Phytochemistry and Biological Effects of the Juglans regia “Sorrento” Walnut Husk Extract on Human Keratinocyte Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extract
2.2. Phytochemical Analysis
2.2.1. Spectroscopic Characterization
2.2.2. Total Phenolic Content (TPC) Determination
2.2.3. U HPLC–Q-Orbitrap HRMS Analysis of the Extract
2.3. Antioxidant Activity
2.3.1. ABTS Assay
2.3.2. DPPH Assay
2.4. Cell Lines and Culture Treatments
2.5. Hydrogen Peroxide and Peroxidase Assays
2.6. Western Blotting
2.7. Seahorse XF Oxygen Consumption Rate (OCR) Assay
3. Results
3.1. Spectroscopic Characterization of WHE
3.2. Phytochemical Profile by UHPLC–Q-Orbitrap HRMS
3.3. Antioxidant Activity of WHE
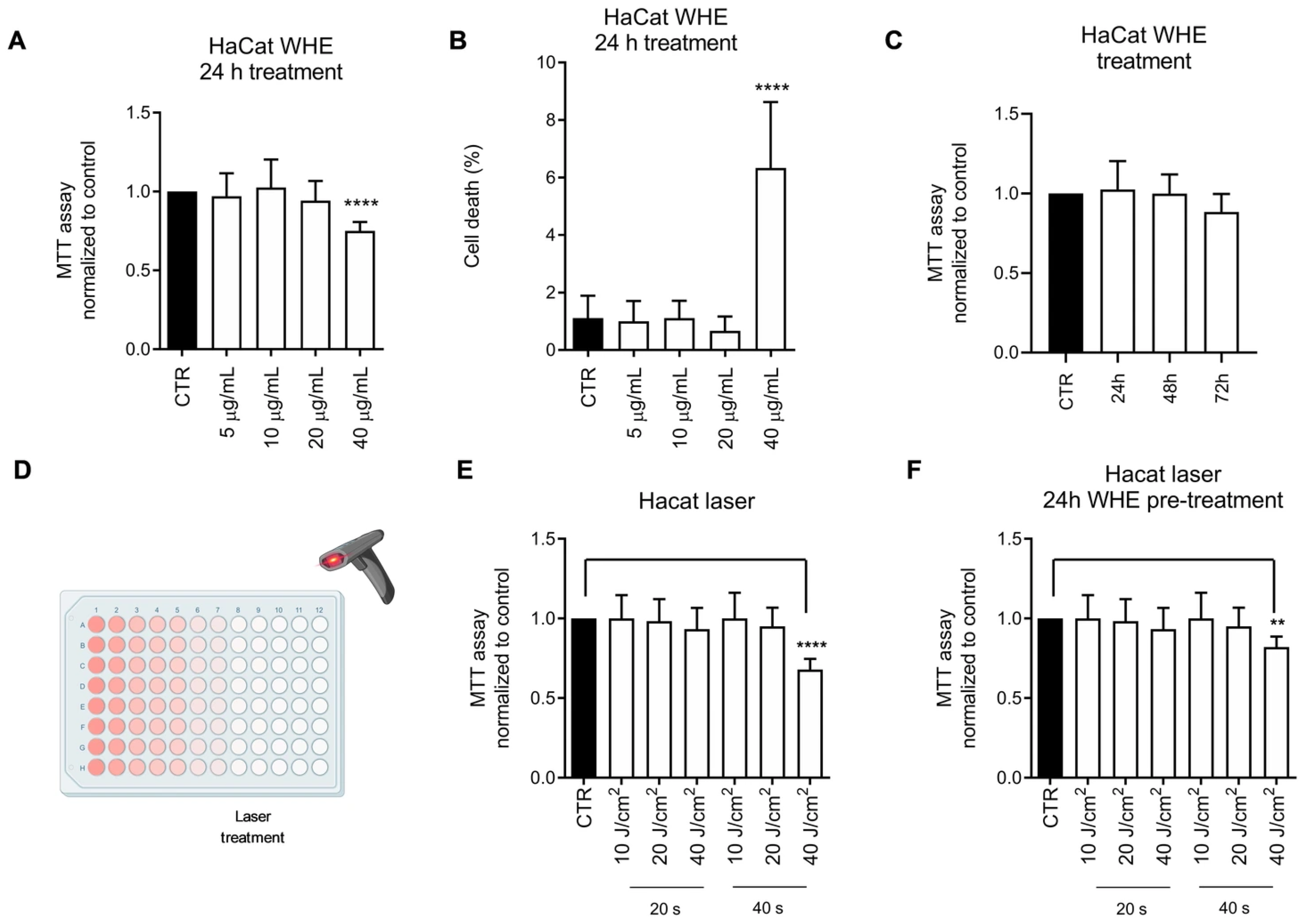
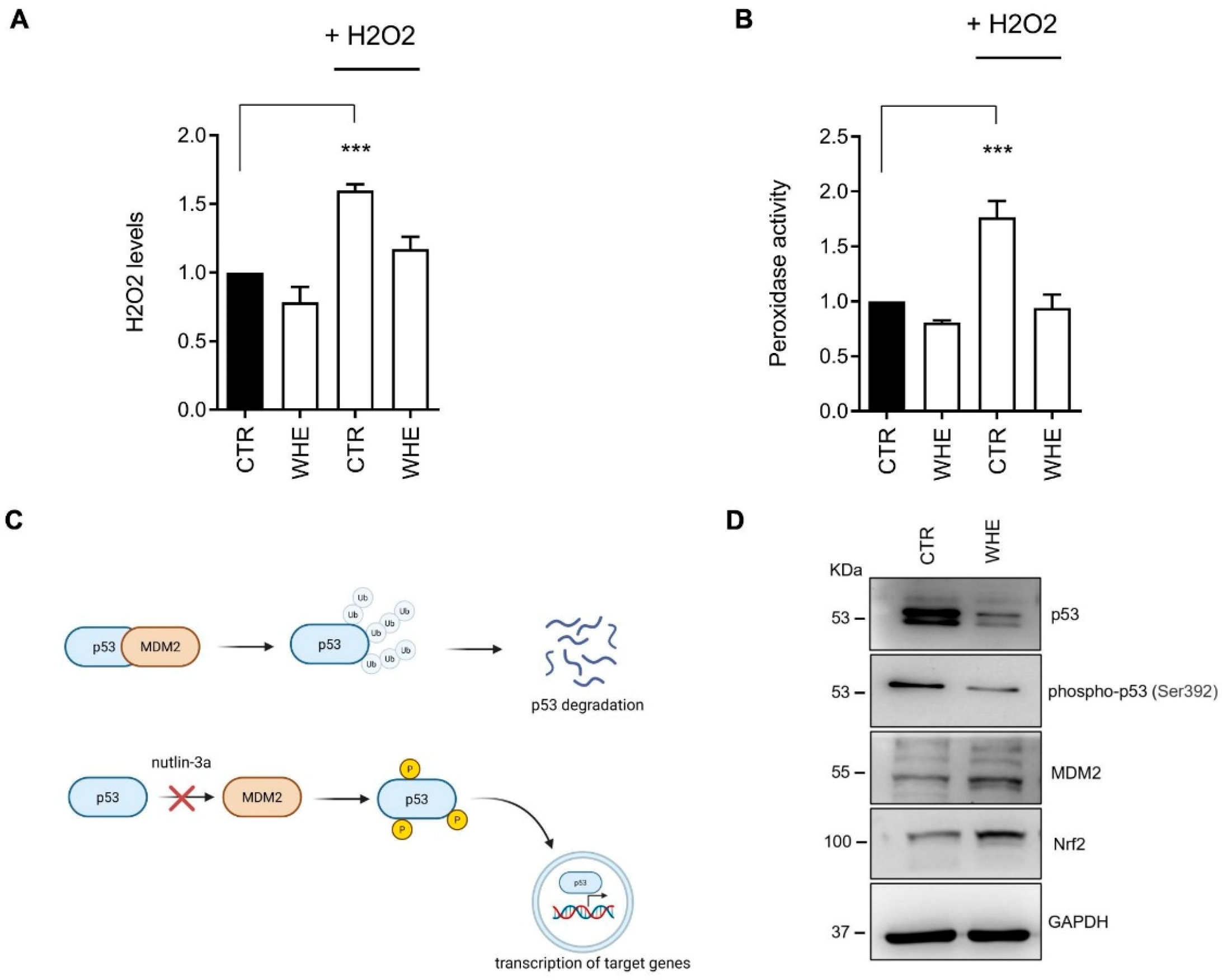
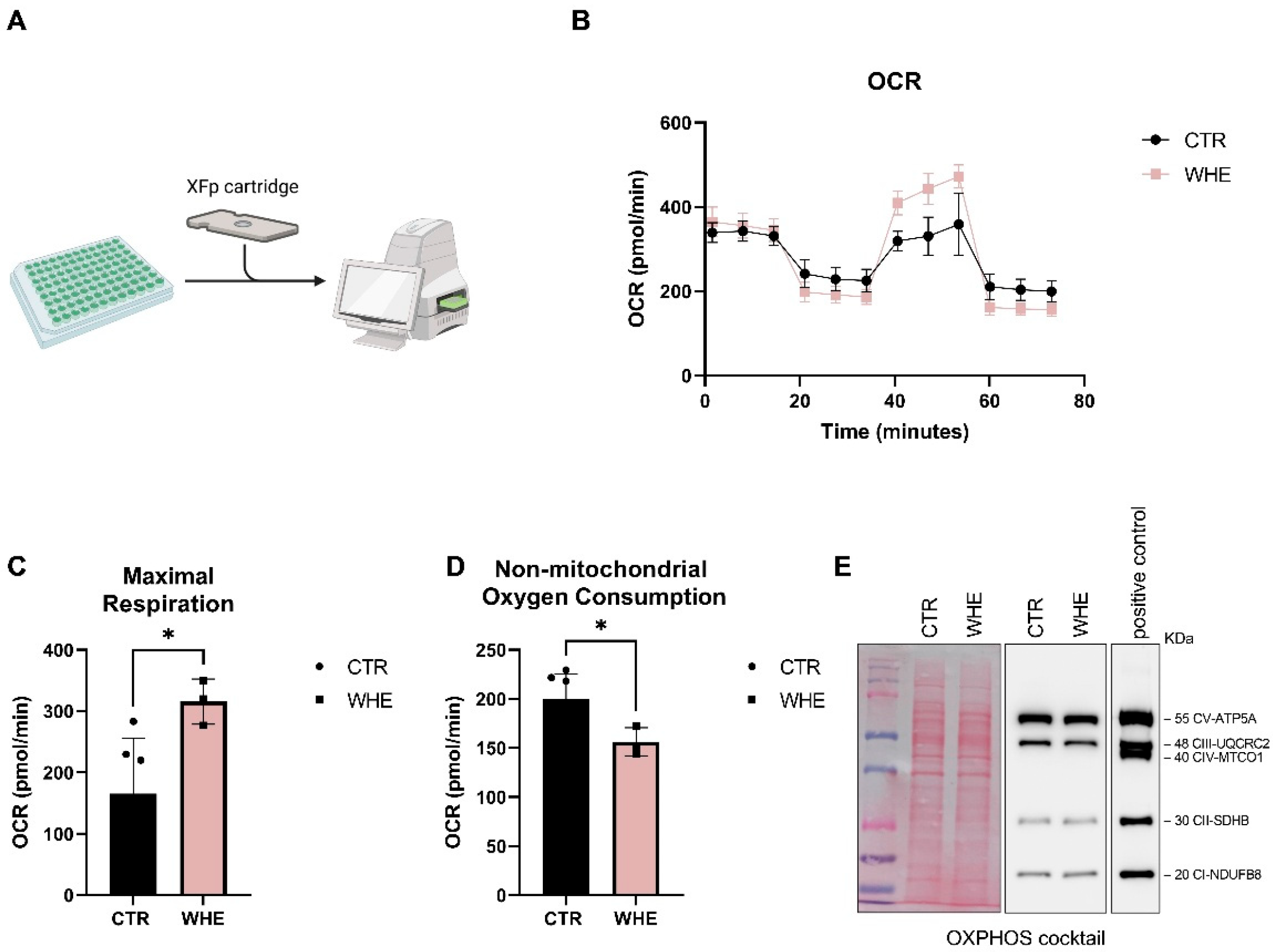
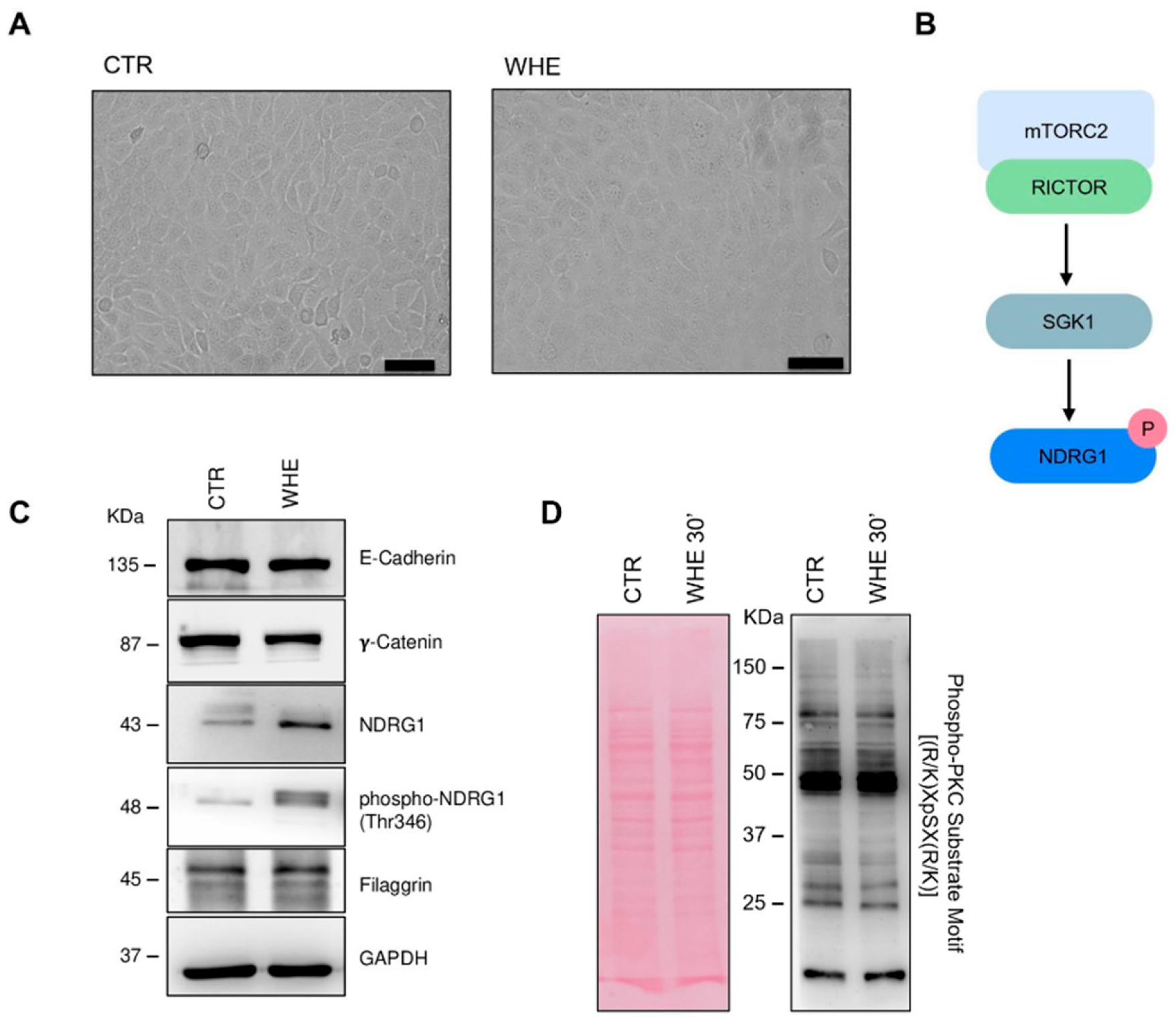
3.4. Biological Evaluation of the Walnut Husk Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OCR | Oxygen Consumption Rate |
| PKC | Protein Kinase C |
| NDRG1 | N-myc downstream regulated 1 |
| TPC | Total phenolic content |
| UHPLC–HRMS | The ultra-high performance liquid chromatography–high-resolution mass spectrometry system |
| WHE | Walnut Husk Extract |
References
- Gǎlbǎu, C.-Ş.; Irimie, M.; Neculau, A.E.; Dima, L.; Pogačnik Da Silva, L.; Vârciu, M.; Badea, M. The Potential of Plant Extracts Used in Cosmetic Product Applications—Antioxidants Delivery and Mechanism of Actions. Antioxidants 2024, 13, 1425. [Google Scholar] [CrossRef]
- Mukarram, S.A.; Wandhekar, S.S.; Ahmed, A.E.M.; Pandey, V.K.; Csaba, O.; Lajos, D.; József, P.; Harsányi, E.; Bela, K. Exploring the Ecological Implications, Gastronomic Applications, and Nutritional and Therapeutic Potential of Juglans regia L. (Green Walnut): A Comprehensive Review. Nutrients 2024, 16, 1183. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent Advances in the Use of Walnut (Juglans regia L.) Shell as a Valuable Plant-Based Bio-Sorbent for the Removal of Hazardous Materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, E.; Cice, D.; Piccolella, S.; Esposito, A.; Petriccione, M.; Pacifico, S. ‘Sorrento’ and ‘Tulare’ Walnut Cultivars: Morphological Traits and Phytochemical Enhancement of Their Shell Waste. Molecules 2024, 29, 805. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Solar, A.; Hudina, M.; Veberic, R. Walnut (J. regia) Agro-Residues as a Rich Source of Phenolic Compounds. Biology 2021, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, M.; Sharma, M. A Comprehensive Review on Ethnobotanical, Medicinal and Nutritional Potential of Walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comprehensive Review on the Chemical Constituents and Functional Uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Adamovic, M.; Adamovic, A.; Andjic, M.; Dimitrijevic, J.; Zdravkovic, N.; Kostic, O.; Pecarski, D.; Pecarski, T.; Obradovic, D.; Tomovic, M. The Botany, Phytochemistry and the Effects of the Juglans regia on Healthy and Diseased Skin. Cosmetics 2024, 11, 163. [Google Scholar] [CrossRef]
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A Review Study on Phytochemistry and Pharmacology Applications of Juglans regia Plant. Pharmacogn. Rev. 2017, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Behl, T.; Panichayupakaranan, P. A Review of Phytochemistry and Pharmacology Profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhao, C.; Sui, H.; Li, C.F.; Zhong, L.; Zhou, Q.; Bai, Y.; An, S.; Du, X.; et al. Green Walnut Husk Extracts Proliferation and Migration in Gastric Cancer. J. Cancer 2022, 13, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Hasan, T.N.; Shafi, G.; Syed, N.A.; Al-Assaf, A.H.; Alamri, M.S.; Al-Khalifa, A.S. Validation of the Antiproliferative Effects of Organic Extracts from the Green Husk of Juglans regia L. on PC-3 Human Prostate Cancer Cells by Assessment of Apoptosis-Related Genes. Evid. Based Complement. Alternat. Med. 2012, 2012, 103026. [Google Scholar] [CrossRef] [PubMed]
- Beiki, T.; Najafpour, G.D.; Hosseini, M. Evaluation of Antimicrobial and Dyeing Properties of Walnut ( Juglans regia L.) Green Husk Extract for Cosmetics. Color. Technol. 2018, 134, 71–81. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Zeb, A.; Ali, G.; Al-Babili, S. Comparative UHPLC-MS/MS-Based Untargeted Metabolomics Analysis, Antioxidant, and Anti-Diabetic Activities of Six Walnut Cultivars. Food Biosci. 2024, 59, 103885. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and Decoction of Juglans regia L.: Different Performances Regarding Bioactive Compounds and in Vitro Antioxidant and Antitumor Effects. Ind. Crops Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef] [PubMed]
