The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Real-Time Quantitative PRC Analysis
2.3. ELISA Assay
2.4. Immunofluorescence
2.5. C. elegans Strains and Lifespan Analysis
2.6. Body Size Analysis
2.7. Fluorescence Analysis of C. elegans Transgenic Strains
2.8. Evaluation of Reactive Oxygen Species (ROS) Levels
2.9. Statistical Analyses
3. Results
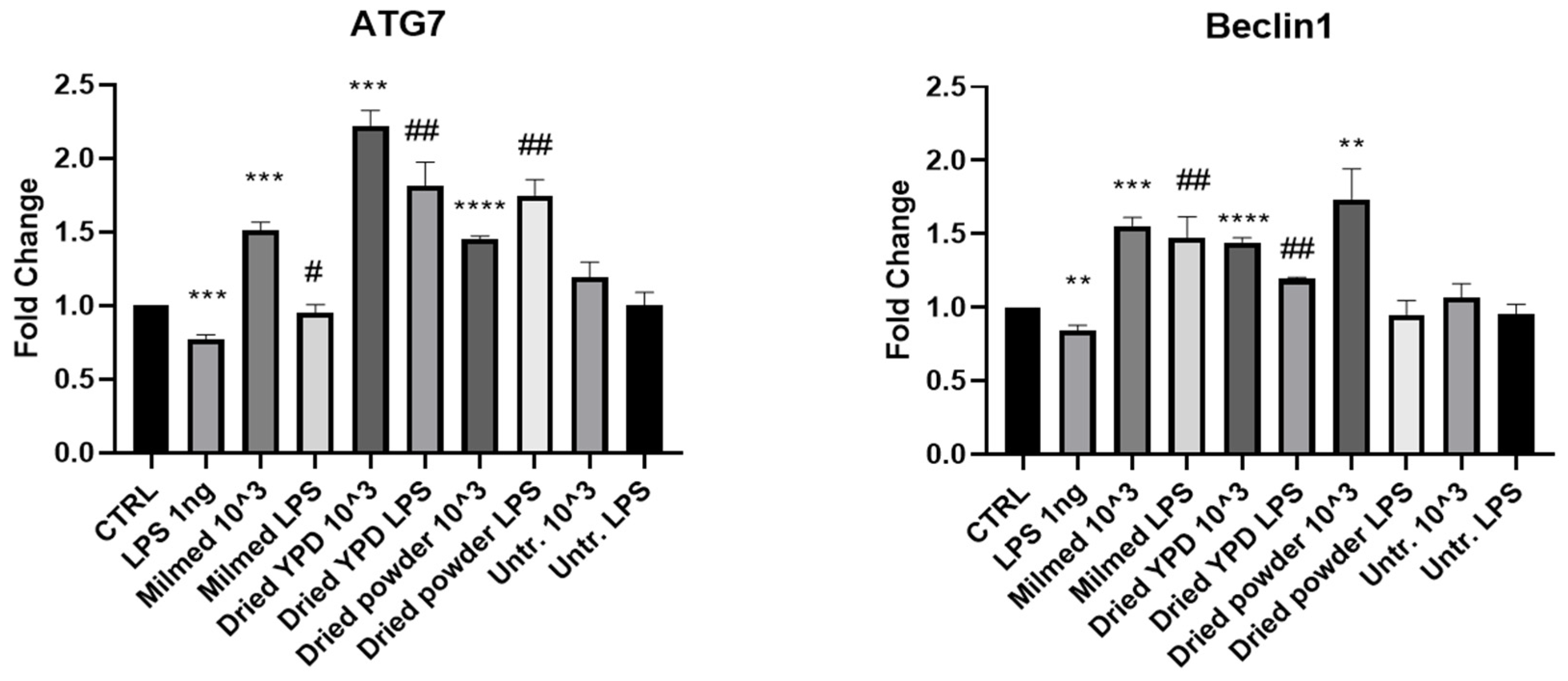
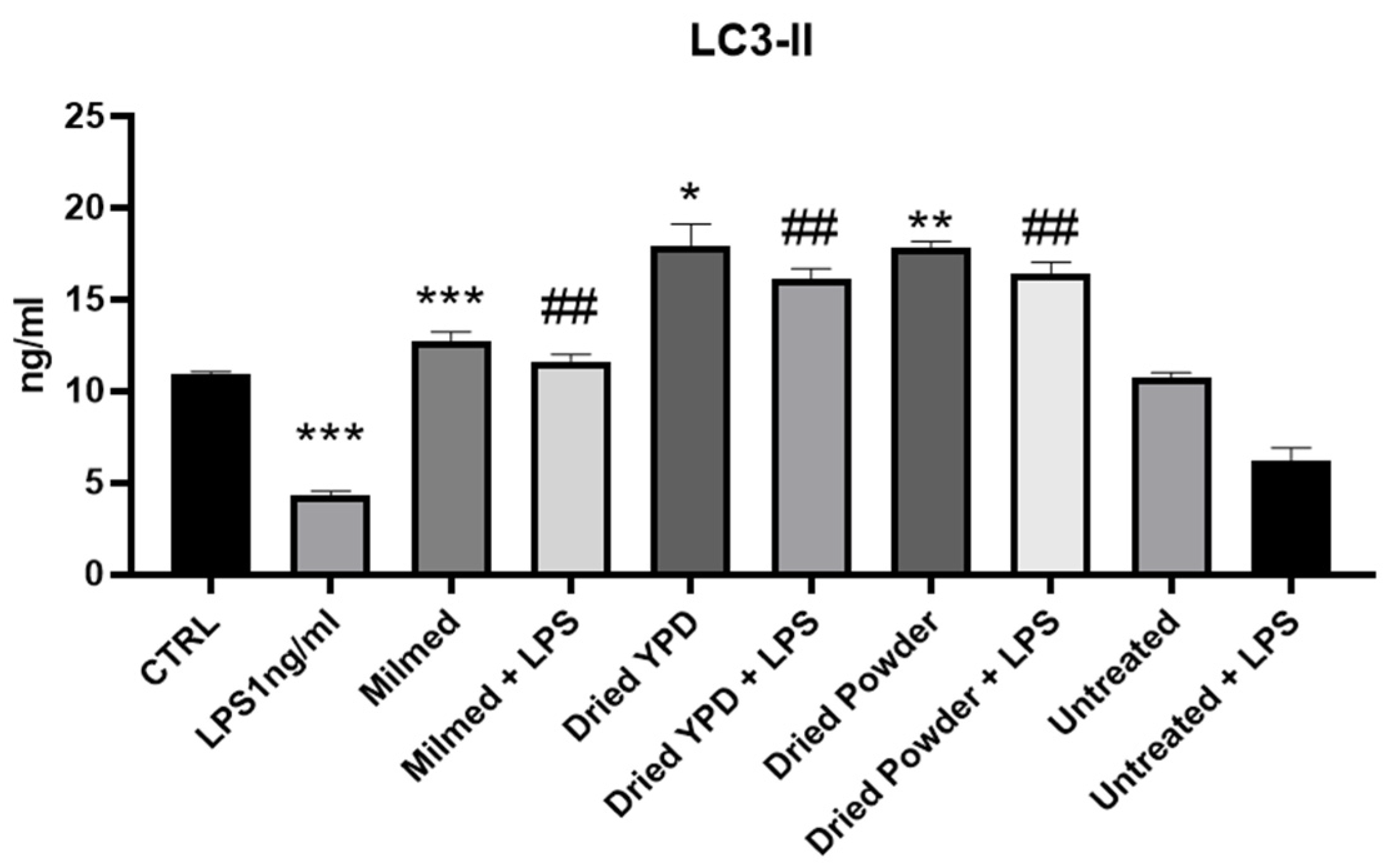
3.1. MILMED Restores Autophagic Processes in LPS-Treated BV2 Cells
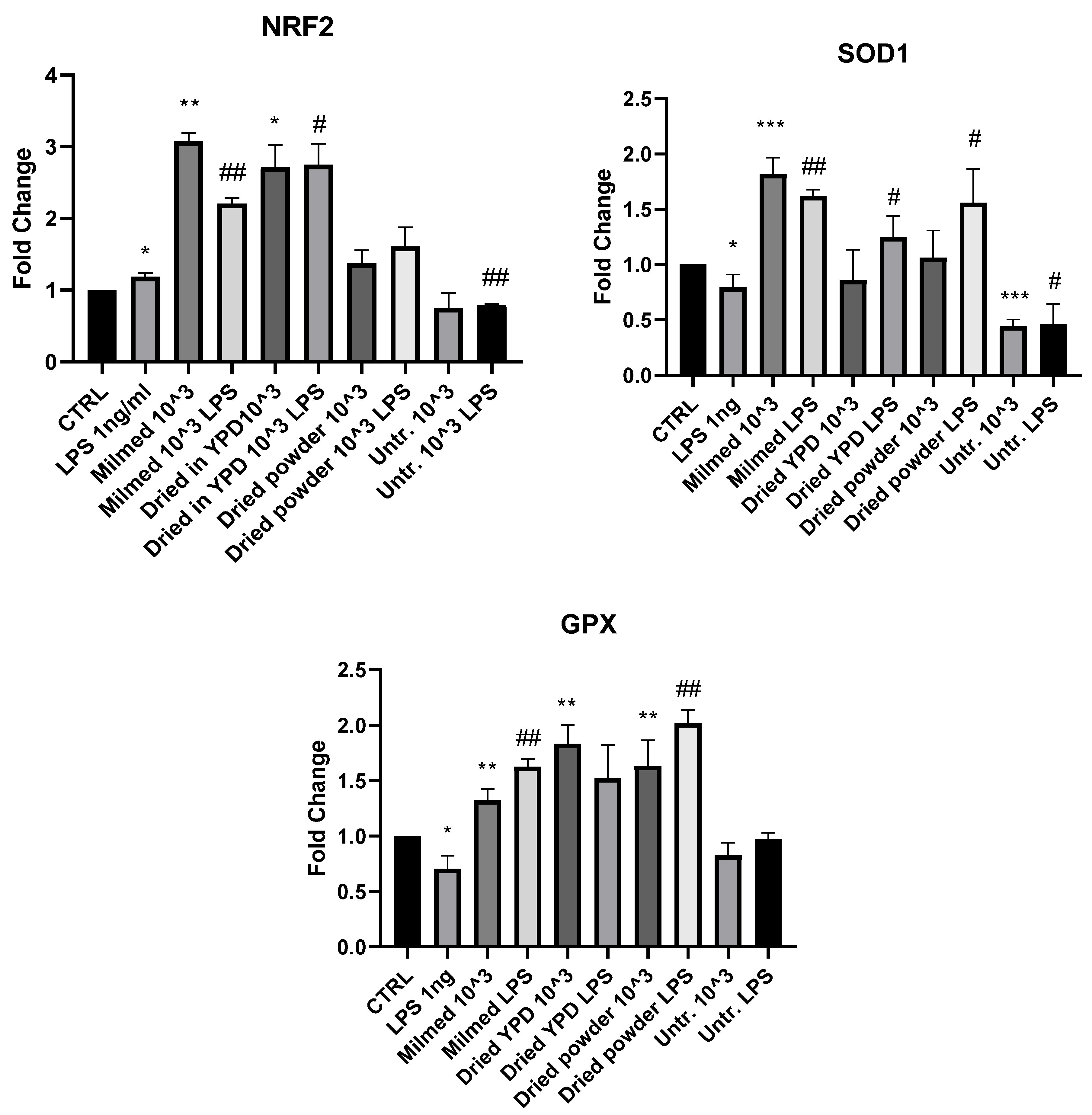
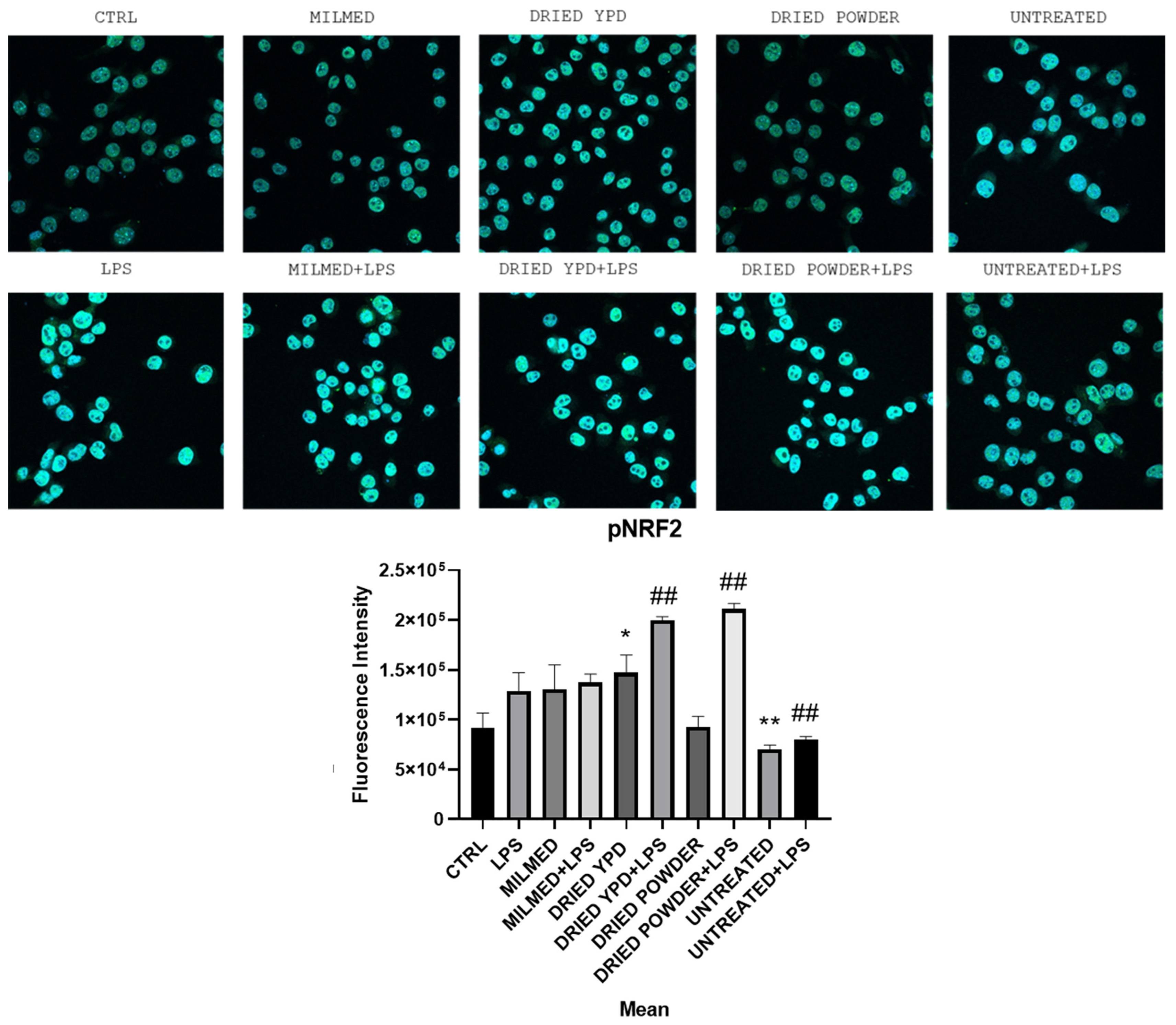
3.2. Antioxidant Effect of Milmed in BV2 Cells
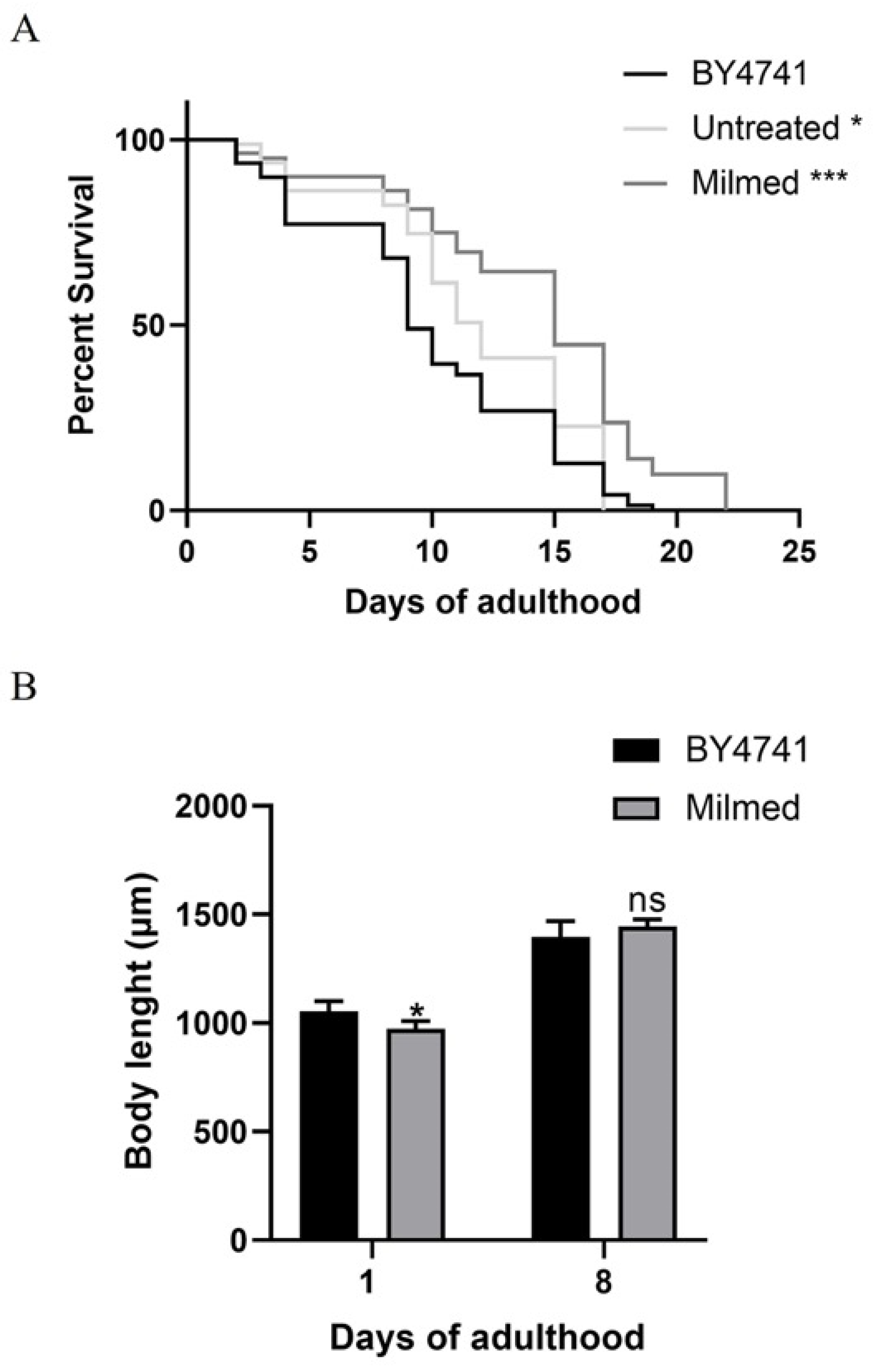
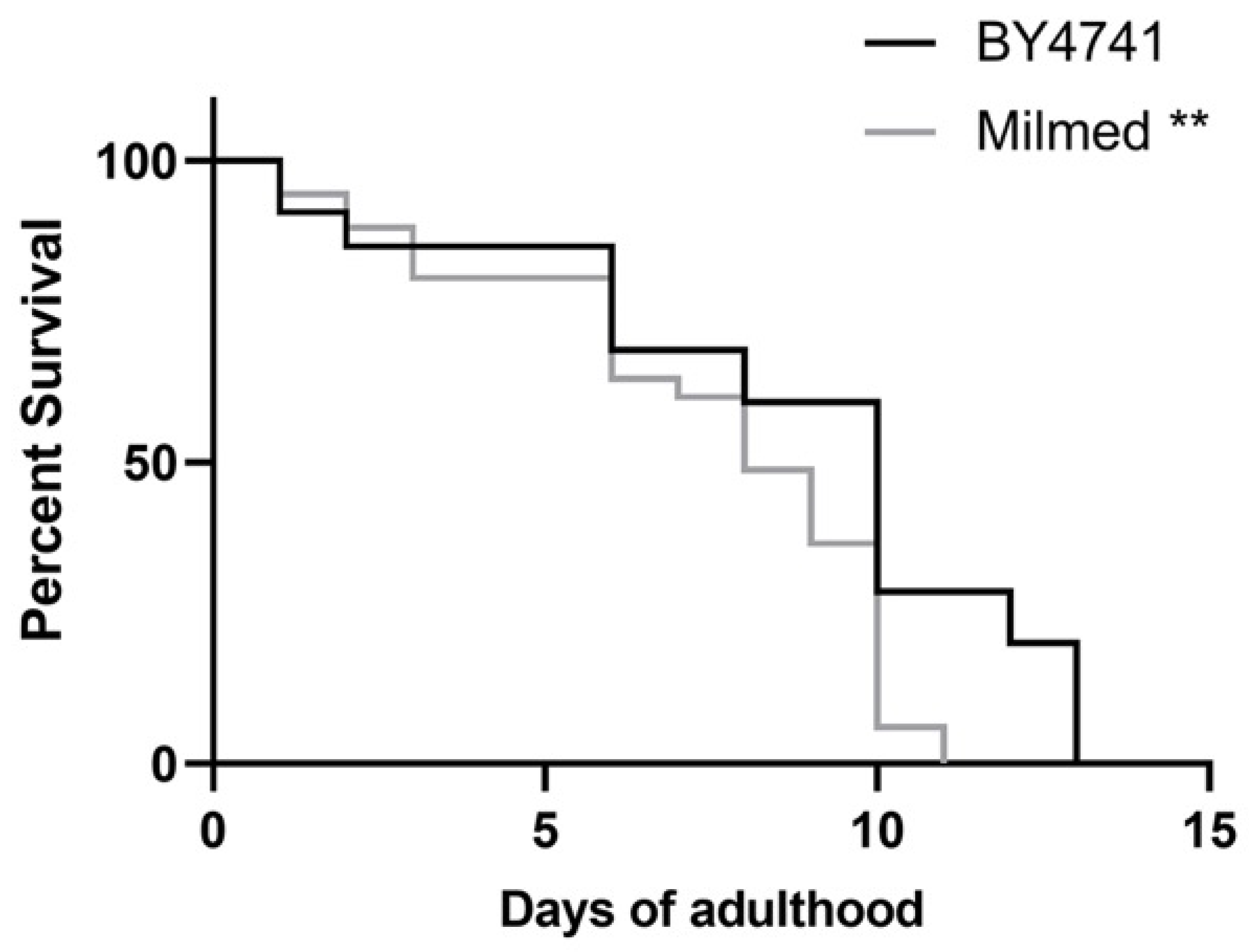
3.3. Milmed Yeast Extends Lifespan and Modulates Growth in C. elegans
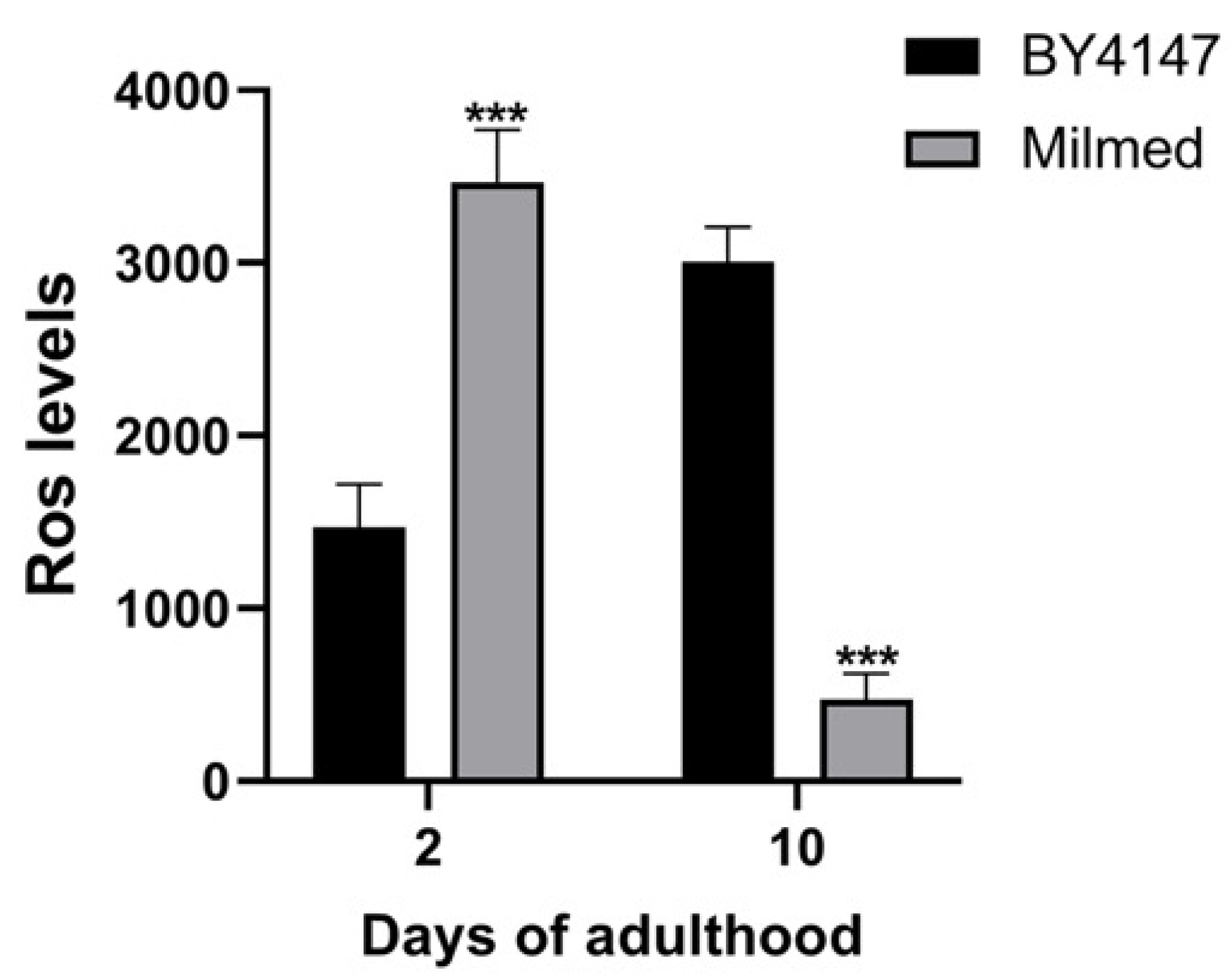
3.4. Milmed Yeast Supplementation Reduces Age-Related Oxidative Stress in C. elegans
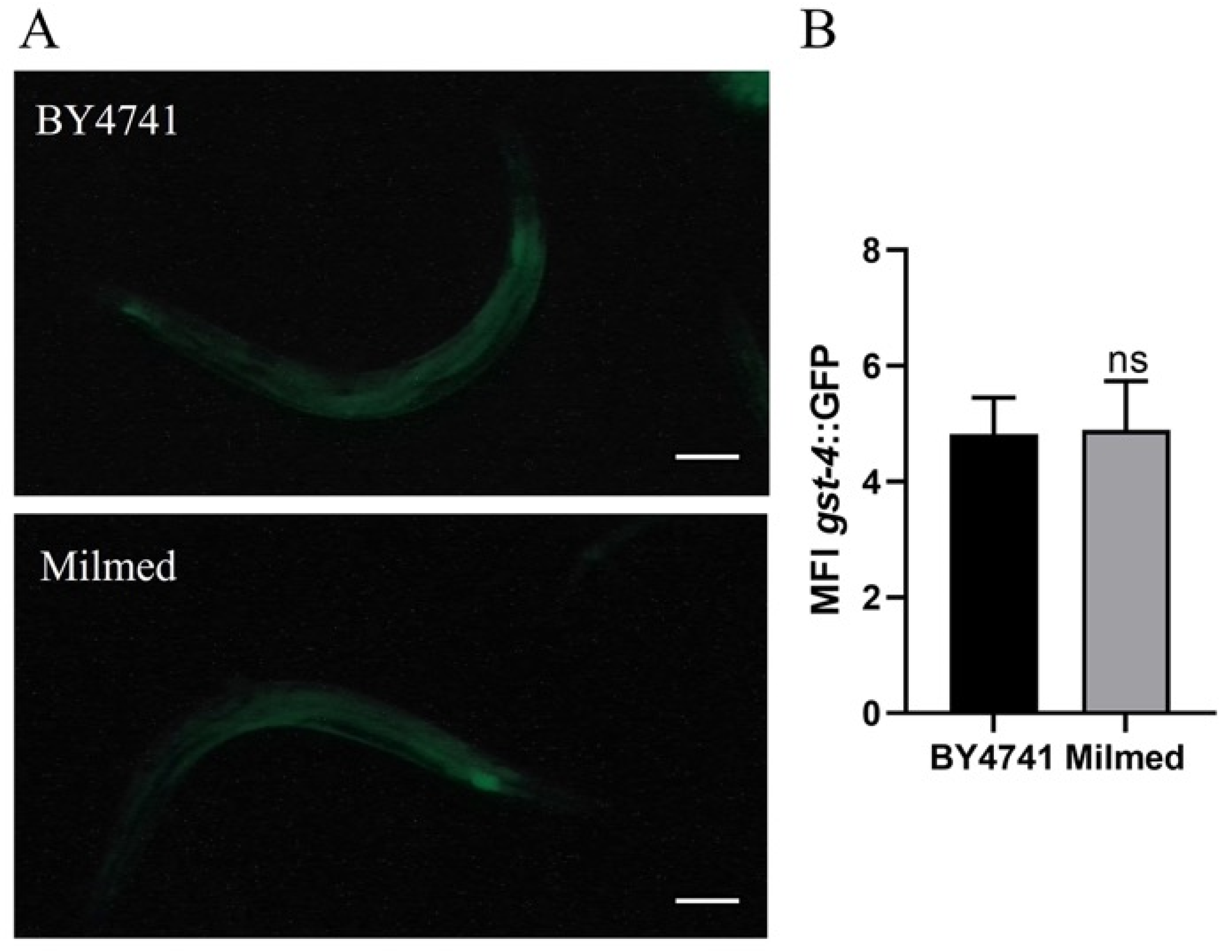
3.5. Milmed Yeast Extends Lifespan in C. elegans Through Activation of SKN-1/Nrf2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. Autophagy in Metabolic Disease and Ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef]
- Pinto, C.; Ninfole, E.; Benedetti, A.; Marzioni, M.; Maroni, L. Involvement of Autophagy in Ageing and Chronic Cholestatic Diseases. Cells 2021, 10, 2772. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the Hallmarks of Aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Q.; Kumar, A.V.; Mills, J.; Lapierre, L.R. Autophagy in Aging and Longevity. Hum. Genet. 2020, 139, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.A.; Botelho, M.; Carmo-Silva, S.; Pascoal, J.F.; Ferreira-Marques, M.; Nóbrega, C.; Cortes, L.; Valero, J.; Sousa-Ferreira, L.; Álvaro, A.R.; et al. Neuropeptide Y Stimulates Autophagy in Hypothalamic Neurons. Proc. Natl. Acad. Sci. USA 2015, 112, E1642–E1651. [Google Scholar] [CrossRef]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef]
