Abstract
Consumer demand for low-alcohol acidic beers is driving the use of non-conventional yeasts in the brewing process. In this study, the addition of mixed berries and fermentation with L. thermotolerans L31 are performed in crafting a low-alcohol acidic beer. Four different beers were brewed in the primary stage with either Saccharomyces cerevisiae or L. thermotolerans and with or without added berry mixture. Beer was fermented for 8 days at 20 °C, stored, and bottled. pH, density, alcoholic content, bitterness, and color of final beer were analyzed for all samples using analytical methods. Volatile compounds, anthocyanin content, and antioxidant activity were also evaluated. Sensory analysis was performed and correlated (PCA) with the analytical results. The obtained data indicated that beers brewed with L. thermotolerans were significantly more acidic and less bitter than S. cerevisiae beers. No difference in alcoholic content was found. Fruity aroma-associated compounds were present in L. thermotolerans beers, which correlated with the sensory analysis. Fruit beers were also redder and showed higher anthocyanin content and stronger antioxidant activity due to the presence of anthocyanins such as cyanidin, delphinidin, and malvidin from fruit, and other antioxidant compounds.
1. Introduction
Lately, the brewing industry has experienced an increase in the production of craft beers as an alternative to traditional brewing [1]. There are currently more than 150 different types of craft beer. Although the craft brewing process resembles many of the steps performed in traditional brewing, there may be some differences amongst them [2]. Due to its efficiency in ethanol production, its ability to metabolize maltose [3], and its ethanol tolerance [1], Saccharomyces cerevisiae is the main yeast used in beer making [4]. However, other non-Saccharomyces yeasts are now being studied and beginning to be used to produce craft beers [5] with unique flavors and aromas [6].
Belgian lambic beer is a type of sour traditional beer characterized by a higher concentration of organic acids, resulting in low pH [7] and acidic taste [8]. Traditionally, Belgian lambic beers are obtained by spontaneous fermentation carried out by bacteria and yeast that naturally exist in oak barrels were the wort is left to mature. The global process can take up to several months [9] and it is only performed during winter months, among many other limitations [7]. Therefore, the brewing industry is seeking innovative methods that allow enhanced production under controlled conditions and reduced-time processes, including controlled mixed fermentations and use of lactic acid bacteria (BAL) and acetic acid bacteria (AAB) [7,10].
An interesting alternative is the use of special non-Saccharomyces yeasts [11] that have low fermentative capacity and high enzymatic activity related to the production of volatile acids [12] and metabolites. These yeasts can produce low-alcohol beers [13] while contributing sensory characteristics similar to those of sour beers [14]. Among these yeasts, Lachancea thermotolerans, Torulaspora delbrueckii, and Metschnikowia pulcherrima stand out [15].
Lachancea thermotolerans (formerly Kluyveromyces thermotolerans) is a yeast species from the genus Lachancea that is found in several ecological niches, notably grape must [16]. It is used in winemaking to lower pH via lactic acid production and, therefore, to enhance sensory properties [17]. This heterofermentative yeast converts sugars into ethanol and lactic acid [18], metabolizing glucose, fructose, and sucrose similarly to Saccharomyces cerevisiae, although it cannot ferment maltotriose [18,19]. It exhibits the Crabtree effect [20], favoring fermentation over respiration in aerobic, glucose-rich environments, leading to ethanol production over biomass growth [21,22]. It consumes more sugar than S. cerevisiae to produce the same ethanol yield [17] and can produce lactic acid, reaching 1–9 g/L depending on the strain [20,23]. It is also appreciated for its ability to produce more glycerol than other yeasts [15,17]. Its growth is not significantly inhibited by hops or ethanol (up to 10% v/v) [24,25]. Additionally, it produces volatiles like 2-phenylethanol, ethyl lactate, and fruity esters, contributing to sweet and fruity aromas that are desirable in beers. These traits make L. thermotolerans an appealing starter culture for craft sour beer production [15,26,27].
In this study, Lachancea thermotolerans was used as a non-Saccharomyces yeast to produce a sour fruit beer by substituting bacterial acidification with yeast acidification, obtaining a final product with sensorial characteristics similar to those of lambic beers. This combination of traditional and modern methods in craft beer brewing opens new possibilities for the creation of differentiated, high-quality products.
2. Materials and Methods
2.1. Preparation of the Wort
The mashing process was carried out in a 40 L mash tun (Grainfather, Auckland, New Zealand), to which was added 6 kg of dehydrated and milled Château Pilsen 2RS malt (Castle Malting, Beloeil, Belgium), 37.5 g of roasted Carafa Type 1 malt (Weyermann, Bamberg, Germany) ground with a manual disc mill, and 18 L of tap water previously boiled and conditioned with calcium sulphate di-hydrate (CaSO4-2H2O) to a concentration of 90 mg/L Ca. The water was heated to 72 °C and added to the mash tun, and the mash tun was controlled to maintain a constant temperature of 68 °C for 60 min. The wort was washed in the same mash tun by lifting the inner lauter tun and adding 16.5 L of water previously adjusted to a calcium concentration of 90 mg/L with calcium sulphate dihydrate, heated to 77 °C, and added in three batches, two of 6.5 L and one of 4.5 L, allowing the water to drain between each batch. Once the wort had been washed, the bagasse was removed and the boiling process began, raising the temperature of the wort to 100 °C and boiling for 75 min. After the first 15 min of boiling, 30 g of Lemondrop hop pellets was added. After boiling, the wort was stirred and left to settle for 25 min.
2.2. Yeast Strains and Culture Media
Two yeasts strains, Saccharomyces cerevisiae 7VA and Lachancea thermotolerans L31 (Blizz™, Lallemand, https://www.lallemandwine.com/es/spain/productos/levaduras-enologicas/blizz/ (accessed on 1 May 2025)), were used for the first fermentation of the must. The yeasts were isolated in Ribera de Duero, Valladolid, and preserved by the microbiology department (ETSIAAB, UPM, Madrid, Spain) in tubes in YPD medium (1% yeast extract, 2% peptone, and 2% glucose) and 1.5% agar in the freezer. These strains were selected for several reasons. S. cerevisiae 7VA was selected for his high fermentative capacity, suitable fermentative kinetics, and low production of volatile acids. L. thermotolerans L31 was selected for its capacity to produce lactic acid. An initial growth was then carried out on the same medium in Petri dishes. The two yeasts were then inoculated in previously sterilized YPD liquid medium prepared for the growth of both yeasts separately. The inoculum was left in an oven at 28 °C for two days to promote growth.
2.3. Fermentation and Storage
The must at 20 °C was pumped into 12 different 2 L bottles to be used as fermenters, which had previously been autoclaved at 121 °C for 15 min, to a volume of 1700 mL each. Fermentations were carried out in triplicates. Berries were added to 6 of the 12 bottles, weighing 195 g of a mixture of Hacendado (Valencia, Spain) brand berries produced by Congelados de Navarra (Fustiñana, Navarra, Spain), containing strawberries, redcurrants, raspberries, blackberries, blackcurrants, and blueberries. The bottles were inoculated with 34 mL of yeast culture, corresponding to 2% of the final volume. The yeast inoculum concentration was 8 log CFU/mL. Six of the bottles were inoculated with S. cerevisiae and the other six with L. thermotolerans. Each bottle was fitted with a fermentation stopper that allowed CO2 from the fermentation to escape but did not allow microbial contaminants to enter the fermenter.
