Boosting Antioxidant Quality in Cucumber Beverages with Encapsulated Tomato Carotenoids
Abstract
1. Introduction
2. Materials and Methods
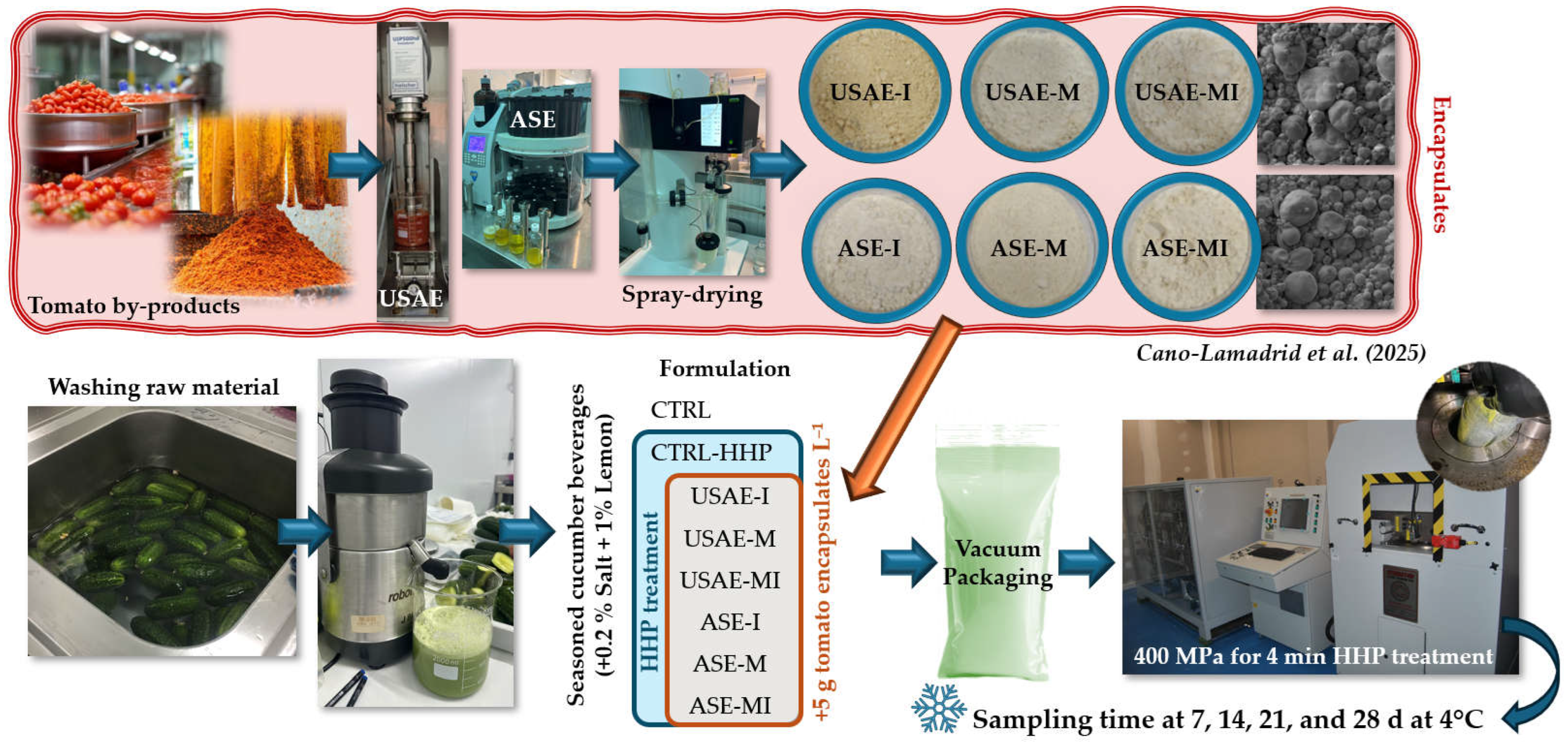
2.1. Obtention and Encapsulation of Antioxidant Tomato By-Product
2.2. Reformulation of the Seasoned Cucumber Beverage
2.3. Microbial Analysis
2.4. Physicochemical Analysis
2.5. Extraction and Analysis of Main Bioactive Compounds with Antioxidant Properties
2.5.1. Free Phenolic Content and Total Antioxidant Capacity
2.5.2. Carotenoid Content
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Evolution During Shelf Life
3.2. Microbial Load During Shelf Life
3.3. Antioxidant Bioactive Compounds Evolution During Refrigerated Storage
3.3.1. Free Phenolic Content and Antioxidant Capacity
3.3.2. Carotenoid Profile and Content
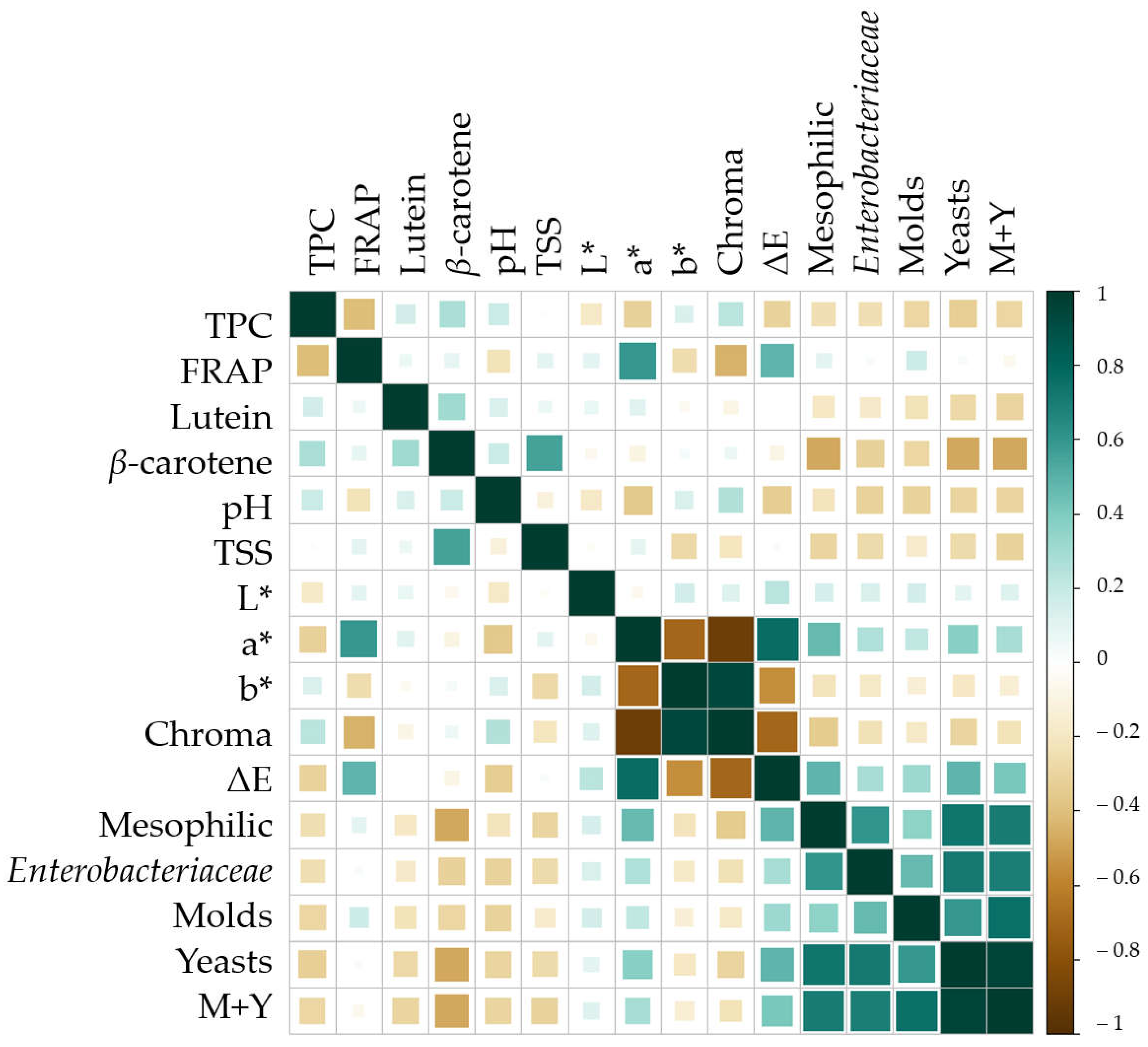
3.4. Statistic Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 15 January 2025).
- Hasan, M.M.; Islam, M.R.; Haque, A.R.; Kabir, M.R.; Khushe, K.J.; Hasan, S.M.K. Trends and Challenges of Fruit By-Products Utilization: Insights into Safety, Sensory, and Benefits of the Use for the Development of Innovative Healthy Food: A Review. Bioresour. Bioprocess. 2024, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Recovery of Carotenoids from Tomato Processing By-Products—A Review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- López Bermúdez, Y.N.; Aldana Heredia, J.F.; Sánchez-Camargo, A.d.P.; Hernández-Carrión, M. Valorization Strategies for a By-Product of Organic Tomato Processing as Potential Ingredient in Functional Food Formulations. Front. Food Sci. Technol. 2022, 2, 893795. [Google Scholar] [CrossRef]
- Szabo, K.; Emőke Teleky, B.; Ranga, F.; Simon, E.; Lelia Pop, O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian Vodnar, D. Bioaccessibility of Microencapsulated Carotenoids, Recovered from Tomato Processing Industrial by-Products, Using in Vitro Digestion Model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- Jiří, B.; Lenka, V.; Josef, S.; Věra, K. Exploring Carotenoids: Metabolism, Antioxidants, and Impacts on Human Health. J. Funct. Foods 2024, 118, 106284. [Google Scholar]