- Savić, I.M.; Savić Gajić, I.M. Extraction and Characterization of Antioxidants and Cellulose from Green Walnut Husks. Foods 2025, 14, 409. [Google Scholar] [CrossRef]
- Rajković, K.M.; Stanković, M.; Markićević, M.; Zavišić, G.; Vranješ-Đurić, S.; Janković, D.; Obradović, Z.; Stanković, D. Chemical Composition and Protective Possibilities of Juglans Nigra Leaves and Green Husks Extracts: DNA Binding and Micronucleus Assay in Human Lymphocytes. Plants 2024, 13, 1669. [Google Scholar] [CrossRef]
- Du, H.; Li, C.; Wen, Y.; Tu, Y.; Zhong, Y.; Yuan, Z.; Li, Y.; Liang, B. Secondary Metabolites from Pericarp of Juglans regia. Biochem. Syst. Ecol. 2014, 54, 88–91. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Durak, A.; Pecio, Ł.; Kowalska, I. Nutraceutical Potential of Tinctures from Fruits, Green Husks, and Leaves of Juglans regia L. Sci. World J. 2014, 2014, 501392. [Google Scholar] [CrossRef]
- Barekat, S.; Nasirpour, A.; Keramat, J.; Dinari, M.; Meziane-Kaci, M.; Paris, C.; Desobry, S. Phytochemical Composition, Antimicrobial, Anticancer Properties, and Antioxidant Potential of Green Husk from Several Walnut Varieties (Juglans regia L.). Antioxidants 2022, 12, 52. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A Comprehensive Review of Natural Products against Atopic Dermatitis: Flavonoids, Alkaloids, Terpenes, Glycosides and Other Compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-H.; Jiang, Z.-T.; Liu, T.; Li, R. Flavonoids in Juglans regia L. Leaves and Evaluation of In Vitro Antioxidant Activity via Intracellular and Chemical Methods. Sci. World J. 2014, 2014, 303878. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.; Dai, S.; Che, X.; Liu, W. Identification and Quantification of Bioactive Compounds in Diaphragma Juglandis Fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef]
- Xi, M.; Hou, Y.; Cai, Y.; Shen, H.; Ao, J.; Li, M.; Wang, J.; Luo, A. Antioxidant and Antimicrobial Characteristics of Ethyl Acetate Polar Fractions from Walnut Green Husk. J. Food Sci. 2023, 88, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Lainé, A.L.; Gennetay, D.; Porte, C.; Chesneau, D.; Laclie, C.; Goudet, G.; Meunier, M.; Delmas, M.; Greil, M.L.; et al. The Walnut Tree as a Source of Progesterone for Reproductive Control in Goats. Animal 2025, 19, 101392. [Google Scholar] [CrossRef]