The fermenters were placed in an oven to keep them at a constant temperature of 20 °C for 8 days, with density, pH, and glucose samples taken every two days to monitor the fermentation, which was stopped when the density of the samples reached a value of around 1012 kg/m3.
Beers inoculated with L31 without added fruit did not complete fermentation and their density stalled above the target density. To complete the fermentation, they were re-inoculated with 0.4 g of yeast per liter of beer, using S. cerevisiae QA23™ active dry yeast (Lallemand Iberia, Madrid, Spain).
The final fermented beers were transferred to a cold room at 4 °C and stored for 7 days. The content of each bottle was then transferred to a clean, sterile bottle and re-transferred to clean bottles every two days to remove the solids and yeast residues. Table 1 shows the key to the experiment, indicating the characteristics, fermentation parameters, and number or replicates of each beer.

Table 1.
Primary fermentation parameters.
2.4. Bottling and Secondary Fermentation
The beer was transferred to sterilized 330 mL amber bottles. In order to produce CO2 in the beer, a secondary fermentation was performed. To achieve this, 0.4 g/L of active dry yeast S. cerevisiae QA23™ (Lallemand Iberia, Madrid, Spain) was added to each bottle. Sucrose (Hacendado, Spain) was dissolved in water and added to each bottle to a final concentration of 5 g/L. Finally, the bottles were capped with crown corks using a manual capper and the beers were stored in a cold room at 4 °C for 15 days for secondary fermentation.
2.5. Instrumental Analysis
2.5.1. Density
The densities of both the wort and finished beer were measured at 20 °C in a 50 mL cylinder using densimeters in the ranges of 1000 to 1050 kg/m3 and 1050 to 1100 kg/m3, respectively.
2.5.2. pH
All wort and finished beer samples were measured at 20 °C using a pre-calibrated Crison micropH 2000 pH meter (Barcelona, Spain).
2.5.3. Glucose
Analysis of glucose concentration was carried out using the Food Quality Enology enzyme kit (Biosystems, Barcelona, Spain) by measuring the absorbance of the samples at a wavelength of 500 nm using a J.P. SELECTA UV-2005 UV–Visible spectrophotometer (Barcelona, Spain).
2.5.4. Color
The color of the beer was measured spectrophotometrically using a J.P. SELECTA UV-2005 UV–Visible spectrophotometer (Barcelona, Spain), according to ASBC protocol number 10 [28]. The samples were filtered through a mixed cellulose ester (MCE) syringe filter with a pore size of 0.45 μm, and their absorbance was measured at 700 nm. The color was calculated in two different ways: the SRM and EBC scales [29].
2.5.5. Alcoholic Content
The ethanol concentration of each sample was determined using combined methods of distillation and densimetry. First, 200 mL of each beer was distilled with 7 mL of lime slurry to neutralize it, and 100 mL of distilled water was added. The distilled hydroalcoholic solution was collected in a receiving flask until it was two-thirds full. The flask was made up to 200 mL with distilled water and transferred to a 250 mL graduated cylinder, wherein the temperature and alcohol content were measured using an alcoholmeter. As the temperature of the samples was not 20 °C, a correction was made using the correlation table of the International Organization of Vine and Wine (OIV).
2.5.6. Bitterness
Bitterness analysis was carried out using a spectrophotometric method with a J.P. SELECTA UV-2005 UV–Visible spectrophotometer (Barcelona, Spain) by determining the α-acid concentration according to ASBC protocol number 23A [30]. First, 10 mL of each beer, 1 mL of 3M HCl, 20 mL of isooctane, and 50 µL of octanol were mixed in a 50 mL tube. The samples were vortexed and centrifuged to ensure complete separation of the phases, and the absorbance was measured at a wavelength of 275 nm using a 1 cm quartz cuvette. Bitterness was calculated in IBUs (International Bitterness Units) from the absorbance.
2.5.7. Determination of Volatile Compounds
An Agilent Technologies 6850 gas chromatograph (GC System Network) equipped with a flame ionization detector (Hewlett-Packard, Palo Alto, CA, USA) was used to determine the concentration of the different volatile compounds present in the beers. A DB-624 column was used. The column temperature was 40 °C for the first 5 min, which was then increased by 10 °C per minute until 250 °C was reached. The injector temperature was 250 °C and the detector temperature was 300 °C. Hydrogen was used as the carrier gas. The method used to analyze the volatile compounds in the sample corresponded to the OIV protocol of volatile compounds (Resolution OIV-OENO 553-2016, https://www.oiv.int/public/medias/4968/oiv-oeno-553-2016-es.pdf, accessed on 20 January 2025).
The method used was calibrated by elaborating calibration curves of the following compounds in standard solutions: acetaldehyde, methanol, 1-propanol, diacetyl, ethyl acetate, 2-butanol, 2-butanol, isobutyl alcohol, 1-butanol, acetoin, 2-methyl-1butanol, 3-methyl-1-butanol, isobutyl acetate, ethyl butyrate, ethyl lactate, 2,3-butanediol, isoamyl acetate, hexanol, 2-phenylethanol, and 2-phenylethyl acetate (r2 > 0.999 for all compounds, LOD = 0.1 mg/L). Calibration curves were obtained in the form of hydroalcoholic solutions, with 13% v/v ethanol in water and different concentrations of between 1.000 and 500.000 mg/L of the compounds. 4-Methyl-2-pentanol (50 mg/L; Fluka Chemie GmbH, Buchs, Switzerland) was used as an internal standard (100 µL per sample).
Samples were evaluated in triplicate in the following order: 7VA, L31, 7VAF, and L31F. The column was washed with water between different types of beer to avoid interferences and instrumental soiling. Before measuring, samples were filtered through a 0.45 μm pore mixed cellulose ester (MCE) syringe filter, and 1 mL of the filtered sample was added to 1.5 mL chromatographic vials. The injection volume was 1 µL. The results were obtained in the form of chromatograms, in which the integration of each signal was performed to determine the concentration of each compound.
2.5.8. Determination of Antioxidant Activity
The antioxidant activity of beer was determined using the ABTS method, as described in Re et al. (1999) [31]. Production of ABTS˙+ was obtained through the reaction of 7 mM ABTS and 2.45 mM potassium persulfate at room temperature for 24 h in the dark. Additionally, 2.5 mM Trolox (Sigma Aldrich, Merck, Burlington, MA, USA) was used as a standard. Next, 1 mL of diluted ABTS˙+ (A734nm = 0.712) was added to 10 µL of sample/Trolox standard and left in the dark for 4 min. Then, absorbance at 734 nm was measured and used to calculate the antioxidant activity of samples in terms of Trolox equivalent antioxidant activity. Samples were analyzed in triplicate.
