198 ([M-H]−) and elemental composition C26H36O11 (Δm/z = 2.3 ppm). Its tandem mass spectrum (Figure S8) showed the elimination of a hexose residue (162 Da), which allowed for the identification of this compound as secoisolariciresinol glucoside (1) with a high degree of confidence. The second compound (tR = 10.96 min) formed an adduct with formic acid ([M-H+HCOOH]−) under ESI(–) conditions, which led to the appearance of a signal at m/z 539.2144 (C25H34O10, Δm/z = 1.89 ppm). Its tandem mass spectrum (Figure S9) showed the loss of two neutral fragments corresponding to formic acid (46 Da) and a pentose residue (132 Da), as well as a set of fragment ions that were similar to those of secoisolariciresinol glucoside. On this basis, this lignan was preferably identified as secoisolariciresinol xyloside (2).
The search for the characteristic fragment ion [C21H27O7]− revealed only one representative monomethoxy derivative of secoisolariciresinol with tR = 10.78 min. According to the MS and MS/MS spectra (Figure 1 and Figure S10), this compound also formed an adduct with formic acid in the ion source and lost two neutral fragments (46 Da and 132 Da) during collisionally activated dissociation. On this basis, this metabolite was identified as methoxysecoisolariciresinol xyloside (6).
The greatest diversity was found in lignan derivatives, the aglycone part of which was represented by 5,5-dimethoxysecoisolariciresinol. In total, 3 metabolites (tR = 9.41, 10.68, and 11.13 min) giving the product ion [C21H27O7]− were detected (Figure 1). Only the least retained analyte did not form an adduct with formic acid in the ion source and lost a neutral fragment with a mass of 162 Da in the collision cell (Figure S11). In addition to the sugar fragment (132 Da), the other two metabolites exhibited cleavage of formic acid (Figures S12 and S13). Although the tandem mass spectra of the detected lignans had a similar set of product ions, the described fragmentation patterns and elemental compositions made it possible to identify them as 5,5-dimethoxysecoisolariciresinol glucoside (3), xyloside (4), and rhamnoside (5), in the order of their elution.
All of the proposed structures were consistent with the results of the NMR analysis and confirmed the presence of dibenzylbutane- and aryltetraline-type lignans in the polyphenolic fraction of the plant extract. A literature search revealed that lyoniside and ssioriside were previously found in the stem extracts of Vaccínium myrtíllus [38] and Rhododendron mucronulatum [39]. Thus, they can be considered characteristic lignans for representatives of the family Ericaceae.
Thus, the analytical strategy based on 2D NMR and HPLC-HRMS was proven to be promising for the non-targeted screening and identification of lignans and can be applied to other plant extracts for which the major components belong to the same class of compounds. However, this approach is not suitable for the screening and identification of trace amounts of metabolites due to the limited sensitivity of NMR spectroscopy, particularly for complex mixtures such as plant extracts.
3.3. Isolation and Characterization of Individual Lignans
To obtain pure preparations of the individual lignans detected by NMR and HPLC-HRMS, semi-preparative reversed-phase HPLC was used. The obtained chromatogram of the polyphenolic fraction with the indicated fraction (F1–F4) collection periods, which are presumably related to the elution of lignans, is shown in Figure S5. The achieved chromatographic resolution ensured an acceptable separation of components and their purity (92.7–97.8%), which was suitable for further structural elucidation by NMR spectroscopy techniques. The obtained 1H, 13C, and 2D NMR data are presented in Tables S1 and S2 and in Figures S14–S27.
From the evidence presented in the 1H and 2D NMR spectra, the analytes in fractions F1 and F2 were concluded to be lyoniside (7) and ssioriside (4). The signals from the protons of the aromatic hydroxyl groups (δH ~ 8.0 ppm) observed in the 1H NMR spectra confirmed that an aliphatic hydroxyl was involved in the formation of a simple ether bond with xyloside.