- Zhang, C.; Li, K.; Xu, S.N.; Zhang, J.K.; Ma, M.H.; Liu, Y. Higher Serum Carotenoid Concentrations Were Associated with the Lower Risk of Cancer-Related Death: Evidence from the National Health and Nutrition Examination Survey. Nutr. Res. 2024, 126, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Özkaynak Kanmaz, E. Humic Acid Formation during Subcritical Water Extraction of Food By-Products Using Accelerated Solvent Extractor. Food Bioprod. Process. 2019, 115, 118–125. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Sharma, K.; Paul, R.K. Optimal Extraction and Fingerprinting of Carotenoids by Accelerated Solvent Extraction and Liquid Chromatography with Tandem Mass Spectrometry. Food Chem. 2015, 177, 369–375. [Google Scholar] [CrossRef]
- Mehta, N.; S, J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Mozafari, L.; Cano-Lamadrid, M.; Martínez-Zamora, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Pulsed Ultrasound-Assisted Extraction of Lycopene and β-Carotene from Industrial Grated Tomato by-Products. LWT 2024, 204, 116462. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application—A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Matos Jorge, L.M.; Jorge, R.M.M. Microencapsulation of Bioactive Compound Extracts Using Maltodextrin and Gum Arabic by Spray and Freeze-Drying Techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef]
- Badin, R.; Gaiani, C.; Desobry, S.; Prakash, S.; Bhandari, B.; Rasch, R.; Bostelmann, H.; Burgain, J. Links between Single Maltodextrin Particles Properties and Powder Functionality. Carbohydr. Polym. 2025, 350, 123057. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Fasolin, L.H.; Silva, E.K. Rheological Properties, Microstructure, and Encapsulation Efficiency of Inulin-Type Dietary Fiber-Based Gelled Emulsions at Different Concentrations. Carbohydr. Polym. 2025, 347, 122742. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Xu, B. Differences in Physicochemical, Rheological, and Prebiotic Properties of Inulin Isolated from Five Botanical Sources and Their Potential Applications. Food Res. Int. 2024, 180, 114048. [Google Scholar] [CrossRef] [PubMed]