2.5.9. Analysis of Anthocyanins Using HPLC-DAD
For the identification of anthocyanins, an Agilent Technologies (Palo Alto, CA, USA) series 1260 HPLC chromatograph equipped with a diode array detector (DAD) was used. The mobile phase consisted of a dilution of water/formic acid (95:5 v/v) (solvent A) and methanol/formic acid (95:5 v/v) (solvent B). The gradient was 0–2 min, 25% B; 2–10 min, 25–50% B linear; 10–11 min, 50% B; 11–12 min, 50–2% B linear; and 12–17 min, re-equilibration. The flow rate was set at 1 mL/min and the pressure of the column was higher than 200 bar according to the gradient composition. The temperature was 25 °C. The column used for the separation of pigments was a reverse-phase Poroshell 120 C18 column (Phenomenex, Torrance, CA, USA) with dimensions of 50 mm × 4.6 mm and a particle size 2.7 μm. The monitoring wavelength was set at 525 nm for the identification of anthocyanins. Samples were defrosted and filtered using 0.45 µm syringe filters. The volume of injection was 50 µL. Concentrations were calculated using a calibration curve with malvidin-3-O-glucoside as the external standard (r2 = 0.9999, LOD = 0.1 mg/L)
2.6. Sensory Analysis
Sensory evaluation was carried out with a panel of 10 trained judges from the Department of Chemistry and Food Technology of ETSIAAB (UPM). Each panelist tasted four beers, corresponding to the four different beer formulations. They rated 20 descriptive attributes using a tasting sheet: visual, olfactory, taste, aftertaste, and general perception. The attributes were rated on a five-point scale, with (1) low perception and (5) high perception. Tasting was carried out in a special room with white light, a controlled temperature of 21 °C, ventilation, and a sample preparation room.
2.7. Statistical Analysis
Determination was carried out in triplicate. Data were processed using Statgraphics v.16.2.04 (Graphics Software System, Rockville, MD, USA) and Microsoft Excel (2505 version). Two-way Analysis of Variance (ANOVA, LSD test) was performed considering two factors: yeast species (7VA, L31) and presence of berries (YES/NO). The level of significance was set at p-value < 0.05. In addition, XLSTAT version 1429 (2025.1.1) (Addinsoft, Paris, France) software was used to run a PCA and Pearson’s correlation test between the data obtained from the volatile analysis and the sensory test.
3. Results
3.1. Fermentation
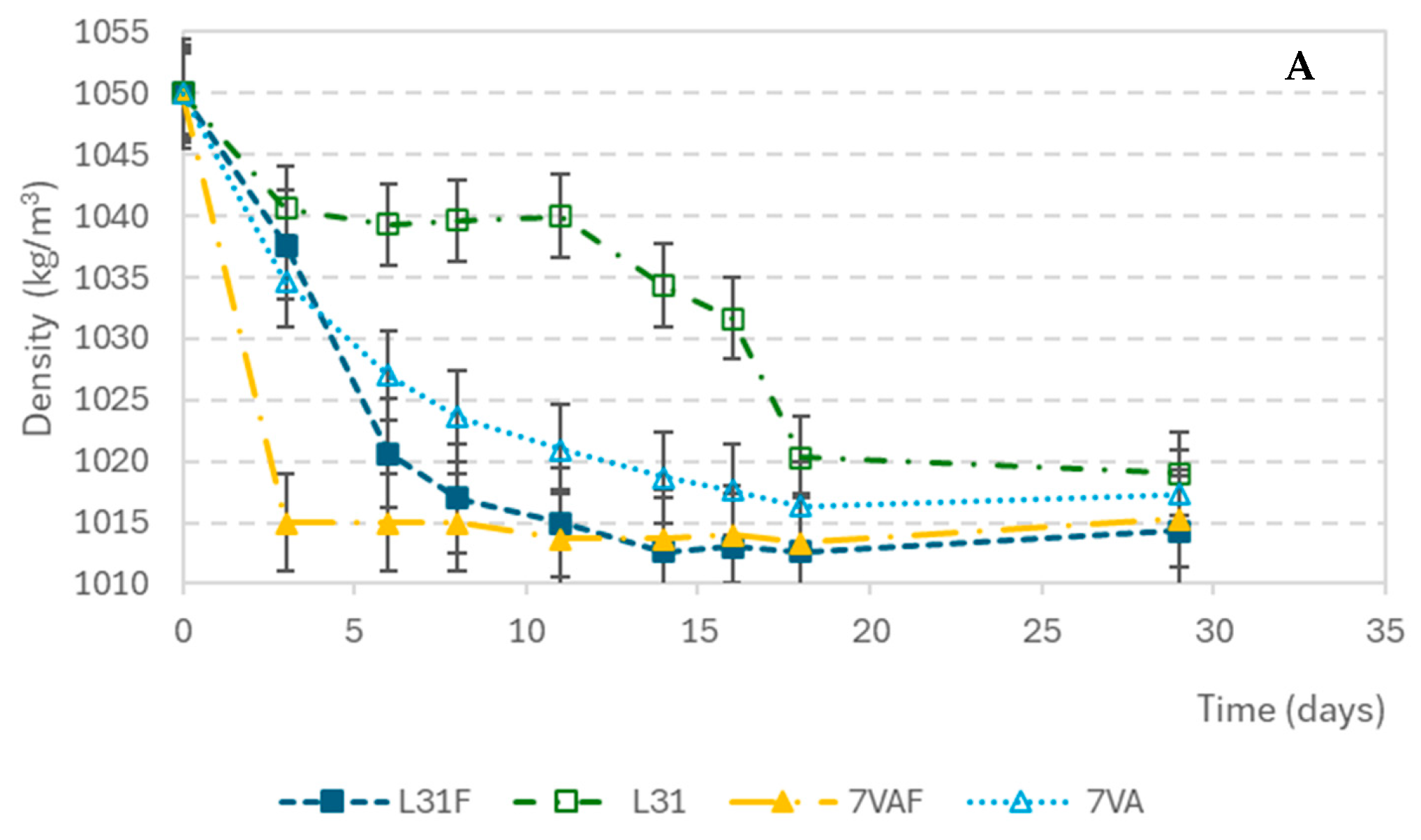
The density variations during fermentation are shown in Figure 1A. The initial density of the wort was 1050 kg/m3 and it decreased progressively in all samples until it stabilized at around 1012 kg/m3 at the end of fermentation. The beers with the fastest decrease in density were those inoculated with S. cerevisiae and fruit, followed by the ones inoculated with L. thermotolerans with fruit, indicating that beers with fruit present the greatest fermentation rate. Something similar seemed to happen with pH variation. The pH decreased from the initial value of 5.27 during the first three days and then remained constant. L31F showed the fastest acidification rate (Figure 1B). As for the glucose concentration drop, shown in Figure 1C, it showed a high consumption rate in the first days, from an initial concentration of 2.83 g/L to values below the sensitivity of the measuring method (0.0023 g/L).