- Cirone, M.; D’Orazi, G. NRF2 in Cancer: Cross-Talk with Oncogenic Pathways and Involvement in Gammaherpesvirus-Driven Carcinogenesis. Int. J. Mol. Sci. 2022, 24, 595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaplan, M.H.; Yang, K. ResTORing Barrier Function in the Skin. J. Allergy Clin. Immunol. 2020, 145, 111–113. [Google Scholar] [CrossRef]
- Goleva, E.; Berdyshev, E.; Leung, D.Y.M. Epithelial Barrier Repair and Prevention of Allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [CrossRef]
- Ding, X.; Bloch, W.; Iden, S.; Rüegg, M.A.; Hall, M.N.; Leptin, M.; Partridge, L.; Eming, S.A. mTORC1 and mTORC2 Regulate Skin Morphogenesis and Epidermal Barrier Formation. Nat. Commun. 2016, 7, 13226. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic Extract of Juglans regia L. Green Husks: A Source of Bioactive Phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica Balsamina Leaf Extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef]
- He, Y.; Mao, S.; Zhao, Y.; Yang, J. Research Advances in the Synthesis, Metabolism, and Function of Chlorogenic Acid. Foods 2025, 14, 1914. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Habtemariam, S. Introduction to Plant Secondary Metabolites—From Biosynthesis to Chemistry and Antidiabetic Action. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–132. ISBN 978-0-08-102922-0. [Google Scholar]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Jung, S.-H.; Heo, H.-Y.; Choe, J.-W.; Kim, J.; Lee, K. Anti-Melanogenic Properties of Velutin and Its Analogs. Molecules 2021, 26, 3033. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The Açaí Flavonoid Velutin Is a Potent Anti-Inflammatory Agent: Blockade of LPS-Mediated TNF-α and IL-6 Production through Inhibiting NF-κB Activation and MAPK Pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef]
- Akbari, V.; Jamei, R.; Heidari, R.; Esfahlan, A.J. Antiradical Activity of Different Parts of Walnut (Juglans regia L.) Fruit as a Function of Genotype. Food Chem. 2012, 135, 2404–2410. [Google Scholar] [CrossRef]
- Biology of Quinoline and Quinazoline Alkaloids. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 88, pp. 1–47. ISBN 978-0-323-98917-6.
