Effect of Microparticle Crystallinity and Food Matrix on the Release Profile and Antioxidant Activity of Encapsulated Gallic and Ellagic Acids During Simulated In Vitro Intestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Central Composite Design for the Preparation of Ellagic Acid-Inulin Microparticles Using Ethanol as Crystallinity Inducer
2.2. Characterization of Ellagic Acid- and Gallic Acid-Inulin Microparticles
2.2.1. Crystallinity Index (CI)
2.2.2. Encapsulation Efficiency (EE)
2.2.3. Particle Size and Morphology
2.2.4. Spray-Drying Yield
2.3. In Vitro Simulated Gastrointestinal Digestion
2.3.1. Formulation of Model Food Matrices with Ellagic Acid- and Gallic Acid-Inulin Microparticles
2.3.2. Preparation of Model Food Matrices
2.3.3. In Vitro Simulated Digestion for the Microparticles and Model Food Matrices
2.3.4. Quantification of Ellagic Acid or Gallic Acid Released During In Vitro Simulated Digestion
2.3.5. Bioaccessibility of Ellagic Acid or Gallic Acid
2.3.6. Determination of Antioxidant Activity
3. Results and Discussion
3.1. Experimental Design for Encapsulation of Ellagic Acid
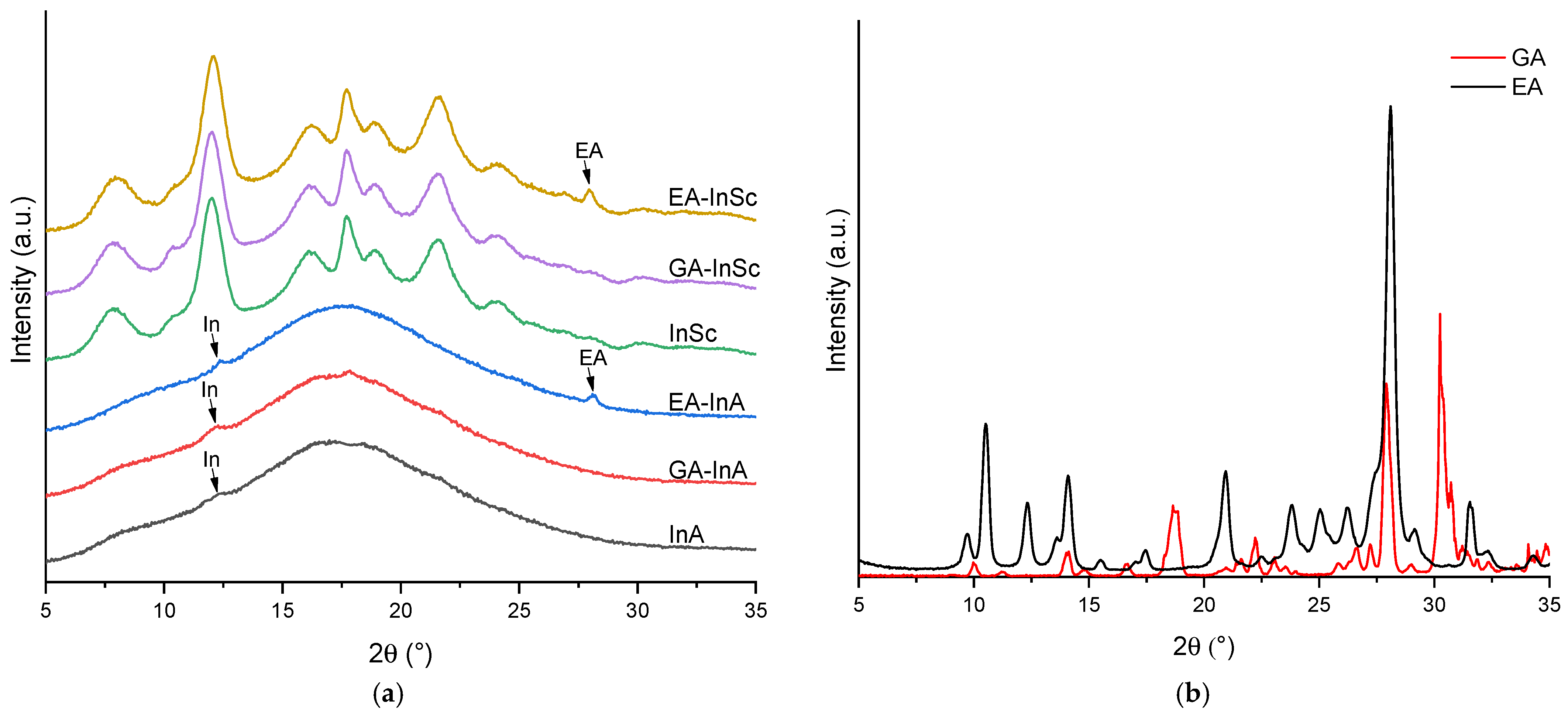
3.2. Characterization of EA- and GA-Inulin Microparticles Obtained Under Optimal Conditions
3.2.1. Crystallinity Index (CI)
3.2.2. Encapsulation Efficiency (EE)
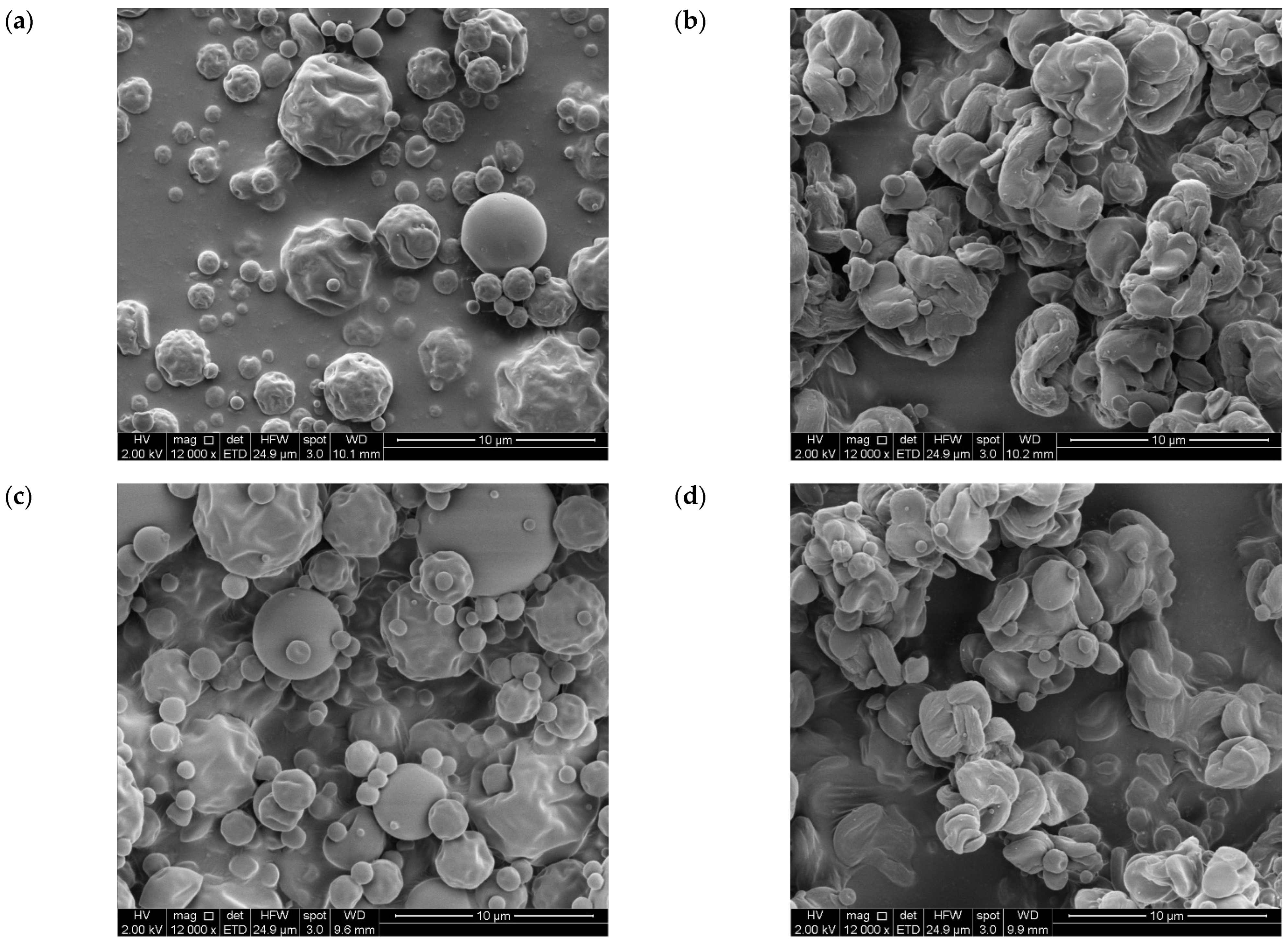
3.2.3. Particle Size and Morphology
3.3. In Vitro Simulated Digestion
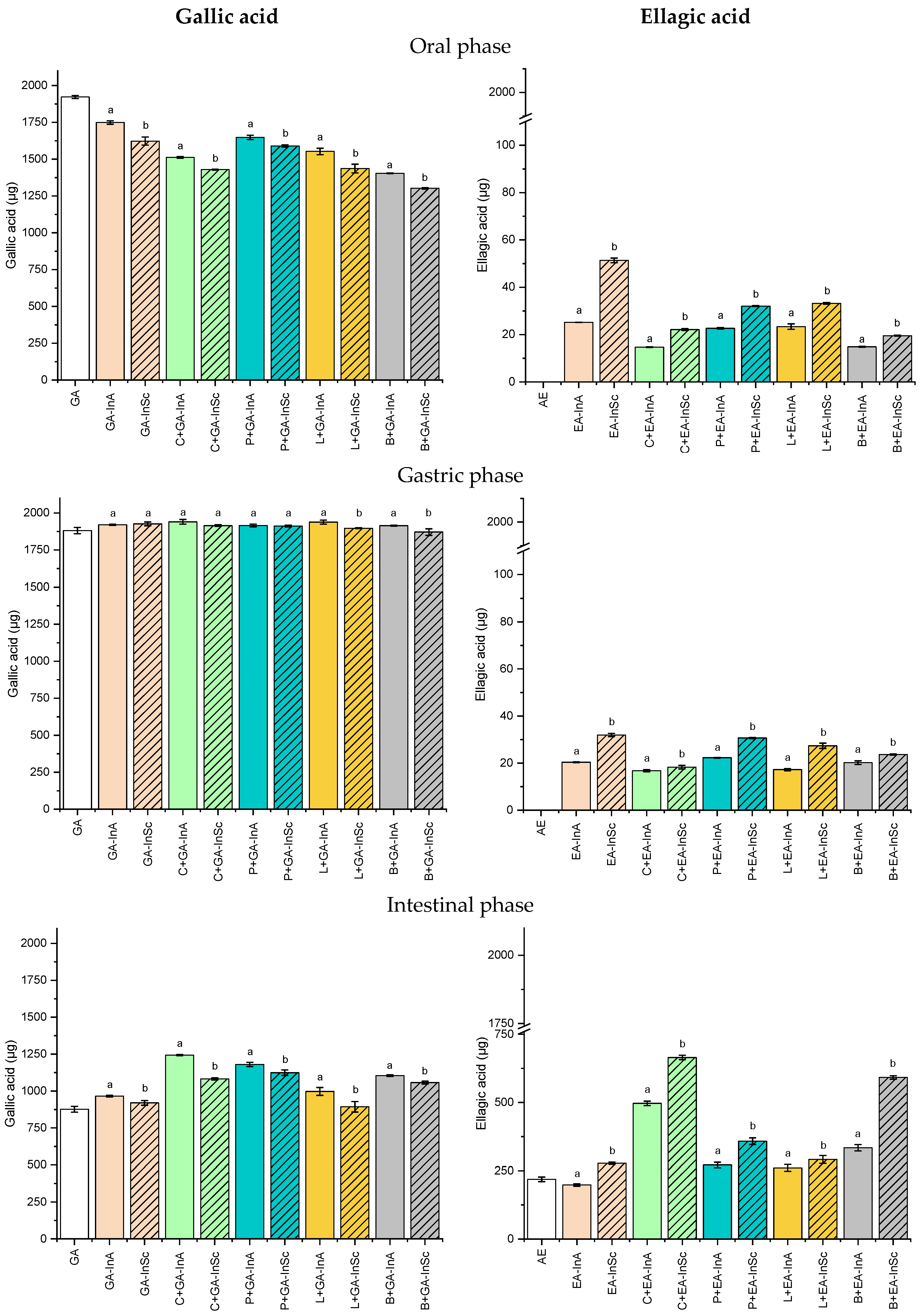
3.3.1. Release Profile of GA and EA
3.3.2. Effect of In Vitro Simulated Digestion on Antioxidant Activity
| Oral Phase (µmol Trolox/g Microparticles) | Gastric Phase (µmol Trolox/g Microparticles) | Intestinal Phase (µmol Trolox/g Microparticles) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | Ferricyanide | DPPH | ABTS | Ferricyanide | DPPH | ABTS | Ferricyanide | |