- Konstantinidis, G.; Tavernarakis, N. Autophagy of the Nucleus in Health and Disease. Front. Cell Dev. Biol. 2022, 9, 814955. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; Elbestawy, A.R.; Shewita, R.S.; Ba-Awadh, H.A.; Alowaimer, A.N.; Abd El-Hamid, H.S. Single and Combined Effects of Clostridium Butyricum and Saccharomyces Cerevisiae on Growth Indices, Intestinal Health, and Immunity of Broilers. Animals 2018, 8, 184. [Google Scholar] [CrossRef]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.C.; Mesquita, E.T. Probiotic Therapy with Saccharomyces Boulardii for Heart Failure Patients: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Whelan, K.; Quigley, E.M.M. Probiotics in the Management of Irritable Bowel Syndrome and Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2013, 29, 184–189. [Google Scholar] [CrossRef]
- Shi, T.; Nishiyama, K.; Nakamata, K.; Aryantini, N.P.D.; Mikumo, D.; Oda, Y.; Yamamoto, Y.; Mukai, T.; Sujaya, I.N.; Urashima, T.; et al. Isolation of Potential Probiotic Lactobacillus Rhamnosus Strains from Traditional Fermented Mare Milk Produced in Sumbawa Island of Indonesia. Biosci. Biotechnol. Biochem. 2012, 76, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Webberley, T.S.; Masetti, G.; Bevan, R.J.; Kerry-Smith, J.; Jack, A.A.; Michael, D.R.; Thomas, S.; Glymenaki, M.; Li, J.; McDonald, J.A.K.; et al. The Impact of Probiotic Supplementation on Cognitive, Pathological and Metabolic Markers in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 843105. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yuan, S.; Kong, Y.; Yang, H.; Wei, H.; Zhang, Y.; Jin, H.; Yu, Q.; Liu, J.; Chen, S.; et al. Effect of Probiotic Fungi against Cognitive Impairment in Mice via Regulation of the Fungal Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2022, 70, 9026–9038. [Google Scholar] [CrossRef]
- Zaylaa, M.; Alard, J.; Al Kassaa, I.; Peucelle, V.; Boutillier, D.; Desramaut, J.; Rosenstiel, P.; Nguyend, H.T.T.; Dabboussi, F.; Pot, B.; et al. Autophagy: A Novel Mechanism Involved in the Anti-Inflammatory Abilities of Probiotics. Cell. Physiol. Biochem. 