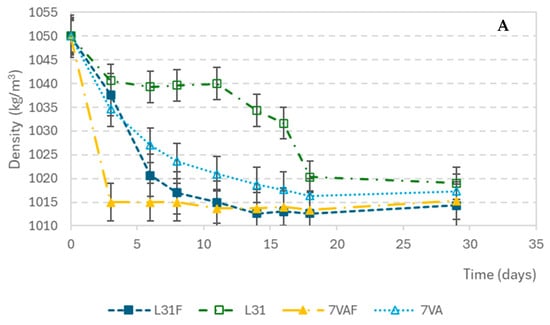
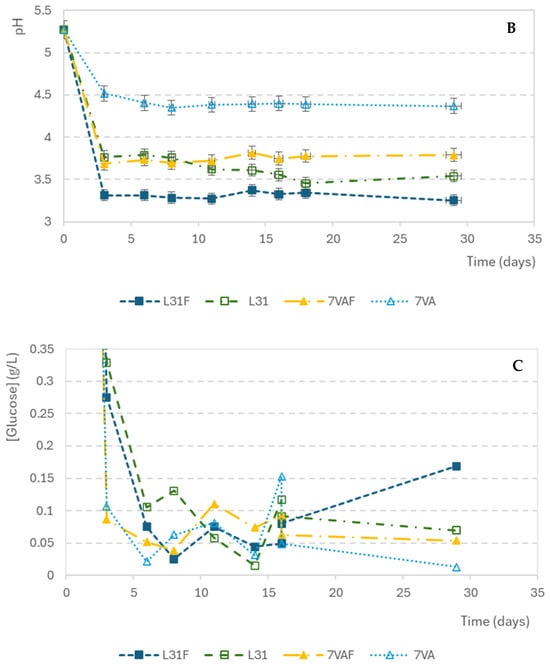
Figure 1.
Evolution of parameters during fermentation: (A) Density (kg/m3); (B) pH; (C) glucose concentration (g/L).
The finished beer analyses showed that both the type of yeast used and the addition of berries had significant effects on several of the measured parameters. The results are presented in Table 2.

Table 2.
Results of finished beer analysis.
Beers brewed with S. cerevisiae were 43.77% more bitter than those fermented with L. thermotolerans. The difference in bitterness may have been due to the influence of the yeast used. In addition, beers without red berries were 53.9% more bitter than beers with berries. Beers brewed with L31 were more acidic, with the pH ranging from 3.3 to 3.6, compared to beers brewed with 7VA, with values between 3.7 and 4.4. The type of yeast used also influenced this parameter, since significant differences could be appreciated between beers inoculated with L31 or 7VA. Also, beers with berries were significantly redder than those without berries. No influence of L31 or 7VA on the final color could be proven.
No significant differences were found in glucose concentration or final density. The residual glucose levels in the final beers were very low, indicating that both yeast strains consumed almost all of the available glucose during fermentation. Although no significant differences were found, a slightly higher concentration of residual glucose could be appreciated amongst beers brewed with the same yeast. This was probably due to the contribution of the sugar in the fruit to the beers. The ethanol contents in the final product of the four beers were similar (3.79–4.37% v/v) and not dependent on the choice of yeast or whether berries were added during the fermentation.
3.2. Volatile Compounds by GC-FID
The results obtained from GC-FID analysis are summarized in Table 3.

Table 3.
Concentrations of volatile compounds (mg/L) in finished beers. Determined by GC-FID analysis. Perception threshold of different compounds in beer correspond to those indicated by Pérez-Peces et al. (2022) [32].
Regarding alcohols, such as methanol and 1-propanol, their concentrations exceeded the perception threshold in all samples. On the other hand, neither 2-butanol nor 1-butanol were detected. The isobutanol concentration was below the perception threshold, which meant that it was not detectable in the sensory profile of the beers. 3-Methyl-1-butanol, related to wine and banana aromas, exceeded the perception threshold only in sample L31, while 2-methyl-1-butanol, associated with similar aromas, showed concentrations above the perception threshold in all beers. Hexanol, related to herbaceous odors, showed concentrations above the perception threshold in beers fermented with L. thermotolerans, while beers fermented with S. cerevisiae showed slightly lower levels. 2-Phenylethyl alcohol, known to contribute to rose petal and perfume aromas, was present in all beers at levels above the detection threshold.
Levels of fruity aroma esters such as ethyl acetate and isobutyl acetate were higher in L. thermotolerans beers. Ethyl butyrate was not detected in any of the samples, while ethyl lactate was present at concentrations below the perception threshold. Isoamyl acetate and phenylethyl were found in all beers at concentrations above the perception threshold. These compounds contribute to aromas such as honey, rose, and apple.
Among carbonyl compounds, acetaldehyde, diacetyl, and acetoin were all present at concentrations over the perception threshold. These are responsible for apple, buttery, and woody aromas, respectively.
3.3. Sensory Analysis
In terms of visual attributes, beers with no berries (7VA and L31) showed goldish colors, this last one being darker. The addition of fruit significantly influenced both color and turbidity, resulting in red and hazy beers due to suspended fruit particles. Beers without fruit showed higher foam consistency and persistence.
Concerning the olfactory profile, the addition of fruit significantly affected the intensity and aromatic quality. Beers with no berries had higher aromatic intensity, with 7VA considered to be the best amongst the jury. In beer with berries, the aroma intensity was lower and fruitier, while beers without berries exhibited floral and malt as the principal aromas.
Finally, speaking of flavor attributes, all beers were rated as medium to low bodied, although L31 and L31F seemed to be fuller bodied. Samples with berries added were sweeter and more acidic. L31F showed the highest score, correlating to acid production by L. thermotolerans. By contrast, beers without fruit showed higher bitterness and effervescence. Astringency was low and similar in all samples. The aftertaste was more pronounced in beers without fruit and those fermented with L. thermotolerans, especially in sample L31.