To the best of our knowledge, compound (6), constituting fraction F3, is a new lignan xyloside first discovered in plants. Detailed NMR data analysis suggested that its carbon skeleton was similar to that of ssioriside and secoisolariciresinol xyloside. However, the HMBC correlations (Figure S28) of H-2 and H-6 (δH 6.34) with C-3 and C-5 (δC 147.6) atoms indicated that one aromatic ring had a methoxy group not only at position 3 but also at position 5. Moreover, δH 6.34 was correlated with C-7 (δC 33.56), and H-7 (δH 2.54) was correlated with δH 2.01 (H-8) and δH 3.81 and 3.31 (H-9) in the TOCSY spectrum (Figure S23 and S28). Additionally, H-1” of the xyloside moiety (δH 4.06) correlated with C-9 of aglycone (δC 68.7), according to the HMBC data. This confirmed that the aliphatic hydroxyl group from the dimethoxylated structure was involved in the formation of ether bonds. Thus, compound (6) was identified as 5-methoxysecoisolariciresinol 9-xyloside.
Fraction F4 contained two lignans. One of them, secoisolariciresinol xyloside (2), was successfully identified by HPLC-ESI(–)-HRMS. The second component also possessed the xyloside moiety in its structure, but the carbon skeleton of the aglycone differed significantly from those of the aryltetralin and dibenzylbutane types of lignans. The HMBC spectrum (Figure S26) indicated that both aromatic rings had methoxy groups at positions 3 and 5, but one of them (δH 6.56; H-2′ and H-5′) was correlated with an oxygenated CH2 group (δC 81.5; C-7), which was involved in the formation of an ether bond with the oxygen of the propane chain from the second aromatic ring. The main correlations between the propane chains were analyzed by TOCSY NMR (Figure S27 and S28). Thus, this aglycone was identified as lariciresinol and, in conjunction with xyloside (H-1” → C-9; 66.06/4.16 ppm), it formed the well-known plant metabolite prupaside (8).
The main signals distinguishing the identified lignans resonated from the atoms in the aliphatic chain at positions 7 (7′), 8 (8′), and 9 (9′). In Tables S1 and S2, the diagnostic NMR signals that were used to distinguish these lignans from each other are highlighted in bold for subsequent studies on the composition of plant extracts using NMR spectroscopy.
3.4. Antioxidant Activity
The antioxidant activities of the isolated fractions of individual compounds (F1–F4) and the whole polyphenolic fraction of the extract (E2) were evaluated by two independent methods. These included (i) detecting the ability of an antioxidant to neutralize free radicals by donating hydrogen atoms (DPPH), and (ii) determining the ability of an antioxidant to reduce any compound [40] through electron transfer. Standard samples of secoisolariciresinol and ascorbic acid were used for comparison purposes. The results are presented in Table 2.

Table 2.
Antioxidant activity in the polyphenolic fraction of the extract and lignan fractions isolated from the stems of Rhododendron tomentosum measured by two antioxidant assays (DPPH and FRAP). Results are expressed as mean ± standard deviation (p < 0.05, n = 3).
The DPPH assay revealed a moderate (relative to ascorbic acid) antioxidant capacity of all of the isolated lignan xylosides close to that of secoisolaricresinol: F2 > F1 > F4 > F3. The best result was observed for ssioriside (2), the IC50 value of which was ~2.5 and 2 times greater than that of fraction E2 and secoisolariciresinol, respectively. The results of the FRAP assay revealed a different distribution of AOA between the selected fractions compared to DPPH: F4 > F2 > F3 > F1. The highest value was obtained for F4 (10.5 ± 0.4 mM Fe2+/g sample), which was close to that of secoisolariciresinol and ~2 times greater than that of E2 (5.63 ± 0.23 mM Fe2+/g sample).
Since the existing protocols for AOA determination may vary significantly, a straightforward comparison of the obtained results and literature data may be difficult. However, in general, the AOA values measured in this study were consistent with those presented in other publications. Lee and co-authors [39] also tested the antioxidant properties of isolated ssioriside and lyoniside against DPPH radicals and came to the same conclusion that these compounds showed moderate activity (IC50 values of 60 and 112 µM, respectively), comparable to ascorbic acid (IC50 = 36.9 µM) as a control sample. In addition, both secoisolariciresinol and its diglucoside exhibited strong activity against the stable free radical DPPH [41]. However, in the case of FRAP, glycosides demonstrate weaker activity than aglycones, as described by Polat Kose and Gulcin [42].