2019, 53, 774–793. [Google Scholar] [CrossRef]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of Probiotic Lactobacillus and Bifidobacterium Strains with Human Intestinal Epithelial Cells: Adhesion Properties, Competition against Enteropathogens and Modulation of IL-8 Production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Wang, J.; Zhao, H.; Xie, X.; Wu, Q. Probiotic or Probiotics Add Supplement Interferes with Coronary Heart Disease: A Meta-analysis of Randomized Controlled Trials. eFood 2023, 4, e120. [Google Scholar] [CrossRef]
- Vitetta, L.; Gobe, G. Uremia and Chronic Kidney Disease: The Role of the Gut Microflora and Therapies with pro- and Prebiotics. Mol. Nutr. Food Res. 2013, 57, 824–832. [Google Scholar] [CrossRef]
- Jin, S.; Chen, P.; Yang, J.; Li, D.; Liu, X.; Zhang, Y.; Xia, Q.; Li, Y.; Chen, G.; Li, Y.; et al. Phocaeicola Vulgatus Alleviates Diet-Induced Metabolic Dysfunction-Associated Steatotic Liver Disease Progression by Downregulating Histone Acetylation Level via 3-HPAA. Gut Microbes 2024, 16, 2309683. [Google Scholar] [CrossRef]
- Nemati, M.; Omrani, G.R.; Ebrahimi, B.; Montazeri-Najafabady, N. The Beneficial Effects of Probiotics via Autophagy: A Systematic Review. BioMed Res. Int. 2021, 2021, 2931580. [Google Scholar] [CrossRef] [PubMed]
- Legon, L.; Rallis, C. Genome-Wide Screens in Yeast Models towards Understanding Chronological Lifespan Regulation. Brief. Funct. Genom. 2022, 21, 4–12. [Google Scholar] [CrossRef]
- Archer, T.; Fredriksson, A. The Yeast Product Milmed Enhances the Effect of Physical Exercise on Motor Performance and Dopamine Neurochemistry Recovery in MPTP-Lesioned Mice. Neurotox. Res. 2013, 24, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.; Garcia, D.; Fredriksson, A. Restoration of MPTP-Induced Deficits by Exercise and Milmed® Co-Treatment. PeerJ 2014, 2, e531. [Google Scholar] [CrossRef] [PubMed]
- Archer, T. Milmed Treatment Alleviates Symptoms of Allergy and Improves General Health. J. Immunol. Allergy 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Archer, T. Anti-Inflammatory Action of the Treated-Yeast, Milmed, Under IBS-IBD Conditions. J. Immunol. Allergy 2022, 3. [Google Scholar] [CrossRef]