- Heena; Kumar, V.; Kaur, J.; Gat, Y.; Chandel, A.; Suri, S.; Panghal, A. Optimization of the Different Variables for the Development of a Cucumber-Based Blended Herbal Beverage. Beverages 2017, 3, 50. [Google Scholar] [CrossRef]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable Functional Cucumber Juices Supplemented with Polyphenols-Rich Herbal Extracts. LWT 2021, 148, 111668. [Google Scholar] [CrossRef]
- Salazar-Bermeo, J.; Moreno-Chamba, B.; Heredia-Hortigüela, R.; Lizama, V.; Martínez-Madrid, M.C.; Saura, D.; Valero, M.; Neacsu, M.; Martí, N. Green Technologies for Persimmon By-Products Revalorisation as Sustainable Sources of Dietary Fibre and Antioxidants for Functional Beverages Development. Antioxidants 2023, 12, 1085. [Google Scholar] [CrossRef]
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Bañón, S.; Ros, J.M. Shelf-Life Extension of Multi-Vegetables Smoothies by High-Pressure Processing Compared with Thermal Treatment. Part I: Microbial and Enzyme Inhibition, Antioxidant Status, and Physical Stability. J. Food Process Preserv. 2019, 43, e14139. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Mozafari, L.; Martínez-Zamora, L.; Lorca, F.; García-Gómez, P.; Artés-Hernández, F. Obtaining Carotenoid Encapsulates with Polysaccharides Carriers after Pilot Scale Accelerated Solvent Extraction and Ultrasound-Assisted Extraction from Industrial Tomato by-Product. Food Res. Int. 2025, 203, 115908. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the Effects of High Hydrostatic Pressure and Thermal Pasteurization Combined with Nisin on the Quality of Cucumber Juice Drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Regulation (EC) No 2073/2005 of the Comission of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Parliament and of the Council: Brussels, Belgium, 2005; Volume 13, pp. 141–166. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analyticial Chemistry: Arlington, VA, USA, 2002. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A Rapid and Sensitive Method for Determination of Carotenoids in Plant Tissues by High Performance Liquid Chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Mohd Rosli, N.N.H.; Harun, N.H.; Abdul Rahman, R.; Ngadi, N.; Samsuri, S.; Amran, N.A.; Safiei, N.Z.; Ab Hamid, F.H.; Zakaria, Z.Y.; Jusoh, M. Preservation of Total Phenolic Content (TPC) in Cucumber Juice Concentrate Using Non-Thermal Progressive Freeze Concentration: Quantitative Design Characteristics and Process Optimization. J. Clean. Prod. 2022, 330, 129705. [Google Scholar] [CrossRef]
- Babajide, J.M.; Olaluwoye, A.A.; Taofik Shittu, T.A.; Adebisi, M.A. Physicochemical Properties and Phytochemical Components of Spiced Cucumber-Pineapple Fruit Drink. Niger. Food J. 2013, 31, 40–52. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent Advances in Food Processing Using High Hydrostatic Pressure Technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, Encapsulation, and Health Benefits of β-Carotene—A Review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Elsadek, M.F.; Mohamed, A.S.; Taha, A.E.; Ahmed, B.M.; Saad, A.M. Effects of Chemical and Natural Additives on Cucumber Juice’s Quality, Shelf Life, and Safety. Foods 2020, 9, 639. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Gao, Z.; Cheng, Y.; Yang, X.; Mu, S.; Qu, K. Effect of Dynamic High-Pressure Microfluidization on the Quality of Not-from-Concentrate Cucumber Juice. Foods 2024, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Zhao, L.; Wang, Y.; Liao, X. Potential of High-Pressure Processing and High-Temperature/Short-Time Thermal Processing on Microbial, Physicochemical and Sensory Assurance of Clear Cucumber Juice. Innov. Food Sci. Emerg. Technol. 2016, 34, 51–58. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Parada, J.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Role of Maltodextrin and Inulin as Encapsulating Agents on the Protection of Oleuropein during in Vitro Gastrointestinal Digestion. Food Chem. 2020, 310, 125976. [Google Scholar] [CrossRef]