| Semicrystalline microparticles | |||||||||
| GA-InSc | 23.7 ± 0.7b | 26.3 ± 0.1 a | 17.5 ± 0.1 b | 24.4 ± 0.2 a | 30.9 ± 0.2 cd | 18.3 ± 0.1 d | 16.4 ± 0.1 b | 11.4 ± 0.1 d | 10.2 ± 0.3 b |
| C+GA-InSc | 22.9 ± 0.2 bc | 24.4 ± 0.3 b | 17.7 ± 0.1 b | 23.7 ± 0.5 a | 30.4 ± 0.3 d | 18.5 ± 0.1 d | 16.6 ± 0.4 b | 11.9 ± 0.0 c | 9.6 ± 0.2 b |
| P+GA-InSc | 26.8 ± 0.2 a | 26.6 ± 0.2 a | 19.5 ± 0.1 a | 24.0 ± 0.5 a | 51.9 ± 0.3 a | 34.7 ± 0.7 a | 18.1 ± 0.2 a | 14.5 ± 0.0 a | 12.4 ± 0.1 a |
| L+GA-InSc | 22.1 ± 0.4 c | 24.5 ± 0.2 b | 15.5 ± 0.1 c | 21.1 ± 0.2 b | 31.6 ± 0.3 c | 19.9 ± 0.0 c | 12.9 ± 0.2 c | 11.8 ± 0.0 c | 5.9 ± 0.3 c |
| B+GA-InSc | 15.6 ± 0.1 d | 18.6 ± 0.2 c | 13.1 ± 0.3 d | 20 ± 0.5 b | 39.1 ± 0.2 b | 33.2 ± 0.1 b | 14.2 ± 0.1 d | 13.8 ± 0.0 b | 10.2 ± 0.3 b |
| Amorphous microparticles | |||||||||
| GA-InA | 27.0 ± 0.3 a | 28.4 ± 0.3 b | 18.6 ± 0.1 b | 25.7 ± 0.2 a | 34.7 ± 0.2 c | 19.9 ± 0.2 d | 18.2 ± 0.2 c | 12.4 ± 0.0 c | 11.0 ± 0.5 c |
| C+GA-InA | 25.5 ± 0.0 b | 26.2 ± 0.2 c | 18.8 ± 0.0 b | 25.7 ± 0.2 a | 33.5 ± 0.0 d | 21.5 ± 0.4 c | 18.7 ± 0.0 b | 13.6 ± 0.2 b | 10.7 ± 0.2 c |
| P+GA-InA | 27.6 ± 0.1 a | 28.8 ± 0.2 a | 20.4 ± 0.2 a | 25.4 ± 0.1 a | 53.8 ± 0.6 a | 36.6 ± 0.1 a | 19.3 ± 0.1 a | 14.9 ± 0.2 a | 13.4 ± 0.1 a |
| L+GA-InA | 24.5 ± 0.4 c | 26.5 ± 0.0 c | 16.2 ± 0.0 c | 23.3 ± 0.2 b | 35.4 ± 0.2 c | 22.3 ± 0.6 c | 13.5 ± 0.1 d | 12.5 ± 0.4 c | 9.3 ± 0.3 d |
| B+GA-InA | 19.2 ± 0.2 d | 21.3 ± 0.1 d | 15.1 ± 0.2 d | 22.7 ± 0.1 c | 50.3 ± 0.3 b | 34.7 ± 0.3 b | 15.0 ± 0.2 e | 15.0 ± 0.1 a | 12.1 ± 0.4 b |
| Oral Phase (µmol Trolox/g Microparticles) | Gastric Phase (µmol Trolox/g Microparticles) | Intestinal Phase (µmol Trolox/g Microparticles) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | Ferricyanide | DPPH | ABTS | Ferricyanide | DPPH | ABTS | Ferricyanide | |