- Armeli, F.; Mengoni, B.; Maggi, E.; Mazzoni, C.; Preziosi, A.; Mancini, P.; Businaro, R.; Lenz, T.; Archer, T. Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to form M2 Anti-Inflammatory Phenotype. Biomedicines 2022, 10, 3116. [Google Scholar] [CrossRef]
- Golant, M.B.; Kuznetsov, A.P.; Bozhanova, T.P. The mechanism of synchronizing yeast cell cultures with EHF-radiation. Biofizika 1994, 39, 490–495. [Google Scholar]
- Golant, M.B.; Mudrik, D.G.; Kruglyakova, O.P.; Izvol’skaya, V.E. Effect of EHF-Radiation Polarization on Yeast Cells. Radiophys. Quantum Electron. 1994, 37, 82–84. [Google Scholar] [CrossRef]
- Archer, T. Physiologic And Subjective Health Benefits of Milmed Among Racehorses in Training. J. Immunol. Allergy 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Maggi, E.; Armeli, F.; Mengoni, B.; Leo, M.; Filetici, P.; Mancini, P.; Lenz, T.; Businaro, R.; Archer, T. Milmed Saccharomyces Cerevisiae Activity on Central Nervous System Cells. J. Toxicol. Pharmacol. 2022, 5, 1–8. [Google Scholar]
- Zhang, Z.; Yang, X.; Song, Y.-Q.; Tu, J. Autophagy in Alzheimer’s Disease Pathogenesis: Therapeutic Potential and Future Perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. Role of the Apg12 Conjugation System in Mammalian Autophagy. Int. J. Biochem. Cell Biol. 2003, 35, 553–561. [Google Scholar] [CrossRef]
- Liu, J.; Li, L. Targeting Autophagy for the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Front. Mol. Neurosci. 2019, 12, 203. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Yao, J.; Mi, N.; Yang, A. Phase Separation of P62: Roles and Regulations in Autophagy. Trends Cell Biol. 2025, 25, s0962-8924(25)00033-9. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 Antioxidant Defense System in Intervertebral Disc Degeneration: Molecular Insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.-S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting Natural Antioxidant Polyphenols to Protect Neuroinflammation and Neurodegenerative Diseases: A Comprehensive Review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Lo Vecchio, F.; Angelillis, V.S.; Gravina, C.; D’Onofrio, G.; Greco, A. Reinforcing Nrf2 Signaling: Help in the Alzheimer’s Disease Context. Int. J. Mol. Sci. 2025, 26, 1130. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, C.; Cao, Y.; Chen, Y. Caenorhabditis Elegans as an in Vivo Model for the Identification of Natural Antioxidants with Anti-Aging Actions. Biomed. Pharmacother. 2023, 167, 115594. [Google Scholar] [CrossRef]
- Chen, X.; Bahramimehr, F.; Shahhamzehei, N.; Fu, H.; Lin, S.; Wang, H.; Li, C.; Efferth, T.; Hong, C. Anti-Aging Effects of Medicinal Plants and Their Rapid Screening Using the Nematode Caenorhabditis Elegans. Phytomedicine 2024, 129, 155665. [Google Scholar] [CrossRef]
- Schifano, E.; Conta, G.; Preziosi, A.; Ferrante, C.; Batignani, G.; Mancini, P.; Tomassini, A.; Sciubba, F.; Scopigno, T.; Uccelletti, D.; et al. 2-Hydroxyisobutyric Acid (2-HIBA) Modulates Ageing and Fat Deposition in Caenorhabditis Elegans. Front. Mol. Biosci. 2022, 9, 986022. [Google Scholar] [CrossRef] [PubMed]
- Ficociello, G.; Schifano, E.; Di Nottia, M.; Torraco, A.; Carrozzo, R.; Uccelletti, D.; Montanari, A. Silencing of the Mitochondrial Ribosomal Protein L-24 Gene Activates the Oxidative Stress Response in Caenorhabditis Elegans. Biochim. Biophys. Acta BBA-Gen. Subj. 2023, 1867, 130255. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Lee, M.-H.; Cha, D. Measurement of Intracellular ROS in Caenorhabditis Elegans Using 2’,7’-Dichlorodihydrofluorescein Diacetate. BIO-Protoc. 2018, 8, e2774. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive Oxygen Species and Aging in Caenorhabditis Elegans: Causal or Casual Relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; E, X.; Jung, J.U. Downregulation of Autophagy by Herpesvirus Bcl-2 Homologs. Autophagy 2008, 4, 268–272. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Armeli, F.; Mengoni, B.; Laskin, D.L.; Businaro, R. Interplay among Oxidative Stress, Autophagy, and the Endocannabinoid System in Neurodegenerative Diseases: Role of the Nrf2-P62/SQSTM1 Pathway and Nutraceutical Activation. Curr. Issues Mol. Biol. 2024, 46, 6868–6884. [Google Scholar] [CrossRef]