4. Conclusions
The stems of Rhododendron tomentosum have been recognized as a new source for eight of lignan glyco- and xylosides (predominantly). This has been achieved through the use of targeted 2D nuclear magnetic resonance (NMR) and high-performance liquid chromatography-electrospray ionization-high resolution mass spectrometry (HPLC-ESI-HRMS).
Fractionation of the extract using reversed-phase high-performance liquid chromatography (HPLC) made it possible to obtain three lignans with a purity of more than 90%. In addition, a fraction consisting of two lignans was obtained with a purity of 98%. The main components were lyoniside (~0.37% of the weight of the vegetable raw materials) and ssioriside (~0.21% of the weight of vegetable raw materials). These are representatives of the aryltetraline and dibenzylbutane classes, respectively.
Studies using the DPPH and FRAP methods have shown that isolated glycosylated lignans exhibit moderate antioxidant capacity, comparable to that of secoisolariciresinol, and approximately 2 times greater than the antioxidant activity of the extract.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14040447/s1, Figure S1: 1H-13C HSQC NMR spectrum of the polyphenolic fraction; Figure S2: The carbon skeleton of two types of lignans identified from 2D NMR spectra; Figure S3: An example of the 2D-NMR workflow for unknown compound structure elucidation in a complex mixture; Figure S4: An example of the 2D-NMR workflow for unknown compound structure elucidation in a complex mixture; Figure S5: Preparative LC-PDA (280 nm) chromatogram of Rhododendron tomentosum stems extract; Figure S6: Tandem mass spectra of lyoniside; Figure S7: Tandem mass spectra of prupaside; Figure S8: Tandem mass spectra of secoisolariciresinol glucoside; Figure S9: Tandem mass spectra of secoisolariciresinol xyloside; Figure S10: Tandem mass spectra of methoxysecoisolariciresinol xyloside; Figure S11: Tandem mass spectra of 5,5-dimethoxy-secoisolariciresinol glucoside; Figure S12: Tandem mass spectra of ssioriside; Figure S13: Tandem mass spectra of 5,5-dimethoxy-seco-isolariciresinol rhamnoside; Figure S14: 1H NMR spectrum of F1; Figure S15: 1H-13C HSQC NMR spectrum of F1; Figure S16: 1H-13C HMBC NMR spectrum of F1; Figure S17: 1H NMR spectrum of F2; Figure S18: 1H-13C HSQC NMR spectrum of F2; Figure S19: 1H-13C HMBC NMR spectrum of F2; Figure S20: 1H NMR spectrum of F3; Figure S21: 1H-13C HSQC NMR spectrum of F3; Figure S22: 1H-13C HMBC NMR spectrum of F3; Figure S23: 1H-1H TOCSY spectrum of F3; Figure S24: 1H NMR spectrum of F4; Figure S25: 1H-13C HSQC NMR spectrum of F4; Figure S26: 1H-13C HMBC NMR spectrum of F4; Figure S27: 1H-1H TOCSY spectrum of F4; Figure S28: 1H–1H TOCSY and 13C–1H HMBC correlations of compounds 6 and 8. Table S1: 1H NMR spectroscopic data; Table S2: 13C NMR spectroscopic data.