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional Walnut Liqueur–Cocktail of Phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Ionica, M.; Tutulescu, F. Phenolics Content, Antioxidant Activity and Color of Green Walnut Extracts for Preparing Walnut Liquor. Not. Bot. Horti Agrobot. 2014, 42, 551–555. [Google Scholar] [CrossRef]
- Jakopic, J.; Solar, A.; Colaric, M.; Hudina, M.; Veberic, R.; Stampar, F. The Influence of Ethanol Concentration on Content of Total and Individual Phenolics in Walnut Alcoholic Drink. Acta Aliment. 2008, 37, 233–239. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total Phenols, Antioxidant Potential and Antimicrobial Activity of Walnut (Juglans regia L.) Green Husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of Solvent on the Antioxidant and Antimicrobial Properties of Walnut (Juglans regia L.) Green Husk Extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Gagliardi, S.; Mitruccio, M.; Di Corato, R.; Romano, R.; Aloisi, A.; Rinaldi, R.; Alifano, P.; Guerra, F.; Bucci, C. Defects of Mitochondria-Lysosomes Communication Induce Secretion of Mitochondria-Derived Vesicles and Drive Chemoresistance in Ovarian Cancer Cells. Cell Commun. Signal. 2024, 22, 165. [Google Scholar] [CrossRef]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.P.; Ferrick, D.; Singal, A.K.; Ballinger, S.W.; Bailey, S.M.; et al. The Bioenergetic Health Index: A New Concept in Mitochondrial Translational Research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in Skin Health, Aging, and Disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Martic, I.; Papaccio, F.; Bellei, B.; Cavinato, M. Mitochondrial Dynamics and Metabolism across Skin Cells: Implications for Skin Homeostasis and Aging. Front. Physiol. 2023, 14, 1284410. [Google Scholar] [CrossRef]
- Gaetano, V.; Gagliardi, A.; Giuliano, E.; Longo, E.; Cosco, D. Chitosan Nanoparticles Loaded with Polyphenols for Cosmeceutical Applications: A State-of-the-Art Review. Pharmaceutics 2025, 17, 1068. [Google Scholar] [CrossRef] [PubMed]
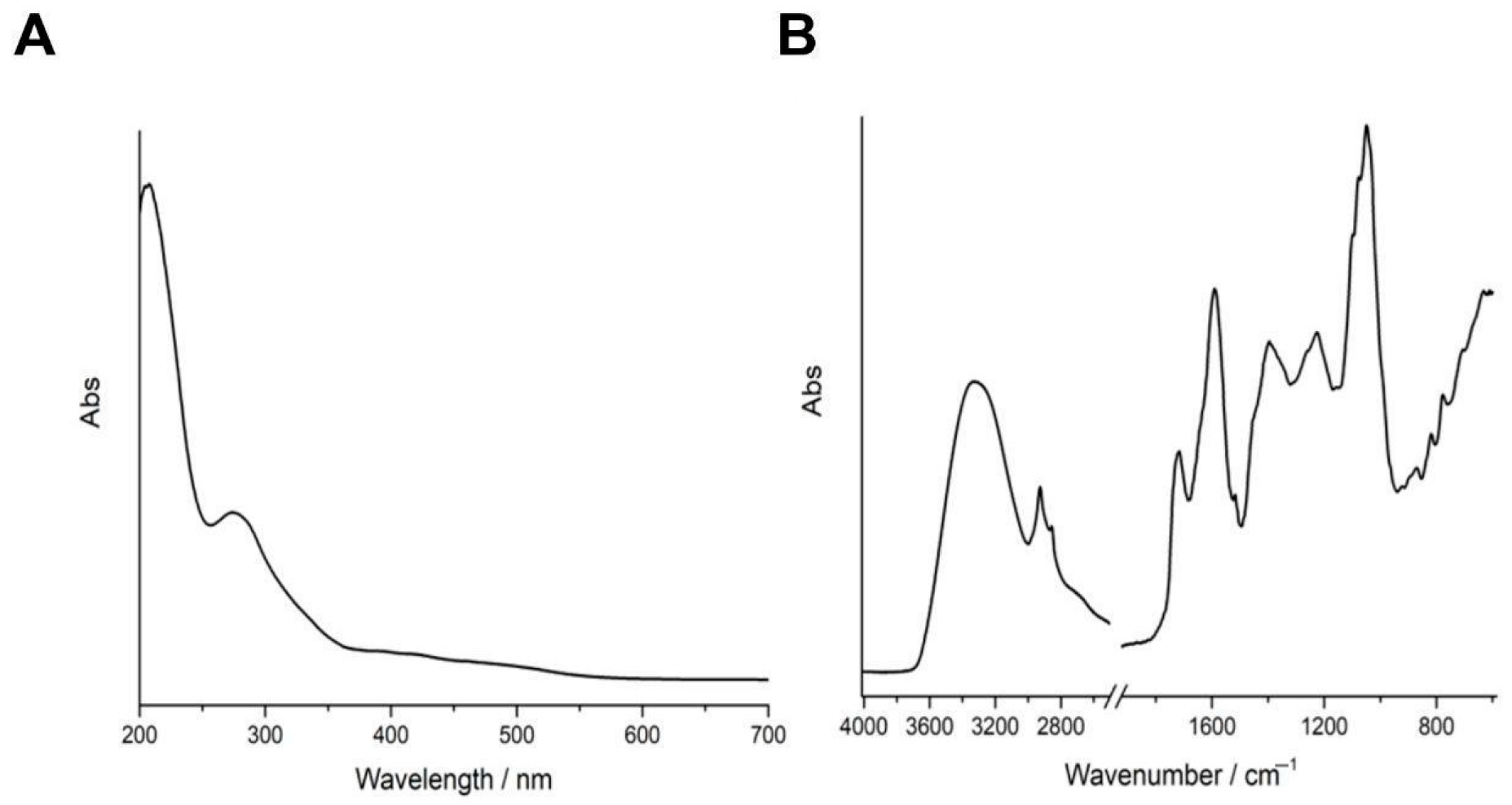
| Extract | Extract Concentration (mg/mL) | GAE Detected at 0.02 mg/mL (μg/mL) | TPC (mg GAE/g Dry Extract) | TPC (μg GAE/mL of Extract) |
|---|---|---|---|---|
| WHE | 13.3 | 0.028 ± 0.02 | 1.45 ± 0.03 | 18.6 ± 0.4 |
| N. | RT (min) | Compound Identification | Ione Mode | (m/z) | Δ ppm | Formula | Classification | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.576 | Adenine | [M─H]− | 134.047 | 1.079 | C5H5N5 | 6-Aminopurines | [15] |
| 2 | 1.616 | Shikimic Acid | [M─H]− | 173.045 | 1.105 | C7H10O5 | Phenolic acids | [16] |
| 3 | 1.712 | D-Glyceric Acid | [M─H]− | 105.019 | 1.057 | C3H6O4 | Sugar Acids and Derivatives | - |
| 4 | 1.812 | Gallic Acid isomer | [M─H]− | 169.014 | 1.105 | C7H6O5 | Phenolic acids (hydroxybenzoic acids) | [5,17] |
| 5 | 1.982 | L-Malic Acid | [M─H]− | 133.014 | 1.073 | C4H6O5 | Beta Hydroxy Acids and Derivatives | [17,18,19] |
| 6 | 2.043 | Arabinofuranosyluracil | [M─H]− | 243.062 | 1.139 | C9H12N2O6 | Pyrimidine Nucleosides | - |
| 7 | 2.071 | Citric Acid | [M─H]− | 191.01 | 1.104 | C6H8O7 | Tricarboxylic Acids and Derivatives | [17,18,19] |
| 8 | 2.097 | Gallic Acid isomer | [M─H]− | 169.014 | 1.105 | C7H6O5 | Phenolic acids (hydroxybenzoic acids) | [5,17] |