- Pompa, L.; Montanari, A.; Tomassini, A.; Bianchi, M.M.; Aureli, W.; Miccheli, A.; Uccelletti, D.; Schifano, E. In Vitro Probiotic Properties and In Vivo Anti-Ageing Effects of Lactoplantibacillus Plantarum PFA2018AU Strain Isolated from Carrots on Caenorhabditis Elegans. Microorganisms 2023, 11, 1087. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Lin, Z.; Chen, X.; Jiao, Q.; Du, X.; Jiang, H. Targeting the Interplay Between Autophagy and the Nrf2 Pathway in Parkinson’s Disease with Potential Therapeutic Implications. Biomolecules 2025, 15, 149. [Google Scholar] [CrossRef]
- Fabi, J.P. The Connection between Gut Microbiota and Its Metabolites with Neurodegenerative Diseases in Humans. Metab. Brain Dis. 2024, 39, 967–984. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Jia, X.; Yang, Y.; Fang, Y.; Ying, X.; Li, H.; Zhang, M.; Wei, J.; Pan, Y. Microglial Polarization in Alzheimer’s Disease: Mechanisms, Implications, and Therapeutic Opportunities. J. Alzheimer’s Dis. 2025, 104, 3–13. [Google Scholar] [CrossRef]
- Humeau, J.; Leduc, M.; Cerrato, G.; Loos, F.; Kepp, O.; Kroemer, G. Phosphorylation of Eukaryotic Initiation Factor-2α (eIF2α) in Autophagy. Cell Death Dis. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Romanin, D.E.; Llopis, S.; Genovés, S.; Martorell, P.; Ramón, V.D.; Garrote, G.L.; Rumbo, M. Probiotic Yeast Kluyveromyces Marxianus CIDCA 8154 Shows Anti-Inflammatory and Anti-Oxidative Stress Properties in in Vivo Models. Benef. Microbes 2016, 7, 83–94. [Google Scholar] [CrossRef]
- Goyache, I.; Yavorov-Dayliev, D.; Milagro, F.I.; Aranaz, P. Caenorhabditis Elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 1321. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of Life Span Extension by Rapamycin in the Fruit Fly Drosophila Melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef]
- Madeo, F.; Tavernarakis, N.; Kroemer, G. Can Autophagy Promote Longevity? Nat. Cell Biol. 2010, 12, 842–846. [Google Scholar] [CrossRef]
- Bové, J.; Martínez-Vicente, M.; Vila, M. Fighting Neurodegeneration with Rapamycin: Mechanistic Insights. Nat. Rev. Neurosci. 2011, 12, 437–452. [Google Scholar] [CrossRef]