Author Contributions
Conceptualization, A.V.F., N.V.U. and D.S.K.; methodology, A.V.F., D.I.F. and N.V.U.; formal analysis, A.V.F., D.I.F. and N.V.U.; investigation, A.V.F., N.V.U., D.I.F. and A.A.O.; writing—original draft preparation, A.V.F. and N.V.U.; writing—review and editing, D.S.K. and N.V.U.; visualization, A.V.F. and D.I.F.; supervision, N.V.U. and D.S.K.; funding acquisition, A.V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (grant no. 23-73-01078).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
This study was performed using the instrumentation of the Core Facility Center “Arktika” of the Northern (Arctic) Federal University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 2D NMR | Two-dimensional nuclear magnetic resonance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| FRAP | Ferric reducing antioxidant power |
| GC | Gas chromatography |
| HMBC | Heteronuclear multiple bond correlation |
| HPLC | High-performance liquid chromatography |
| HSQC | Heteronuclear single quantum coherence |
| MS | Mass spectrometry |
| RM | Raw materials |
| TOCSY | Total correlation spectroscopy |
References
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Dampc, A.; Luczkiewicz, M. Labrador tea—The aromatic beverage and spice: A review of origin, processing and safety. J. Sci. Food Agric. 2015, 95, 1577–1583. [Google Scholar] [CrossRef]
- Mamedov, N.; Gardner, Z.; Craker, L.E. Medicinal plants used in Russia and Central Asia for the treatment of selected skin conditions. J. Herbs Spices Med. Plants 2004, 11, 191–222. [Google Scholar] [CrossRef]
- Shikov, A.N.; Tsitsilin, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Heinrich, M. Traditional and Current Food Use of Wild Plants Listed in the Russian Pharmacopoeia. Front. Pharmacol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Singh, B.; Gupta, J.; Mohan, R.; Gupta, R.; Pal, K. Hypouricemic significance of Ledum palustre (Marsh tea). J. Pharm. Technol. 2021, 14, 2907–2912. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef]
- Baananou, S.; Bagdonaite, E.; Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Boughattas, N.A. Supercritical CO2 extract and essential oil of aerial part of Ledum palustre L.-Chemical composition and anti-inflammatory activity. Nat. Prod. Res. 2015, 29, 999–1005. [Google Scholar] [CrossRef]
- Baldwin, T.A.; Oberbauer, S.F. Essential oil content of Rhododendron tomentosum responds strongly to manipulation of ecosystem resources in Arctic Alaska. Arct. Sci. 2022, 8, 916–934. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Sender, J.; Danuta, U.; Maślanko, W.; Canale, A.; Barboni, L.; Petrelli, R.; Zeppa, L.; et al. Ascaridole-rich essential oil from marsh rosemary (Ledum palustre) growing in Poland exerts insecticidal activity on mosquitoes, moths and flies without serious effects on non-target organisms and human cells. Food Chem. Toxicol. 2020, 138, 111184. [Google Scholar] [CrossRef]
- Jesionek, A.; Poblocka-Olech, L.; Zabiegala, B.; Bucinski, A.; Krauze-Baranowska, M.; Luczkiewicz, M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Kokotkiewicz, A.; Mikosik-Roczynska, A.; Ciesielska-Figlon, K.; Luczkiewicz, P.; Bucinski, A.; Daca, A.; Witkowski, J.M.; Bryl, E.; Zabiegala, B.; et al. Chemical variability of Rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia 2019, 139, 104402. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, A.; Zabiegała, B.; Buciński, A.; Łuczkiewicz, M. From Harvesting to Distillation—Effect of Analytical Procedures on The Yield and Chemical Composition of Rhododendron tomentosum (Ledum palustre) Essential Oil. Acta Pol. Pharm.—Drug Res. 2019, 76, 83–92. [Google Scholar] [CrossRef]
- Judžentienė, A. Marsh rosemary (Rhododendron tomentosum Harmaja (ex Ledum palustre Linn) growing in Lithuania) essential oils and their properties. Chemija 2020, 31, 269–277. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential oils and supercritical CO2 extracts of arctic angelica (Angelica archangelica L.), marsh labrador tea (Rhododendron tomentosum) and common tansy (Tanacetum vulgare)—Chemical compositions and antimicrobial activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Q.; Yuan, G.; Xu, F.; Li, B.; Wang, J.; Ouyang, J. Comparison of the Essential Oil Composition of Wild Rhododendron tomentosum Stems, Leaves, and Flowers in Bloom and Non-bloom Periods from Northeast China. J. Essent. Oil-Bear. Plants 2016, 19, 1216–1223. [Google Scholar] [CrossRef]