| Semicrystalline microparticles | |||||||||
| EA-InSc | 0.7 ± 0.04 bc | 1.0 ± 0.03 bc | 0.6 ± 0.02 a | 0.4 ± 0.03 c | 0.4 ± 0.04 bc | 0.3 ± 0.01 d | 1.5 ± 0.03 c | 1.8 ± 0.02 c | 2.3 ± 0.12 d |
| C+EA-InSc | 0.6 ± 0.01 c | 0.7 ± 0.01 d | 0.4 ± 0.03 c | 0.5 ± 0.05 bc | 0.3 ± 0.02 c | 0.4 ± 0.01 c | 3.3 ± 0.05 a | 3.6 ± 0.14 a | 6.5 ± 0.22 b |
| P+EA-InSc | 0.6 ± 0.01 c | 1.1 ± 0.06 b | 0.5 ± 0.01 b | 0.5 ± 0.02 bc | 0.5 ± 0.01 b | 0.3 ± 0.02 d | 1.1 ± 0.05 d | 2.1 ± 0.05 b | 3.6 ± 0.20 c |
| L+EA-InSc | 0.7 ± 0.00 b | 0.9 ± 0.02 cd | 0.5 ± 0.01 b | 0.8 ± 0.05 a | 0.7 ± 0.04 a | 0.7 ± 0.02 a | 1.1 ± 0.01 d | 1.0 ± 0.01 d | 1.8 ± 0.14 e |
| B+EA-InSc | 0.8 ± 0.02 a | 1.4 ± 0.04 a | 0.5 ± 0.03 b | 0.5 ± 0.06 b | 0.5 ± 0.02 b | 0.6 ± 0.01 b | 2.2 ± 0.1 b | 3.5 ± 0.02 a | 7.0 ± 0.05 a |
| Amorphous microparticles | |||||||||
| EA-InA | 0.5 ± 0.00 b | 0.8 ± 0.03 b | 0.2 ± 0.01 c | 0.3 ± 0.00 c | 0.3 ± 0.01 b | 0.1 ± 0.03 c | 1.1 ± 0.07 c | 1.4 ± 0.08 d | 1.6 ± 0.06 d |
| C+EA-InA | 0.5 ± 0.03 b | 0.7 ± 0.01 c | 0.3 ± 0.02 ab | 0.4 ± 0.05 ab | 0.2 ± 0.02 b | 0.3 ± 0.01 b | 3.1 ± 0.07 a | 2.5 ± 0.08 b | 5.4 ± 0.08 b |
| P+EA-InA | 0.6 ± 0.01 a | 0.6 ± 0.01 d | 0.4 ± 0.02 a | 0.4 ± 0.01 ab | 0.2 ± 0.01 b | 0.1 ± 0.01 c | 1.0 ± 0.03 c | 1.8 ± 0.07 c | 2.7 ± 0.16 c |
| L+EA-InA | 0.5 ± 0.01 b | 0.7 ± 0.01 c | 0.3 ± 0.01 b | 0.5 ± 0.05 a | 0.4 ± 0.03 a | 0.4 ± 0.01 a | 1.1 ± 0.02 c | 1.1 ± 0.01 e | 1.5 ± 0.08 d |
| B+EA-InA | 0.7 ± 0.01 a | 1.2 ± 0.03 a | 0.4 ± 0.02 a | 0.4 ± 0.03 bc | 0.2 ± 0.01 b | 0.2 ± 0.00 bc | 1.5 ± 0.07 b | 2.7 ± 0.04 a | 5.8 ± 0.18 a |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EA | Ellagic acid |
| GA | Gallic acid |
| In | Inulin |
| EtOH | Ethanol |
| Te | Inlet air temperature to the dryer |
| EE | Encapsulation efficiency |
| CI | Crystallinity index |