3.4. Correlation Between Volatile Compounds Identified by GC-FID and Sensory Analysis
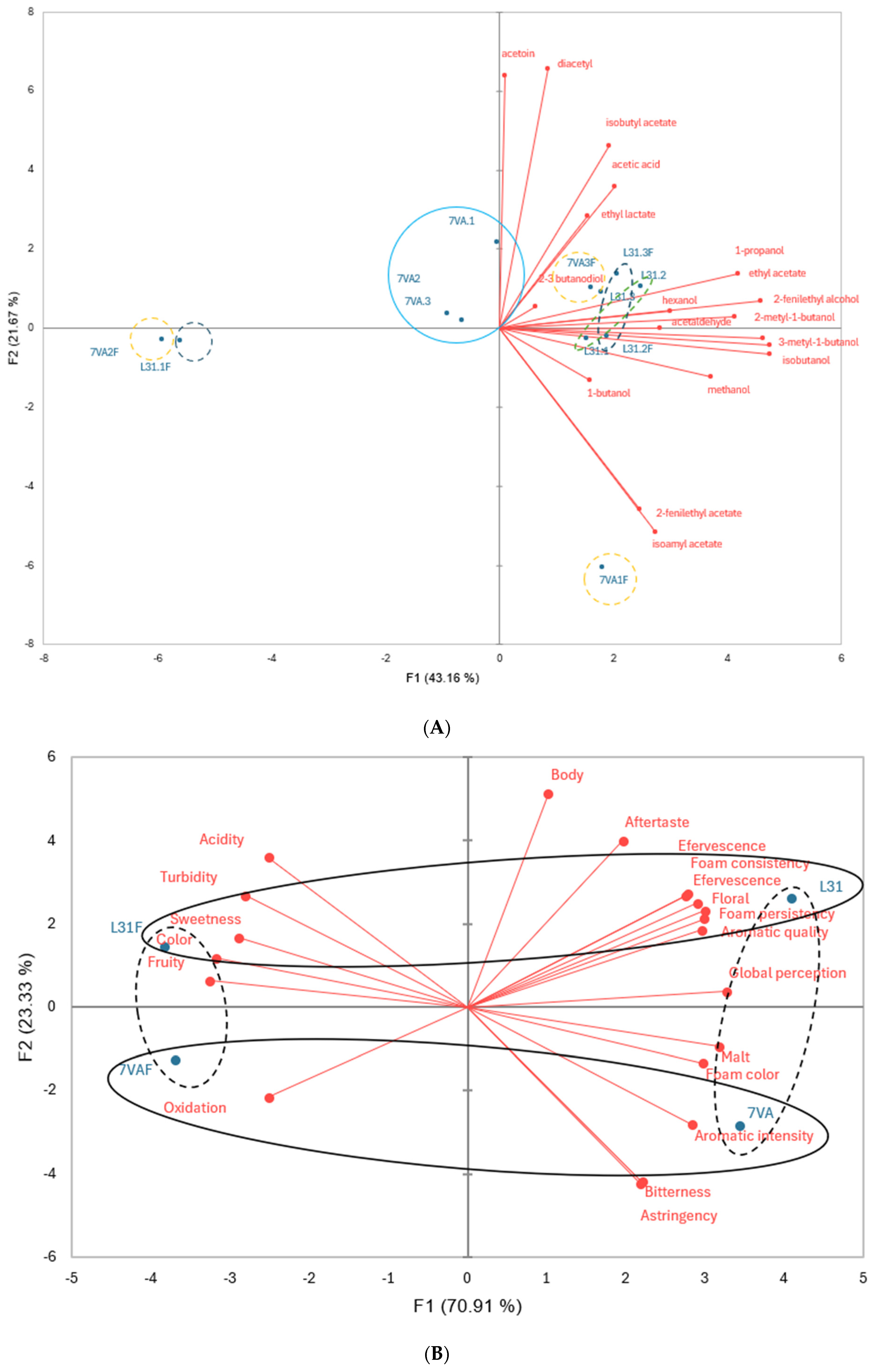
For a better understanding of these results, principal component analysis (PCA) was performed and the results are shown in Figure 2. The first PCA, based on volatile compounds, identified seven groups of beers with distinct chemical profiles, while the second PCA, focusing on sensory analysis, reduced the number to two separated groups. The results clearly indicated differences in sensory attributes between beers depending on the presence of fruit and the yeast used during the fermentation step.
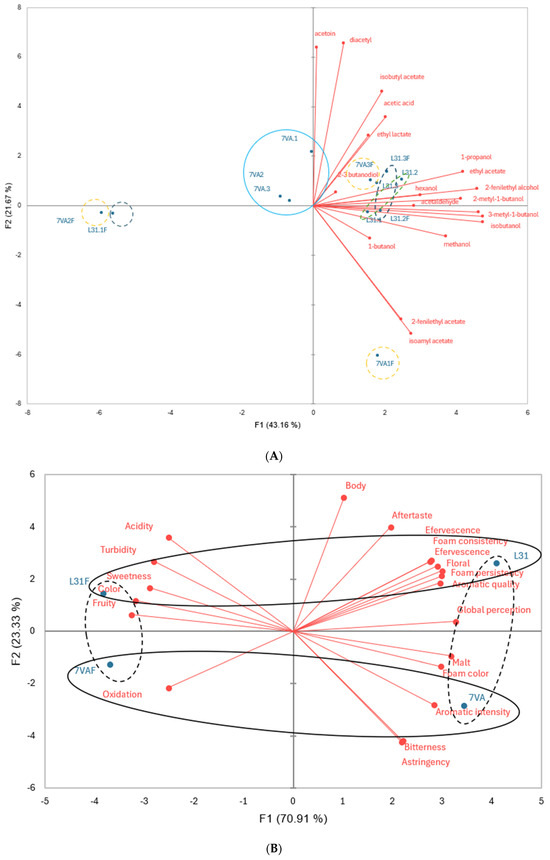
Figure 2.
Principal component analysis of (A) volatile compounds in beers by GC-FID; (B) of sensory analysis. Different colors indicate different groups of beers.
The correlation between the volatile compounds and the sensory analysis of the different beers was analyzed using Pearson’s correlation coefficient. The results are summarized in Table 4.

Table 4.
Correlations (Pearson’s test) between volatile analysis and sensory analysis.
Positive correlations were found between aromatic intensity and compounds such as diacetyl, acetic acid, isobutyl acetate, and 2,3-butanediol. Aromatic quality also showed positive correlations with several volatile compounds, while floral aroma was related to many alcohols and esters present in samples analyzed by GC-FID. Contrary to expectations, the fruity aroma attribute did not show positive correlations with compounds typically associated with fruity aromas.
3.5. Anthocyanin Levels and Antioxidant Activity
Total anthocyanin content analysis, HPLC, and the ABTS essay were performed to determine the antioxidant activity of beer. The data are compiled in Table 5.

Table 5.
Antioxidant activity (µmol Trolox Equivalent/mL) and anthocyanin content (mg/L) in finished beers.
While beers 7VA, 7VAF, and L31 had similar antioxidant activities, beers fermented with L. thermotolerans and with additional berries seemed to have the strongest radical scavenging power. This may have been due to the presence of berries and related to the higher anthocyanin content in beer with berries. The highest anthocyanin concentration (9.42 ± 5.61 for 7VAF) did not match the beer with highest antioxidant activity (1.49 ± 0.38 for L31F), contrary to expectations.
According to Table 5, the anthocyanin content in beers with no berries could not be determined by HPLC-DAD since the concentration was below the limit of detection (LOD = 0.1 mg/L). Beers with berries did have anthocyanins, although the concentration was very low. 7VAF showed the highest anthocyanin content of all samples (9.42 ± 5.61) but had similar antioxidant activity levels to 7VA and L31, while these two had no fruit in their composition. Although the differences seemed to be significant for antioxidant activity (p-value < 0.05), we could not ensure that the increased antioxidant activity was caused by the addition of fruit, since the variation was minor and could be consequence of many other factors.