| 9 | 2.259 | Citramalic Acid | [M─H]− | 147.029 | 1.084 | C5H8O5 | Hydroxy Fatty Acids | - |
| 10 | 2.299 | Tyramine | [M─H]+ | 138.091 | −0.912 | C8H11NO | Phenethylamines | - |
| 11 | 2.836 | Gallic Acid isomer | [M─H]− | 169.01425 | 1.105 | C7H6O5 | Phenolic acids (hydroxybenzoic acids) | [5,17] |
| 12 | 3.022 | L-Malic Acid Derivate 2 | [M─H]− | 133.014 | 1.073 | C4H6O5 | Beta Hydroxy Acids and Derivatives | [17,18,19] |
| 13 | 3.083 | Gallic Acid hexoside | [M─H]− | 169.014 | 1.105 | C7H6O5 | Phenolic acids (hydroxybenzoic acids) | [5,17] |
| 14 | 3.554 | Vanillylmandelic Acid | [M─H]− | 197.045 | 1.127 | C9H10O5 | Methoxyphenols | - |
| 15 | 4.331 | D-Pantothenic Acid | [M─H]− | 218.103 | 1.132 | C9H17NO5 | Secondary Alcohols | [15] |
| 16 | 4.479 | D-Pantothenic Acid isomer | [M─H]− | 218.103 | 1.132 | C9H17NO5 | Secondary Alcohols | [15] |
| 17 | 4.69 | 2-Hydroxyquinoline isomer | [M─H]− | 144.045 | 1.113 | C9H7NO | Hydroquinolones | - |
| 18 | 4.922 | Hyacinthacine | [M─H]+ | 220.117 | −0.883 | C9H17NO5 | Alkaloid | [15] |
| 19 | 6.388 | Pyrocatechuic Acid | [M─H]− | 153.019 | 1.101 | C7H6O4 | Phenolic acids (hydroxybenzoic acids) | [20] |
| 20 | 6.4 | 2-Hydroxyquinoline | [M─H]+ | 146.060 | −0.902 | C9H7NO | Hydroquinolones | - |
| 21 | 6.482 | 2-Hydroxyquinoline | [M─H]− | 144.045 | 1.113 | C9H7NO | Hydroquinolones | - |
| 22 | 6.636 | Quinic Acid | [M─H]− | 191.056 | 1.11 | C7H12O6 | Phenolic acids (Quinic Acids and Derivatives) | [17] |
| 23 | 6.848 | Chlorogenic Acid | [M─H]+ | 355.102 | −0.794 | C16H18O9 | Phenolic acids (hydroxycinnamic acid derivative) | [21] |
| 24 | 6.98 | Chlorogenic Acid isomer | [M─H]+ | 355.102 | −0.794 | C16H18O9 | Phenolic acids (hydroxycinnamic acid derivative) | [21] |
| 25 | 7.218 | Quinic Acid Derivate 2 | [M─H]− | 191.056 | 1.11 | C7H12O6 | Phenolic acids (Quinic Acids and Derivatives) | [17] |
| 26 | 7.304 | Tryptophan | [M─H]+ | 205.097 | −0.872 | C11H12N2O2 | Indolyl carboxylic acids and derivatives | [15] |
| 27 | 7.436 | (-)-Epicatechin | [M─H]+ | 291.086 | −0.818 | C15H14O6 | Flavanols (Catechins) | [17,22] |
| 28 | 7.663 | 1,6-Digalloyl-Beta-D-Glucopyranose | [M─H]− | 483.078 | 1.286 | C20H20O14 | Tannins | [17,23] |
| 29 | 7.904 | Dihydrocoumaroyl Hexoside | [M─H]− | 327.108 | 1.206 | C15H20O8 | Phenolic acids | - |
| 30 | 7.916 | (-)-Epicatechin isomer | [M─H]+ | 291.086 | −0.818 | C15H14O6 | Flavanols (Catechins) | [17,22] |
| 31 | 8.025 | 1,6-bis-O-galloyl-beta-D-glucose | [M─H]− | 483.078 | 1.286 | C20H20O14 | Tannins | [17,23] |
| 32 | 8.032 | Coumaroylquinic Acid | [M─H]− | 337.092 | 1.216 | C16H18O8 | Phenolic acids (hydroxycinnamic acid derivative) | [5,17,22] |
| 33 | 8.172 | Coumaroylquinic Acid isomer | [M─H]− | 337.092 | 1.216 | C16H18O8 | Phenolic acids (hydroxycinnamic acid derivative) | [5,17,22] |
| 34 | 8.212 | Neochlorogenic Acid | [M─H]− | 353.087 | 1.221 | C16H18O9 | Phenolic acids (hydroxycinnamic acid derivative) | [22] |
| 35 | 8.305 | Catechin | [M─H]− | 289.071 | 1.196 | C15H14O6 | Flavanols (Catechins) | [17,22] |
| 36 | 8.337 | Neochlorogenic Acid isomer | [M─H]− | 353.087 | 1.221 | C16H18O9 | Phenolic acids (hydroxycinnamic acid derivative) | [22] |
| 37 | 8.381 | Pyrocatechuic Acid isomer | [M─H]− | 153.019 | 1.101 | C7H6O4 | Phenolic acids (hydroxybenzoic acids) | [20] |