- Menzies, F.M.; Huebener, J.; Renna, M.; Bonin, M.; Riess, O.; Rubinsztein, D.C. Autophagy Induction Reduces Mutant Ataxin-3 Levels and Toxicity in a Mouse Model of Spinocerebellar Ataxia Type 3. Brain 2010, 133, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Ghillebert, R.; Wilms, T.; Winderickx, J. Molecular Mechanisms Linking the Evolutionary Conserved TORC1-Sch9 Nutrient Signalling Branch to Lifespan Regulation in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 17–32. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Thi Nguyen, N.-H.; Kim, J.H.; Lee, S.-M.; Cho, B.-K.; Kim, Y.-H.; Min, J. Inhibition of Tau Phosphorylation and Aβ Accumulation by S. Cerevisiae-Derived Vacuoles in LPS-Induced SH-SY5Y Cells. J. Biotechnol. 2023, 376, 45–52. [Google Scholar] [CrossRef]
- Hawrysh, P.J.; Gao, J.; Tan, S.; Oh, A.; Nodwell, J.; Tompkins, T.A.; McQuibban, G.A. PRKN/Parkin-Mediated Mitophagy Is Induced by the Probiotics Saccharomyces boulardii and Lactococcus lactis. Autophagy 2023, 19, 2094–2110. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Klionsky, D.J. Life and Death Decisions—The Many Faces of Autophagy in Cell Survival and Cell Death. Biomolecules 2022, 12, 866. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, L.; Makarczyk, M.J.; Feng, P.; Zhang, J. The Anti-Aging Mechanism of Metformin: From Molecular Insights to Clinical Applications. Molecules 2025, 30, 816. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, V.; Khan, H.; Singh, S.; Singh, T.G. Intersecting Molecular Pathways in Synucleinopathies and Amyloidogenesis: Exploring Shared Mechanisms and Therapeutic Potential. Brain Res. 2025, 1855, 149568. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Cenzi, G.; Melo, D.S.; Dias, D.R.; Schwan, R.F. Probiotic Potential, Antioxidant Activity, and Phytase Production of Indigenous Yeasts Isolated from Indigenous Fermented Foods. Probiotics Antimicrob. Proteins 2020, 12, 280–288. [Google Scholar] [CrossRef]