| SEM | Scanning electron microscopy |
| D4,3 | Volume-weighted mean diameter |
| XRD | X-ray diffraction |
| InSc | Semicrystalline inulin |
| InA | Amorphous inulin |
| EA-InSc | Ellagic acid–inulin semicrystalline microparticles |
| EA-InA | Ellagic acid–inulin amorphous microparticles |
| GA-InSc | Gallic acid–inulin semicrystalline microparticles |
| GA-InA | Gallic acid–inulin amorphous microparticles |
| C | Carbohydrate matrix |
| P | Protein matrix |
| L | Lipid matrix |
| B | Blend matrix |
| AA | Antioxidant activity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid |
References
- López-Astorga, M.; Leon-Bejarano, M.; Gámez-Meza, N.; Del Toro-Sánchez, C.L.; Simsek, S.; Ovando-Martínez, M. Microencapsulated Grape Pomace Extract as an Antioxidant Ingredient Added to Greek-Style Yogurt: Storage Stability an in Vitro Bioaccessibility. Food Chem. 2025, 477, 143550. [Google Scholar] [CrossRef] [PubMed]
- De Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of Gallic Acid and Evaluation of Its Cytotoxicity and Antioxidant Activity. Mater. Sci. Eng. C 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Lu, L.-L.; Lu, X.-Y. Solubilities of Gallic Acid and Its Esters in Water. J. Chem. Eng. Data 2007, 52, 37–39. [Google Scholar] [CrossRef]
- Delfanian, M.; Sahari, M.A. Improving Functionality, Bioavailability, Nutraceutical and Sensory Attributes of Fortified Foods Using Phenolics-Loaded Nanocarriers as Natural Ingredients. Food Res. Int. 2020, 137, 109555. [Google Scholar] [CrossRef]
- Choi, Y.R.; Chang, Y.H. Microencapsulation of Gallic Acid through the Complex of Whey Protein Concentrate-Pectic Polysaccharide Extracted from Ulmus Davidiana. Food Hydrocoll. 2018, 85, 222–228. [Google Scholar] [CrossRef]