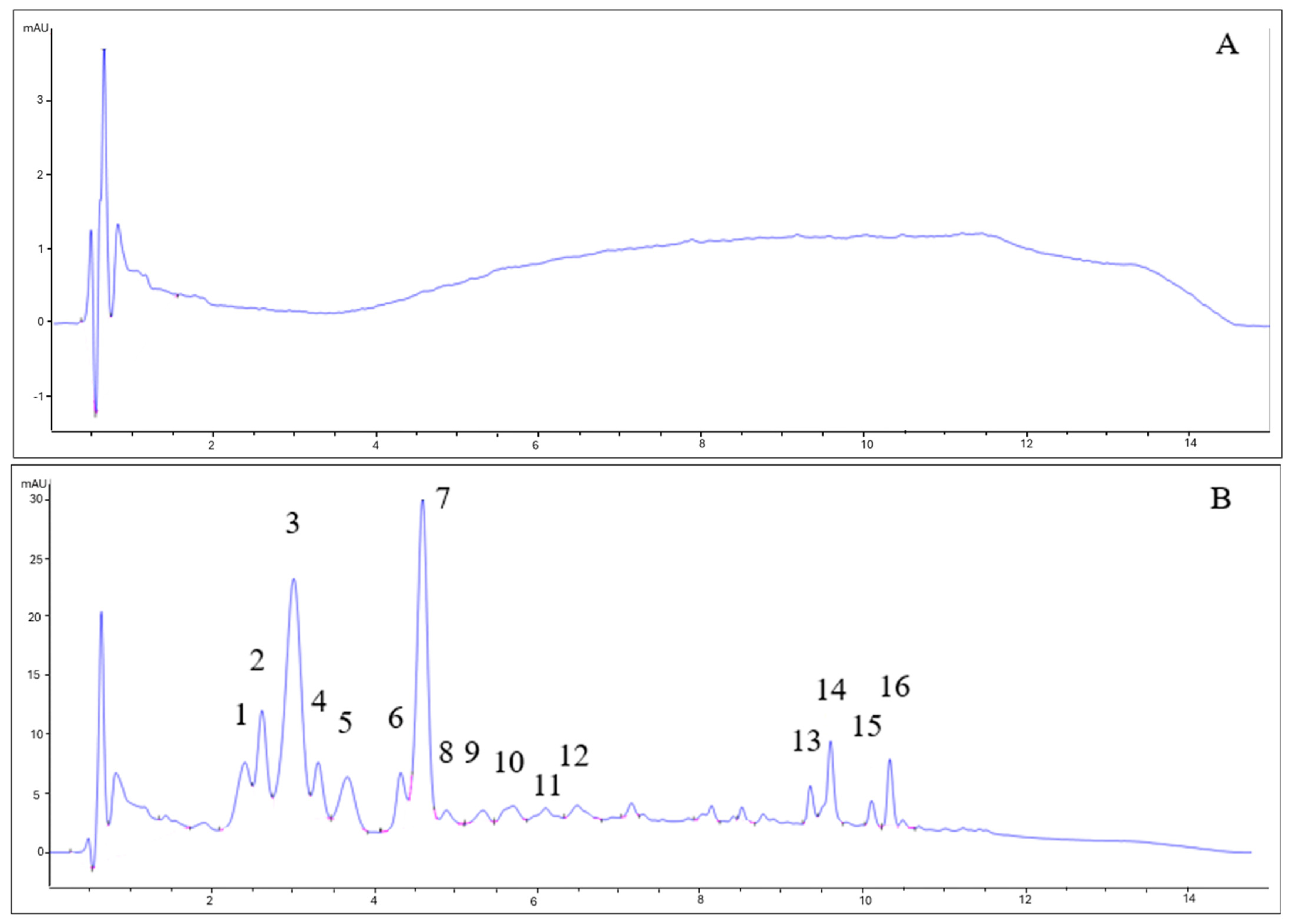
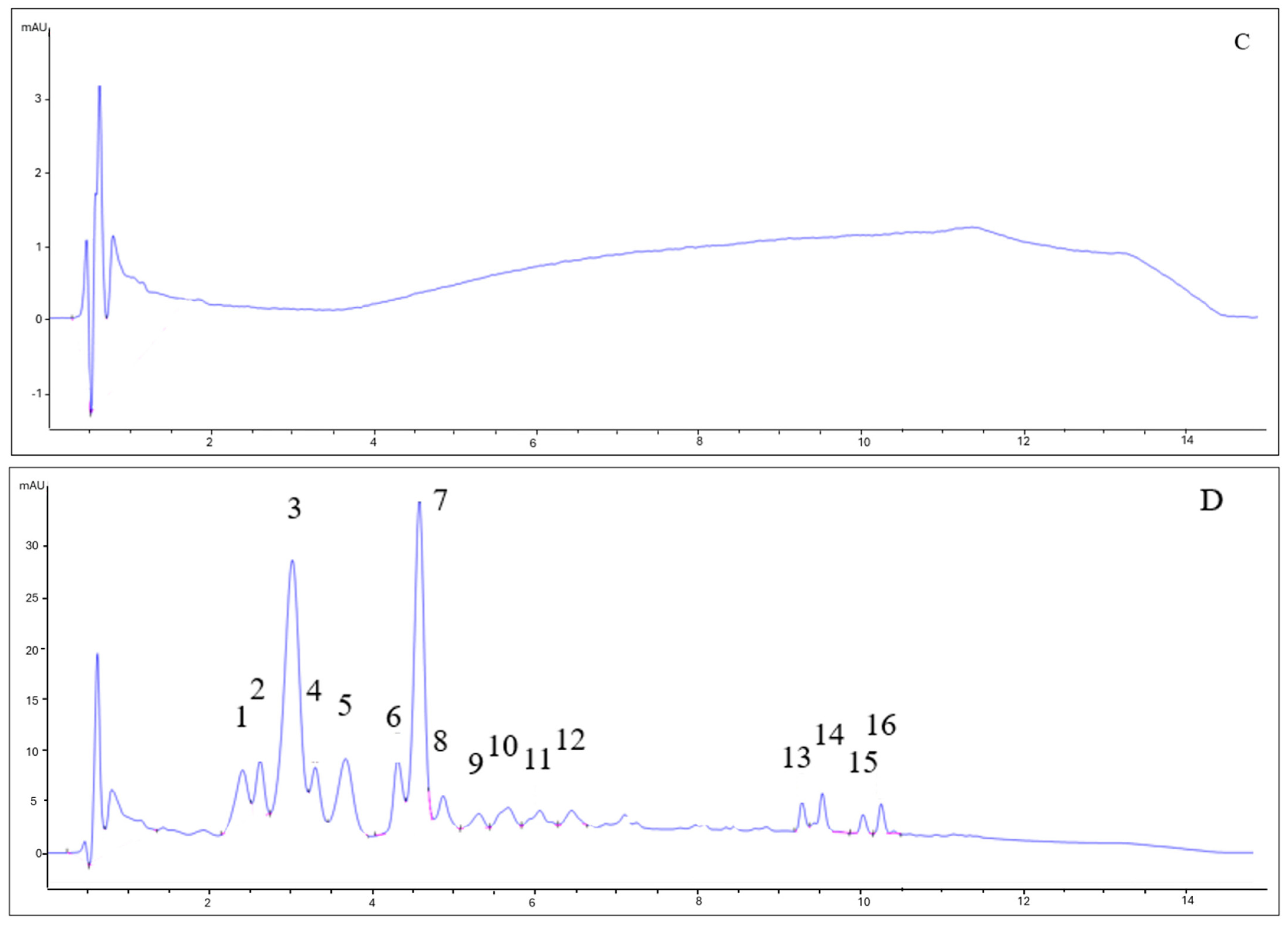
As can be seen in Figure 3, the chromatograms for beers with berries and beers without berries showed completely different patterns. The type of yeast did not influence the anthocyanin profile. While beers without berries (7VA and L31) showed no remarkable peaks (all signals are lower than 1 mA), beers with berries had a very complex profile. Some of the identified peaks for fruit beer samples corresponded to delphinidin-3-O-galactoside (2) delphinidin-3-O-glucoside (3), cyanidin-3-O-glucoside (7), and malvidin-3-O-glucoside (11), by comparison with available data. Due to the low absorbance signal, weak UV–Vis spectra, and complexity, the rest of the peaks could not be properly identified, although peaks 1, 4, and 5 were thought to be delphinidin, cyanidin, and pelargonidin derivatives since these are the main anthocyanins in blueberries, blackcurrants, raspberries, and strawberries [33]. Peaks 13–16 were apolar compounds, probably beer aging products.