- Siesto, G.; Pietrafesa, R.; Infantino, V.; Thanh, C.; Pappalardo, I.; Romano, P.; Capece, A. In Vitro Study of Probiotic, Antioxidant and Anti-Inflammatory Activities among Indigenous Saccharomyces Cerevisiae Strains. Foods 2022, 11, 1342. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Li, H.; Zhang, Z.; Sheng, W.; Zhang, S.; Li, P.; Zhang, X.; Li, X.; Lin, H.; et al. Carboxymethylated Yeast β-Glucan: Biological Activity Screening in Zebrafish, Sprayable Hydrogel Preparation, and Wound Healing Study in Diabetic Mice. Int. J. Biol. Macromol. 2025, 285, 138178. [Google Scholar] [CrossRef] [PubMed]


| GENE | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession Numbers | bp |
|---|---|---|---|---|
| mLC3 | TTCTTCCTCCTGGTGAATGG | GTCTCCTGCGAGGCATAAAC | NM_026160 | 2455 |
| mBeclin-1 | CAGCCTCTGAAACTGGACACGA | CTCTCCTGAGTTAGCCTCTTCC | NM_019584 | 2072 |
| mNrf2 | TCTGAGCCAGGACTACGACG | GAGGTGGTGGTGGTGTCTCTGC | NM_010902 | 2347 |
| mp62 | CCTTGCCCTACAGCTGAGTC | CCACACTCTCCCCCACATTC | NM_001290769 | 1916 |
| mATG7 | CAATGAGATCTGGGAAGCCATAA | AGGTCAAGAGCAGAAACTTGTTGA | NM_001253717 | 3872 |
| mβ-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT | NM_007393.5 | 1935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armeli, F.; Mengoni, B.; Schifano, E.; Lenz, T.; Archer, T.; Uccelletti, D.; Businaro, R. The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. elegans. Antioxidants 2025, 14, 393. https://doi.org/10.3390/antiox14040393
Armeli F, Mengoni B, Schifano E, Lenz T, Archer T, Uccelletti D, Businaro R. The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. elegans. Antioxidants. 2025; 14(4):393. https://doi.org/10.3390/antiox14040393
Chicago/Turabian StyleArmeli, Federica, Beatrice Mengoni, Emily Schifano, Thomas Lenz, Trevor Archer, Daniela Uccelletti, and Rita Businaro. 2025. "The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. elegans" Antioxidants 14, no. 4: 393. https://doi.org/10.3390/antiox14040393
APA StyleArmeli, F., Mengoni, B., Schifano, E., Lenz, T., Archer, T., Uccelletti, D., & Businaro, R. (2025). The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. elegans. Antioxidants, 14(4), 393. https://doi.org/10.3390/antiox14040393