- Sepelevs, I.; Stepanova, V.; Galoburda, R. Encapsulation of Gallic Acid with Acid-Modified Low Dextrose Equivalent Potato Starch Using Spray-and Freeze-Drying Techniques. Pol. J. Food Nutr. Sci. 2018, 68, 273–280. [Google Scholar] [CrossRef]
- Nájera-Martínez, E.F.; Flores-Contreras, E.A.; Araújo, R.G.; Iñiguez-Moreno, M.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Pastrana, L.M.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Microencapsulation of Gallic Acid Based on a Polymeric and pH-Sensitive Matrix of Pectin/Alginate. Polymers 2023, 15, 3014. [Google Scholar] [CrossRef]
- Aydogdu Emir, A.; Yildiz, E.; Aydogdu, Y.; Sumnu, G.; Sahin, S. Gallic Acid Encapsulated Pea Flour-based Nanofibers Produced by Electrospinning as a Potential Active Food Packaging Material. Legume Sci. 2021, 3, e90. [Google Scholar] [CrossRef]
- Xiong, Y.; Feng, Y.; Chang, M.; Wang, Q.; Yin, S.; Jian, L.; Ren, D. Formulated Chitosan-sodium Tripolyphosphate Nanoparticles for Co-encapsulation of Ellagic Acid and Anti-inflammatory Peptide: Characterization, Stability and Anti-inflammatory Activity. J. Sci. Food Agric. 2023, 103, 3447–3456. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Ramana, L.N.; Sethuraman, S.; Krishnan, U.M. Ellagic Acid Encapsulated Chitosan Nanoparticles as Anti-Hemorrhagic Agent. Carbohydr. Polym. 2014, 111, 215–221. [Google Scholar] [CrossRef]
- Kaur, H.; Ghosh, S.; Kumar, P.; Basu, B.; Nagpal, K. Ellagic Acid-Loaded, Tween 80-Coated, Chitosan Nanoparticles as a Promising Therapeutic Approach against Breast Cancer: In-Vitro and In-Vivo Study. Life Sci. 2021, 284, 119927. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elghany, A.A.; Mohamad, E.A. Chitosan-Coated Niosomes Loaded with Ellagic Acid Present Antiaging Activity in a Skin Cell Line. ACS Omega 2023, 8, 16620–16629. [Google Scholar] [CrossRef]