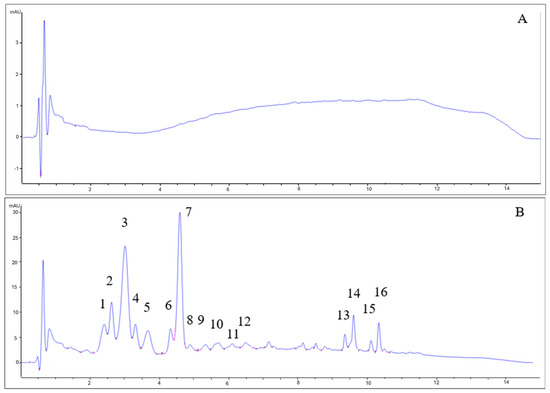
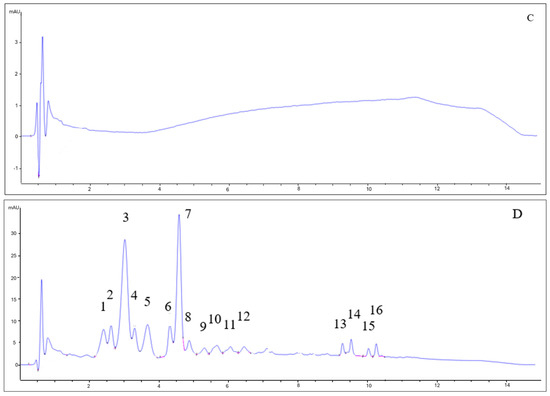
Figure 3.
HPLC-DAD chromatograms of (A) 7VA, (B) 7VAF, (C) L31, and (D) L31F. Anthocyanins were measured at 525 nm. Numbers correspond to: delphinidin derivative (1), delphinidin-3-O-galactoside (2), delphinidin-3-O-glucoside (3), cyanidin derivative (4), pelargonidin derivative (5), cyanidin-3-O-glucoside (7), and malvidin-3-O-glucoside (11). Peaks 13–16 are apolar compounds.
4. Discussion
This study evaluated the effect of different yeasts (S. cerevisiae and L. thermotolerans) as well as the addition of mixed berries in beer production and its significance in the physicochemical and sensory profiles of the final products.
During fermentation, the beers that lost density the fastest were those fermented with S. cerevisiae with fruit, followed by those fermented with L. thermotolerans with fruit, indicating that the samples with mixed berries fermented earlier and confirming the superior fermentative power of S. cerevisiae compared to L. thermotolerans [34,35,36]. Furthermore, during fermentation, L. thermotolerans showed a decrease in fermentative activity due to depletion of available sugars, while S. cerevisiae continued to ferment, possibly using other fermentable sugars remaining in the wort, such as maltose and maltotriose [19,37,38].
Beers fermented with S. cerevisiae were more bitter than those fermented with L. thermotolerans, suggesting that the yeast strain influenced bitterness. According to some studies, the α-acid molecules from hops can bind to yeast cell walls, which are deposited on the bottom of the fermenter, thus reducing the bitterness of the beer [39]. In addition, beers without berries were more bitter, which was consistent with other reviewed studies [17,40]. As previously seen with the type of yeast used, the α-acids may adhere to the cell walls of the fruit, reducing the bitterness of this type of beer.
Beers fermented with L. thermotolerans were more acidic than those fermented with S. cerevisiae. This was caused by the production of lactic acid by L. thermotolerans, which lowers the pH [15,17,32]. The pH values obtained in the four beer configurations were also in the range found by Polshin et al. [41]. The darker red color of beer with berries agreed with results obtained by Ducruet et al. (2017) in their studies on the brewing of craft beer with the addition of berries. These authors observed the extraction of compounds from berries due to the high formation of melanoidins during the Maillard reaction in the boiling phase [42].
The alcohol content of the beers studied showed no significant differences between the different yeast strains used, contrary to other studies such as the study by Callejo et al. (2019), where the ethanol content of beer produced by S. cerevisiae was significantly higher than that of beer produced by L. thermotolerans [19].
As for the volatile compounds, many alcohols (2-methyl-1-butanol and hexanol) were found in concentrations above the threshold and similar to those found in other studies, such as in the study by Peces-Pérez et al. (2022) [32]. A wide variety of esters responsible for the olfactory profile of the final product were found in concentrations similar to those reported by Ocvirk et al. (2018) [43] and even higher than those reported by Pavsler and Buiatti (2009) [44]. Amongst them, we can highlight ethyl acetate, isobutyl acetate, and acetaldehyde. These results also correlated with some recent studies on the matter, such as those by Zapata et al. (2019) [45] and Postigo et al. (2023) [35]. Beers crafted with L. thermotolerans were associated with herbaceous and fruity aromas, similar to lambic-type beers. Beers with S. cerevisiae were noted for higher intensity and malt-like aromas. Data from the sensory analysis also showed that beer brewed with L. thermotolerans L31 and with berries added was more acidic, less bitter, redder, and presented higher aromatic quality due to floral and fruity aromas and therefore had more similarities with lambic beers than 7VA, 7VAF, and L31.
Regarding antioxidant activity, anthocyanins are water-soluble compounds and the anthocyanin level should have been higher in L31F since its alcoholic graduation was lower. However, the antioxidant activity was higher in 7VAF, where the anthocyanin level was slightly lower. This meant that there were other antioxidant compounds, distinct from anthocyanins, that were not detected by the HPLC method but exerted antioxidant activity in the ABTS assay. Some possibilities include phenolic acids, melanoidin, or flavonoids present in malt, hops, and berries [46,47]. The extraction of these compounds is favored by methanolic solvents [48] and, therefore, higher ethanol content may aid the process. Since 7VAF had a greater ethanolic content, this could be an explanation for what we observed in this experiment.
Other studies claim that the ABTS assay is not able to determine the total antioxidant activity of all compounds in samples. Other methods, like the DPPH assay, work similarly and show higher values when performing both tests. According to Sariburun et al. [48], some differences can be appreciated when running one or the other while comparing dark-colored fruits like blackcurrants or blackberries and light red fruits such as strawberries and raspberries.
Some of the identified anthocyanins here, like delphinidin-3-O-galactoside, delphinidin-3-O-glucoside, or cyanidin-3-O-glucoside, are representative anthocyanins present in berries such as blackberries, blackcurrants, raspberries, and strawberries [49,50,51]. The fact that they only appeared in beers with fruits indicated that they had been extracted from the mixed berries to the wort during fermentation, affecting the antioxidant activity and color of these beers.