- Vieira, M.V.; Noore, S.; Tiwari, B.; O’Donnell, C.; Gonçalves, C.; Pastrana, L.M.; Fuciños, P. Enhancing the Stability and Functionality of Phycobiliproteins as Natural Food Colourants through Microparticle Formulation. Food Chem. 2025, 465, 142077. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and Antioxidant Properties of Tomato Processing Byproducts and Their Correlation with the Biochemical Composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- International Commission on Microbi. Microorganisms in Foods 7, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-68458-1. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Martínez, L.; Jongberg, S.; Ros, G.; Skibsted, L.H.; Nieto, G. Plant Derived Ingredients Rich in Nitrates or Phenolics for Protection of Pork against Protein Oxidation. Food Res. Int. 2020, 129, 108789. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant Evaluation Protocols: Food Quality or Health Effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Yan, W.; Wang, J.; Xu, J.; Cai, C.; Qi, X.; Xu, Q.; Yang, X.; Xu, X.; et al. Cucumber Abscisic Acid 8′-Hydroxylase Csyf2 Regulates Yellow Flesh by Modulating Carotenoid Biosynthesis. Plant Physiol. 2023, 193, 1001–1015. [Google Scholar] [CrossRef]
- Navazio, J.P.; Simon, P.W. Diallel Analysis of High Carotenoid Content in Cucumbers. J. Am. Soc. Hortic. Sci. 2001, 126, 100–104. [Google Scholar] [CrossRef]
- De Ancos, B.; Rodrigo, M.J.; Sánchez-Moreno, C.; Pilar Cano, M.; Zacarías, L. Effect of High-Pressure Processing Applied as Pretreatment on Carotenoids, Flavonoids and Vitamin C in Juice of the Sweet Oranges “Navel” and the Red-Fleshed “Cara Cara”. Food Res. Int. 2020, 132, 109105. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Stability of Avocado Paste Carotenoids as Affected by High Hydrostatic Pressure Processing and Storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 121–128. [Google Scholar] [CrossRef]