- Stojiljković, N.; Ilić, S.; Stojanović, N.; Janković-Veličković, L.; Stojnev, S.; Kocić, G.; Radenković, G.; Arsić, I.; Stojanović, M.; Petković, M. Nanoliposome-Encapsulated Ellagic Acid Prevents Cyclophosphamide-Induced Rat Liver Damage. Mol. Cell. Biochem. 2019, 458, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sinha, S.; Seth, D.; Jana, R. Effect of pH on Ellagic Acid and Its Complexation with Gamma-Cyclodextrins. J. Mol. Struct. 2024, 1306, 137894. [Google Scholar] [CrossRef]
- Morelo, G.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices. Antioxidants 2019, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Deroanne, C.; Paquot, M.; Fougnies, C.; Lambrechts, J.-C.; Blecker, C.S. Characterization of the Physical State of Spray-Dried Inulin. Food Biophys. 2007, 2, 83–92. [Google Scholar] [CrossRef]
- Kim, Y.; Faqih, M.N.; Wang, S.S. Factors Affecting Gel Formation of Inulin. Carbohydr. Polym. 2001, 46, 135–145. [Google Scholar] [CrossRef]
- Glibowski, P.; Pikus, S. Amorphous and Crystal Inulin Behavior in a Water Environment. Carbohydr. Polym. 2011, 83, 635–639. [Google Scholar] [CrossRef]
- Harjunen, P.; Lehto, V.-P.; Välisaari, J.; Lankinen, T.; Paronen, P.; Järvinen, K. Effects of Ethanol to Water Ratio in Feed Solution on the Crystallinity of Spray-Dried Lactose. Drug Dev. Ind. Pharm. 2002, 28, 949–955. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Kuhn, F.; Santos Dorneles, M.; Pelayo Zapata Noreña, C. Accelerated Stability Testing and Simulated Gastrointestinal Release of Encapsulated Betacyanins and Phenolic Compounds from Bougainvillea glabra Bracts Extract. Food Chem. 2022, 393, 133391. [Google Scholar] [CrossRef]