5. Conclusions
Overall, the choice of yeast influenced the physicochemical and sensory parameters of the final beer by 23%, while the addition of berries had a more significant impact on sensory attributes at 71%. Although beers without berries were rated better overall, the addition of berries may provide added value and differentiation to appeal to a more targeted audience. The combined use of L. thermotolerans and fruit contributes to producing sour low-alcohol beers, with an intense fruity aromatic profile resembling those of sour lambic beers. Future research could explore the interactions between different yeast strains and the influence of different fruits on the organoleptic and nutritional profiles of beer, contributing to innovation and diversification of the beer market.
Author Contributions
Conceptualization, C.L. and A.M.; methodology, C.L. and A.M.; investigation, R.B. and E.A.; data curation, R.B.; writing—original draft preparation, R.B. and E.A.; writing—review and editing, E.A., C.L. and A.M.; project administration, C.L. and A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Guido, L.F. Brewing and craft beer. Beverages 2019, 5, 51. [Google Scholar] [CrossRef]
- Meußdoerffer, F.; Zarnkow, M. Das Bier: Eine Geschichte von Hopfen und Malz, 1st ed.; C.H. Beck: Munich, Germany, 2014. [Google Scholar]
- Bokulich, N.A.; Bamforth, C.W. The microbiology of malting and brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.J.; Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; De Rouck, G.; Rukke, E.O.; Westereng, B.; Wicklund, T. Microbial dynamics in traditional and modern sour beer production. Appl. Environ. Microbiol. 2020, 86, e00566-20. [Google Scholar] [CrossRef]
- Tonsmeire, M. American Sour Beer: Innovative Techniques for Mixed Fermentations, 1st ed.; Brewers Publications: Boulder, CO, USA, 2014. [Google Scholar]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and non-conventional yeasts in beer production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Microbial acidification, alcoholization, and aroma production during spontaneous lambic beer production. J. Sci. Food Agric. 2019, 99, 25–38. [Google Scholar] [CrossRef]
- Klimczak, K.; Cioch-Skoneczny, M.; Ciosek, A.; Poreda, A. Application of non-Saccharomyces yeast for the production of low-alcohol beer. Foods 2024, 13, 3214. [Google Scholar] [CrossRef]
- Tolosa, J.J.M.; Prieto, S.M. Non-Saccharomyces yeasts: An enzymatic unexplored world to be exploited. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 433–450. [Google Scholar] [CrossRef]
- Palmer, J.J. How to Brew: Everything You Need to Know to Brew Great Beer Every Time, 4th ed.; Brewers Publications: Boulder, CO, USA, 2017. [Google Scholar]
- Anderson, K. The emergence of lower-alcohol beverages: The case of beer. J. Wine Econ. 2023, 18, 66–86. [Google Scholar] [CrossRef]
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Non-conventional yeasts as tools for innovation and differentiation in brewing. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Pirrone, A.; Naselli, V.; Gugino, I.M.; Porrello, A.; Viola, E.; Craparo, V.; Vella, A.; Alongi, D.; Seminerio, V.; Carusi, M.; et al. Use of non-conventional yeasts for enhancing the sensory quality of craft beer. Food Res. Int. 2025, 208, 116164. [Google Scholar] [CrossRef]
- Callejo, M.J.; García Navas, J.J.; Alba, R.; Escott, C.; Loira, I.; González, M.C.; Morata, A. Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Tsaruk, A.; Filip, K.; Sibirny, A.; Ruchala, J. Native and recombinant yeast producers of lactic acid: Characteristics and perspectives. Int. J. Mol. Sci. 2025, 26, 2007. [Google Scholar] [CrossRef]
- Malina, C.; Yu, R.; Björkeroth, J.; Kerkhoven, E.J.; Nielsen, J. Adaptations in metabolism and protein translation give rise to the crabtree effect in yeast. Proc. Natl. Acad. Sci. USA 2021, 118, e2112836118. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An integrative view of the role of Lachancea thermotolerans in wine technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Galaz, V.; Franco, W. Lachancea quebecensis a novel isolate for the production of craft beer. Foods 2023, 12, 3347. [Google Scholar] [CrossRef]
- Franco, W.; Benavides, S.; Valencia, P.; Ramírez, C.; Urtubia, A. Native yeasts and lactic acid bacteria isolated from spontaneous fermentation of seven grape cultivars from the maule region (Chile). Foods 2021, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. Color. In ASBC Methods of Analysis [Protocol]; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar] [CrossRef]
- Koren, D.; Hegyesné Vecseri, B.; Kun-Farkas, G.; Urbin, Á.; Nyitrai, Á.; Sipos, L. How to objectively determine the color of beer? J. Food Sci. Technol. 2020, 57, 1183–1189. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. Beer Bitterness [Protocol]. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Peces-Pérez, R.; Vaquero, C.; Callejo, M.J.; Morata, A. Biomodulation of physicochemical parameters, aromas, and sensory profile of craft beers by using non-Saccharomyces yeasts. ACS Omega 2022, 7, 17822–17840. [Google Scholar] [CrossRef]
- Morata, A.; López, C.; Tesfaye, W.; González, C.; Escott, C. Anthocyanins as Natural Pigments in Beverages. In Value-Added Ingredients and Enrichments of Beverages: The Science of Beverages; Woodhead Publishing: Sawston, UK, 2019; Volume 14, pp. 383–428. [Google Scholar]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Postigo, V.; Esteban, S.; Arroyo, T. Lachancea thermotolerans, an innovative alternative for sour beer production. Beverages 2023, 9, 20. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Heras, J.M.; Carrau, F.; González, C.; Morata, A. Biocompatibility in ternary fermentations with Lachancea thermotolerans, other non-Saccharomyces and Saccharomyces cerevisiae to control pH and improve the sensory profile of wines from warm areas. Front. Microbiol. 2021, 12, 656262. [Google Scholar] [CrossRef]
- Fu, X.; Guo, L.; Li, Y.; Chen, X.; Song, Y.; Li, S. Transcriptional analysis of mixed-culture fermentation of Lachancea thermotolerans and Saccharomyces cerevisiae for natural fruity sour beer. Fermentation 2024, 10, 180. [Google Scholar] [CrossRef]
- Francesca, N.; Pirrone, A.; Gugino, I.; Prestianni, R.; Naselli, V.; Settanni, L.; Todaro, A.; Guzzon, R.; Maggio, A.; Porrello, A.; et al. A novel microbiological approach to impact the aromatic composition of sour loquat beer. Food Biosci. 2023, 55, 103011. [Google Scholar] [CrossRef]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces yeasts for beer fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef]
- Polshin, E.; Rudnitskaya, A.; Kirsanov, D.; Legin, A.; Saison, D.; Delvaux, F.; Delvaux, F.R.; Nicolaï, B.M.; Lammertyn, J. Electronic tongue as a screening tool for rapid analysis of beer. Talanta 2010, 81, 88–94. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Ocvirk, M.; Mlinarič, N.K.; Košir, I.J. Comparison of sensory and chemical evaluation of lager beer aroma by gas chromatography and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2018, 98, 3627–3635. [Google Scholar] [CrossRef]
- Pavsler, A.; Buiatti, S. Lager beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Udine, Italy, 2009; pp. 31–43. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Tulio, A.Z.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Raymond, A. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).