- Obel, H.O.; Cheng, C.; Tian, Z.; Njogu, M.K.; Li, J.; Du, S.; Lou, Q.; Zhou, J.; Yu, X.; Ogweno, J.O.; et al. Transcriptomic and Physiological Analyses Reveal Potential Genes Involved in Photoperiod-Regulated β-Carotene Accumulation Mechanisms in the Endocarp of Cucumber (Cucumis sativus L.) Fruit. Int. J. Mol. Sci. 2022, 23, 12650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Guo, S.; Ren, Y.; Li, M.; Wang, J.; Zhang, H.; Gong, G.; Xu, Y. Decreased Protein Abundance of Lycopene β-Cyclase Contributes to Red Flesh in Domesticated Watermelon. Plant Physiol. 2020, 183, 1171–1183. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid Metabolism and Regulation in Horticultural Crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
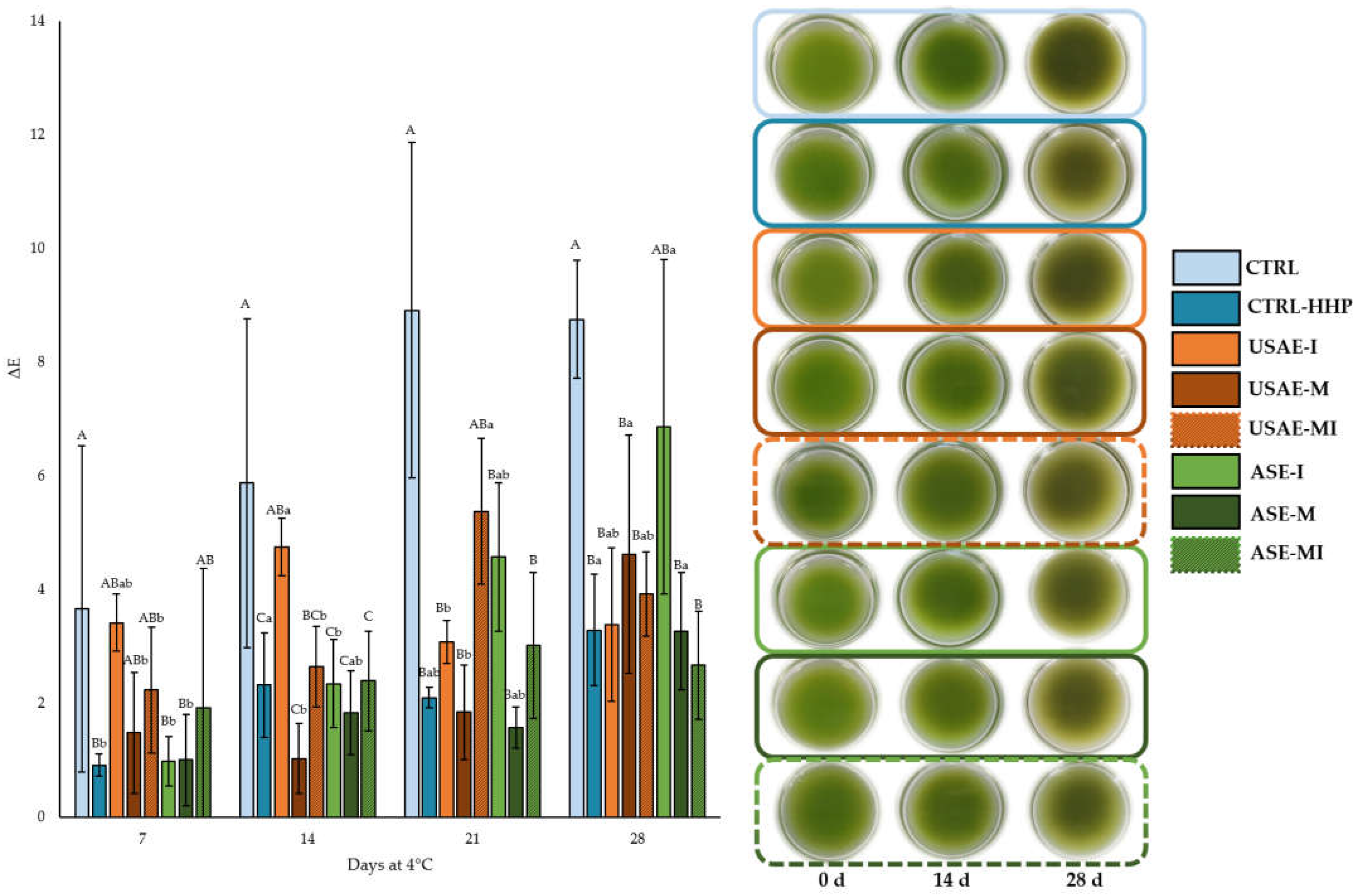



| Treatments | Day at 4 °C | pH | TSS | a* | b* | Chroma |
|---|---|---|---|---|---|---|
| CTRL | 0 | 4.80 ± 0.00 a | 4.50 ± 0.10 C b | −7.65 ± 0.33 BC c | 9.12 ± 0.54 ABCD | 11.90 ± 0.62 ABC a |
| 7 | 4.77 ± 0.06 ab | 4.53 ± 0.06 BC ab | −6.3 ± 0.75 A b | 8.25 ± 0.54 B | 10.38 ± 0.88 B ab | |
| 14 | 4.63 ± 0.06 C cd | 4.73 ± 0.06 E a | −5.95 ± 0.34 A ab | 7.38 ± 1.23 D | 9.51 ± 0.92 E b | |
| 21 | 4.60 ± 0.00 B d | 4.70 ± 0.10 B ab | −4.89 ± 0.46 A a | 7.92 ± 0.53 B | 9.31 ± 0.55 B b | |
| 28 | 4.70 ± 0.00 C bc | 4.53 ± 0.06 B ab | −5.51 ± 0.29 ab | 8.45 ± 0.57 | 10.09 ± 0.63 ab | |
| CTRL-HHP | 0 | 4.87 ± 0.06 a | 4.50 ± 0.10 C ab | −7.31 ± 0.12 AB d | 9.17 ± 0.17 ABC a | 11.72 ± 0.20 ABC a |
| 7 | 4.80 ± 0.00 b | 4.43 ± 0.06 C ab | −6.86 ± 0.17 ABC c | 8.83 ± 0.18 AB ab | 11.18 ± 0.24 AB ab | |
| 14 | 4.77 ± 0.06 AB b | 4.73 ± 0.06 E a | −6.40 ± 0.30 AB b | 8.49 ± 0.40 BC b | 10.63 ± 0.49 BCD b | |
| 21 | 4.70 ± 0.00 AB c | 4.20 ± 0.35 C b | −6.21 ± 0.07 BCD ab | 8.83 ± 0.04 AB ab | 10.79 ± 0.06 AB b | |
| 28 | 4.80 ± 0.00 AB b | 4.43 ± 0.23 B ab | −6.01 ± 0.31 a | 9.16 ± 0.49 a | 10.96 ± 0.58 b | |