| 38 | 8.418 | Ferulic Acid | [M─H]+ | 195.065 | −0.881 | C10H10O4 | Phenolic acids (Hydroxycinnamic acids) | [17] |
| 39 | 8.445 | Catechin isomer | [M─H]− | 289.071 | 1.196 | C15H14O6 | Flavanols (Catechins) | [17,22] |
| 40 | 8.572 | Esculetin | [M─H]− | 177.019 | 1.122 | C9H6O4 | Phenolic acids (coumarin derivative) | [19,24] |
| 41 | 8.58 | 5-O-Feruloylquinic Acid | [M─H]− | 367.103 | 1.232 | C17H20O9 | Phenolic acids (hydroxycinnamic acid derivative) | - |
| 42 | 8.918 | Epiaxifolin | [M─H]+ | 305.065 | −0.814 | C15H12O7 | Flavonoids (Flavanols) | [22] |
| 43 | 9.192 | Hyperoside | [M─H]+ | 465.102 | −0.727 | C21H20O12 | Flavonoids (Flavonoid-3-O-glycosides) | [17,25] |
| 44 | 9.194 | (4S,5Z,6S)-4-(2-Methoxy-2-Oxoethyl)-5-[2-[(E)-3-Phenylprop-2-Enoyl]Oxyethylidene]-6-[(2S,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(Hydroxymethyl)Oxan-2-Yl]Oxy-4H-Pyran-3-Carboxylic Acid | [M─H]+ | 303.049 | −0.814 | C15H10O7 | Null | - |
| 45 | 9.202 | Syringic Acid | [M─H]− | 197.045 | 1.127 | C9H10O5 | Phenolic acids (hydroxybenzoic acids) | [20] |
| 46 | 9.374 | Epicatechin Gallate | [M─H]+ | 443.097 | −0.725 | C22H18O10 | Flavonoids (Flavanols) | [17] |
| 47 | 9.441 | Quercetin-3-Arabinoside | [M─H]+ | 435.092 | −0.742 | C20H18O11 | Flavonoids (Flavonoid-3-O-glycosides) | [17] |
| 48 | 9.539 | Taxifolin | [M─H]+ | 305.065 | −0.814 | C15H12O7 | Flavonoids (Flavanols) | [22] |
| 49 | 9.645 | Ascorbic Acid | [M─H]− | 175.024 | 1.099 | C6H8O6 | Butenolides | [15] |
| 50 | 9.662 | Scopoletin | [M─H]+ | 193.049 | −0.881 | C10H8O4 | Phenolic acids (coumarin derivative) | [17] |
| 51 | 9.693 | Kaempferol 3-O-glucoside | [M─H]+ | 449.107 | −0.731 | C21H20O11 | Flavonoids (Flavonoid-3-O-glycosides) | [17] |
| 52 | 9.863 | Sayaendoside | [M─H]− | 415.160 | 1.259 | C19H28O10 | O-Glycosyl Compounds | - |
| 53 | 9.946 | 1,2,3,6-Tetragalloylglucose | [M─H]− | 787.099 | 1.473 | C34H28O22 | Tannins | [17] |
| 54 | 10.241 | Ellagic Acid | [M─H]− | 300.999 | 1.194 | C14H6O8 | Hydrolyzable Tannins | [17,23] |
| 55 | 10.246 | Myricitrin | [M─H]− | 463.088 | 1.288 | C21H20O12 | Flavonoids (Flavonoid-3-O-Glycosides) | [17,25] |
| 56 | 10.434 | Catechin Gallate | [M─H]− | 441.082 | 1.29 | C22H18O10 | Flavonoids (Flavanols) | [17] |
| 57 | 10.496 | [3,4,5-Trihydroxy-6-[[(E)-3-(4-Hydroxyphenyl)Prop-2-Enoyl]Oxymethyl]Oxan-2-Yl] 3,4,5-Trihydroxybenzoate | [M─H]− | 477.103 | 1.299 | C22H22O12 | Tannins | - |
| 58 | 10.569 | Astilbin | [M─H]− | 449.108 | 1.284 | C21H22O11 | Flavonoids (Flavonoid-3-O-Glycosides) | [17,25] |
| 59 | 10.569 | Quercetin 3-arabinoside | [M─H]− | 433.077 | 1.273 | C20H18O11 | Flavonoids (Flavonoid-3-O-Glycosides) | [17,25] |
| 60 | 10.728 | 4-Hydroxy-3-methoxycinnamic acid isomer | [M─H]− | 193.050 | 1.133 | C10H10O4 | Phenolic acids (Hydroxycinnamic Acids) | [17] |
| 61 | 10.825 | Quercetin-3-o-beta-d-xylopyranoside | [M─H]− | 433.077 | 1.273 | C20H18O11 | Flavonoids (Flavonoid-3-O-Glycosides) | [17,25] |
| 62 | 10.875 | Taxifolin isomer | [M─H]− | 303.051 | 1.201 | C15H12O7 | Flavonoids (Flavanols) | [22] |
| 63 | 10.922 | Quercitrin Derivate 2 | [M─H]− | 447.093 | 1.284 | C21H20O11 | Flavonoids (Flavonoid-3-O-Glycosides) | [17] |
| 64 | 11.111 | Octopine | [M─H]+ | 247.140 | −0.876 | C9H18N4O4 | Arginine and derivatives | - |
| 65 | 11.247 | Eriodictyol | [M─H]+ | 289.07 | −0.818 | C15H12O6 | Flavonoids (Flavanones) | [17] |