- Mikhailova, N.S.; Rybalko, K.S. Chemical constitution of Ledum palustre. Chem. Nat. Compd. 1980, 16, 131–135. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, M.; Wang, M.-J.; Wang, D.; Wang, J.-L.; Zhang, S.-J. Triterpenoids isolated from leaves of Ledum palustre. Chin. Tradit. Herb. Drugs 2018, 49, 1250–1254. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S. Investigation of supercritical CO2-extracts of wild Ledum palustre l. (Rhododendron tomentosum harmaja) and identification of its metabolites by tandem mass spectrometry. Khimiya Rastitel’nogo Syr’ya 2022, 1, 179–191. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.-F.; Zhang, K.; Chen, L.-J.; Pei, S.-C.; Li, J.; Zhang, S.-J. Phenolic constituents of Ledum palustre and their antioxidant activity. Chin. Tradit. Herb. Drugs 2017, 48, 4394–4398. [Google Scholar] [CrossRef]
- Liu, X.-J.; Su, H.-G.; Peng, X.-R.; Bi, H.-C.; Qiu, M.-H. An updated review of the genus Rhododendron since 2010: Traditional uses, phytochemistry, and pharmacology. Phytochemistry 2024, 217, 113899. [Google Scholar] [CrossRef] [PubMed]
- MacRae, W.D.; Towers, G.H.N. Biological activities of lignans. Phytochemistry 1984, 23, 1207–1220. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Sarajlija, H.; Čukelj Mustač, N.; Novotni, D.; Mršić, G.; Brncic, M.; Curic, D. Preparation of Flaxseed for Lignan Determination by Gas Chromatography–Mass Spectrometry Method. Czech J. Food Sci. 2012, 30, 45. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; Venema, D.P.; Lasaroms, J.J.P.; Wähälä, K.; Hollman, P.C.H. Optimization of a liquid chrom-atoraphy-tandem mass spectrometry method for quantification of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in foods. J. Agric. Food Chem. 2004, 52, 4643–4651. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef]
- Popova, I.E.; Hall, C.; Kubatova, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef]
- Falev, D.I.; Faleva, A.V.; Krylov, I.A.; Sukhanov, A.E. Comparative study of four Yucca species by 2D-NMR and LC–MS. Nat. Prod. Res. 2024, 38, 544–548. [Google Scholar] [CrossRef]
- Faleva, A.V.; Ul’yanovskii, N.V.; Falev, D.I.; Onuchina, A.A.; Budaev, N.A.; Kosyakov, D.S. New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity. Metabolites 2022, 12, 974. [Google Scholar] [CrossRef]
- Faleva, A.V.; Ul’yanovskii, N.V.; Onuchina, A.A.; Falev, D.I.; Kosyakov, D.S. Comprehensive Characterization of Secondary Metabolites in Fruits and Leaves of Cloudberry (Rubus chamaemorus L.). Metabolites 2023, 13, 598. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Comprehensive Characterization of Chemical Composition and Antioxidant Activity of Lignan-Rich Coniferous Knotwood Extractives. Antioxidants 2022, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Ovchinnikov, D.V.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Analytical and Preparative Separation of Softwood Lignans by Supercritical Fluid Chromatography. Separations 2023, 10, 449. [Google Scholar] [CrossRef]
- Jerković, I.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R. Bioprospecting of Less-Polar Constituents from Endemic Brown Macroalga Fucus virsoides J. Agardh from the Adriatic Sea and Targeted Antioxidant Effects In Vitro and In Vivo (Zebrafish Model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Botta, B.; Monache, G.; Misiti, D.; Vitali, A.; Zappia, G. Aryltetralin Lignans: Chemistry, Pharmacology and Biotransformations. Curr. Med. Chem. 2001, 8, 1363–1381. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26, 1251. [Google Scholar] [CrossRef]
- Szakiel, A.; Voutquenne-Nazabadioko, L.; Henry, M. Isolation and biological activities of lyoniside from rhizomes and stems of Vaccinium myrtillus. Phytochem. Lett. 2011, 4, 138–143. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeon, W.J.; Yoo, E.S.; Kim, C.M.; Kwon, Y.S. The chemical constituents and their antioxidant activity of the stem of Rhododendron mucronulatum. Nat. Prod. Sci. 2005, 11, 97–102. [Google Scholar] [CrossRef][Green Version]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Rev. Pneumol. Clin. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Polat Kose, L.; Gulcin, İ. Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).