| USAE-I | 0 | 4.80 ± 0.00 a | 5.00 ± 0.00 A b | −6.76 ± 0.03 A c | 8.47 ± 0.03 D | 10.84 ± 0.00 D |
| 7 | 4.80 ± 0.00 a | 4.97 ± 0.06 AB b | −6.33 ± 0.16 A b | 8.38 ± 0.25 B | 10.51 ± 0.29 B | |
| 14 | 4.73 ± 0.06 ABC b | 5.33 ± 0.12 B a | −5.91 ± 0.28 A a | 8.29 ± 0.53 BCD | 10.18 ± 0.59 CDE | |
| 21 | 4.63 ± 0.06 B c | 5.07 ± 0.06 AB b | −6.01± 0.05 BC ab | 8.51 ± 0.18 AB | 10.42 ± 0.17 AB | |
| 28 | 4.70 ± 0.00 C b | 5.07 ± 0.06 A b | −6.11 ± 0.30 ab | 8.74 ± 0.56 | 10.66 ± 0.63 | |
| USAE-M | 0 | 4.83± 0.06 | 4.70 ± 0.35 BC b | −7.35 ± 0.59 B c | 8.95 ± 0.68 BCD a | 11.58 ± 0.90 BCD a |
| 7 | 4.77 ± 0.06 | 4.93 ± 0.06 AB ab | −7.14 ± 0.39 BC bc | 8.96 ± 0.49 AB a | 11.46 ± 0.63 AB a | |
| 14 | 4.83 ± 0.06 A | 4.83 ± 0.06 DE ab | −6.94 ± 0.20 B abc | 8.98 ± 0.34 AB a | 11.34 ± 0.38 AB a | |
| 21 | 4.80 ± 0.10 A | 4.80 ± 0.00 AB ab | −6.59 ± 0.31 CD ab | 8.43 ± 0.46 AB ab | 10.70 ± 0.55 AB ab | |
| 28 | 4.83 ± 0.06 A | 5.07 ± 0.15 A a | −6.25 ± 0.42 a | 7.87 ± 0.61 b | 10.05 ± 0.74 b | |
| USAE-MI | 0 | 4.80 ± 0.00 a | 5.00 ± 0.10 A b | −7.74 ± 0.20 BC c | 9.70 ± 0.35 A a | 12.41 ± 0.38 AB a |
| 7 | 4.73 ± 0.06 b | 5.17 ± 0.06 A b | −6.51 ± 0.27 AB b | 8.31 ± 0.37 B ab | 10.55 ± 0.46 B b | |
| 14 | 4.73 ± 0.06 ABC b | 5.57 ± 0.06 A a | −6.61 ± 0.38 AB b | 7.58 ± 0.30 CD d | 10.06 ± 0.04 DE bc | |
| 21 | 4.60 ± 0.00 B c | 5.17 ± 0.35 A b | −5.54 ± 0.23 AB a | 7.90 ± 0.42 B cd | 9.65 ± 0.47 B c | |
| 28 | 4.70 ± 0.00 C b | 5.07 ± 0.23 A b | −5.80 ± 0.27 a | 8.89 ± 0.30 b | 10.62 ± 0.38 b | |
| ASE-I | 0 | 4.80 ± 0.00 a | 5.03 ± 0.12 A | −8.16± 0.29 C d | 9.59 ± 0.28 AB a | 12.60 ± 0.39 A a |
| 7 | 4.77 ± 0.06 ab | 4.97 ± 0.06 AB | −7.07 ± 0.12 BC c | 8.67 ± 0.23 B ab | 11.19 ± 0.26 AB b | |
| 14 | 4.70 ± 0.00 BC b | 5.00 ± 0.10 CD | −6.31 ± 0.13 AB b | 8.42 ± 0.43 BCD b | 10.52 ± 0.41 BCDE bc | |
| 21 | 4.60 ± 0.00 B c | 4.97 ± 0.06 AB | −5.89 ± 0.19 BC ab | 7.86 ± 0.51 B b | 9.82 ± 0.51 B c | |
| 28 | 4.70 ± 0.00 C b | 5.00 ± 0.00 A | −580 ± 0.14 a | 7.97 ± 0.48 b | 9.86 ± 0.36 c | |