| 66 | 11.77 | Pyrogallin | [M─H]− | 203.035 | 1.144 | C11H8O4 | Tropolones | - |
| 67 | 11.944 | (+/-)-Dihydrokaempferol | [M─H]− | 287.056 | 1.196 | C15H12O6 | Flavonoids (Flavonols) | [17,19,26] |
| 68 | 11.961 | 2 beta-D-glucopyranosyl Phloretin | [M─H]+ | 275.091 | −0.823 | C15H14O5 | Flavonoids (2′-Hydroxy-dihydrochalcones) | [17] |
| 69 | 12.121 | Naringenin | [M─H]+ | 273.075 | −0.823 | C15H12O5 | Flavonoids (Flavanones) | [17] |
| 70 | 13.029 | Quercetin | [M─H]− | 301.035 | 1.2 | C15H10O7 | Flavonoids (Flavonols) | [17] |
| 71 | 13.079 | Sternbin | [M─H]+ | 303.086 | −0.808 | C16H14O6 | Flavonoids (Flavanones) | - |
| 72 | 13.97 | Phloretin | [M─H]− | 273.076 | 1.192 | C15H14O5 | Flavonoids (2′-Hydroxy-Dihydrochalcones) | [17] |
| 73 | 14.127 | Naringenin chalcone | [M─H]− | 271.061 | 1.192 | C15H12O5 | Flavonoids (Flavanones) | [17] |
| 74 | 14.647 | Velutin | [M─H]+ | 315.086 | −0.797 | C17H14O6 | Flavonoids (7-O-methylated flavonoids) | - |
| 75 | 16.358 | Progesterone | [M─H]+ | 315.231 | −0.769 | C21H30O2 | Null | [27] |
| 76 | 17.605 | Luteolin 3′,4′-Dimethyl Ether | [M─H]− | 313.071 | 1.218 | C17H14O6 | Flavonoids (4′-O-Methylated Flavonoids) | [17] |
| 77 | 18.362 | LPE 18:3 | [M─H]− | 474.262 | 1.293 | C23H42NO7P | Lysophosphatidylethanolamine | - |
| 78 | 19.503 | LPI 18:2 | [M─H]− | 595.288 | 1.356 | C27H49O12P | Lysophosphatidylinositol | - |
| 79 | 19.555 | LPE 18:2 | [M─H]− | 476.278 | 1.293 | C23H44NO7P | Lysophosphatidylethanolamine | - |
| 80 | 20.521 | LPE 16:0 | [M─H]− | 452.278 | 1.272 | C21H44NO7P | Lysophosphatidylethanolamine | - |
| 81 | 21.159 | LPE 18:1 | [M─H]− | 478.293 | 1.294 | C23H46NO7P | Lysophosphatidylethanolamine | - |
| 82 | 22.529 | Mannose | [M─H]− | 179.056 | 1.1 | C6H12O6 | Hexoses | [15] |
| Concentration (mg/mL) | ABTS–WHE (% Inhibition) | DPPH–WHE (% Inhibition) |
|---|---|---|
| 0.667 | 100 ± 2 | 68 ± 3 |
| 0.550 | 100 ± 1 | 66 ± 2 |
| 0.333 | 100 ± 2 | 60 ± 3 |
| 0.167 | 100 ± 1 | 64 ± 2 |
| 0.083 | 93 ± 2 | 52 ± 3 |
| 0.040 | 89 ± 3 | 38 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergine, G.; Ottolini, M.; De Benedetto, G.E.; Bettini, S.; Baldassarre, F.; Vergara, D.; Ciccarella, G. Phytochemistry and Biological Effects of the Juglans regia “Sorrento” Walnut Husk Extract on Human Keratinocyte Cells. Antioxidants 2025, 14, 1385. https://doi.org/10.3390/antiox14121385
Vergine G, Ottolini M, De Benedetto GE, Bettini S, Baldassarre F, Vergara D, Ciccarella G. Phytochemistry and Biological Effects of the Juglans regia “Sorrento” Walnut Husk Extract on Human Keratinocyte Cells. Antioxidants. 2025; 14(12):1385. https://doi.org/10.3390/antiox14121385
Chicago/Turabian StyleVergine, Giulia, Michela Ottolini, Giuseppe E. De Benedetto, Simona Bettini, Francesca Baldassarre, Daniele Vergara, and Giuseppe Ciccarella. 2025. "Phytochemistry and Biological Effects of the Juglans regia “Sorrento” Walnut Husk Extract on Human Keratinocyte Cells" Antioxidants 14, no. 12: 1385. https://doi.org/10.3390/antiox14121385
APA StyleVergine, G., Ottolini, M., De Benedetto, G. E., Bettini, S., Baldassarre, F., Vergara, D., & Ciccarella, G. (2025). Phytochemistry and Biological Effects of the Juglans regia “Sorrento” Walnut Husk Extract on Human Keratinocyte Cells. Antioxidants, 14(12), 1385. https://doi.org/10.3390/antiox14121385