| ASE-M | 0 | 4.83 ± 0.06 a | 5.03 ± 0.06 A | −7.80 ± 0.32 BC c | 9.38 ± 0.45 AB | 12.20 ± 0.55 ABC a |
| 7 | 4.80 ± 0.00 a | 4.90 ± 0.30 ABC | −7.31 ± 0.25 C bc | 9.14 ± 0.51 AB | 11.70 ± 0.55 A ab | |
| 14 | 4.70 ± 0.00 BC b | 5.10 ± 0.10 C | −6.82 ± 0.29 B ab | 8.89 ± 0.73 AB | 11.21 ± 0.76 BC ab | |
| 21 | 4.60 ± 0.00 B c | 5.00 ± 0.10 AB | −6.55 ± 0.10 CD a | 8.75 ± 0.21 AB | 10.93 ± 0.22 AB ab | |
| 28 | 4.70 ± 0.00 C b | 5.07 ± 0.06 A | −6.28 ± 0.26 a | 8.60 ± 0.52 | 10.65 ± 0.57 b | |
| ASE-MI | 0 | 4.90 ± 0.00 a | 4.83 ± 0.06 AB | −7.34 ± 0.36 B b | 8.67 ± 0.30 CD | 11.36 ± 0.46 CD ab |
| 7 | 4.80 ± 0.00 b | 4.60 ± 0.69 BC | −7.54 ± 0.59 C b | 9.66 ± 1.01 A | 12.26 ± 1.16 A a | |
| 14 | 4.80 ± 0.00 B b | 4.97 ± 0.15 CD | −7.74 ± 1.01 C b | 9.65 ± 0.23 A | 12.39 ± 0.78 A a | |
| 21 | 4.70 ± 0.00 AB c | 5.00 ± 0.10 AB | −6.95 ± 0.60 D ab | 9.57 ± 1.05 A | 11.83 ± 1.20 A ab | |
| 28 | 4.73 ± 0.06 BC c | 5.07 ± 0.06 A | −6.17 ± 0.20 a | 8.47 ± 0.33 | 10.48 ± 0.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozafari, L.; Martínez-Zamora, L.; Cano-Lamadrid, M.; Gómez, P.A.; Artés-Hernández, F. Boosting Antioxidant Quality in Cucumber Beverages with Encapsulated Tomato Carotenoids. Antioxidants 2025, 14, 354. https://doi.org/10.3390/antiox14030354
Mozafari L, Martínez-Zamora L, Cano-Lamadrid M, Gómez PA, Artés-Hernández F. Boosting Antioxidant Quality in Cucumber Beverages with Encapsulated Tomato Carotenoids. Antioxidants. 2025; 14(3):354. https://doi.org/10.3390/antiox14030354
Chicago/Turabian StyleMozafari, Laleh, Lorena Martínez-Zamora, Marina Cano-Lamadrid, Perla A. Gómez, and Francisco Artés-Hernández. 2025. "Boosting Antioxidant Quality in Cucumber Beverages with Encapsulated Tomato Carotenoids" Antioxidants 14, no. 3: 354. https://doi.org/10.3390/antiox14030354
APA StyleMozafari, L., Martínez-Zamora, L., Cano-Lamadrid, M., Gómez, P. A., & Artés-Hernández, F. (2025). Boosting Antioxidant Quality in Cucumber Beverages with Encapsulated Tomato Carotenoids. Antioxidants, 14(3), 354. https://doi.org/10.3390/antiox14030354