- Li, Y.; Mei, L.; Guan, X.; Hu, Y. Ellagic Acid Solid Dispersion: Characterization and Bioactivity in the Hydroxyl Radical Oxidation System. Food Res. Int. 2021, 142, 110184. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Apak, R. Comparative Evaluation of Fe(III) Reducing Power-Based Antioxidant Capacity Assays in the Presence of Phenanthroline, Batho-Phenanthroline, Tripyridyltriazine (FRAP), and Ferricyanide Reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Tarazi Riess, H.; Shani Levi, C.; Lesmes, U. Inclusion of Phenolic Bioactives in High Amylose Corn Starch for Gastro-Intestinal Delivery. Front. Nutr. 2022, 9, 981408. [Google Scholar] [CrossRef]
- Rajapaksha, D.S.W.; Shimizu, N. Valorization of Spent Black Tea by Recovery of Antioxidant Polyphenolic Compounds: Subcritical Solvent Extraction and Microencapsulation. Food Sci. Nutr. 2020, 8, 4297–4307. [Google Scholar] [CrossRef]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of Tea Tree Oil by Amorphous Beta-Cyclodextrin Powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Shi, M. Effect of Ethanol–Water Solution on the Crystallization of Short Chain Amylose from Potato Starch. Starch Stärke 2016, 68, 683–690. [Google Scholar] [CrossRef]
- Gajendragadkar, C.N.; Gogate, P.R. Ultrasound Assisted Intensified Recovery of Lactose from Whey Based on Antisolvent Crystallization. Ultrason. Sonochem. 2017, 38, 754–765. [Google Scholar] [CrossRef]
- Palma, M.; García, P.; Márquez-Ruiz, G.; Vergara, C.; Robert, P. Release Kinetics of Flavonoids in Methyl Linoleate from Microparticles Designed with Inulin and Channelizing Agent. Food Res. Int. 2014, 64, 99–105. [Google Scholar] [CrossRef]
- Morelo, G.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Robert, P. Design of Flavonoid Microparticles with Channel Forming Properties to Improve Oxidative Stability of Sunflower Oil. Euro. J. Lipid Sci. Technol. 2017, 119, 1700041. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Paquot, M.; Fougnies, C.; Deroanne, C.; Blecker, C.S. Effect of Water Uptake on Amorphous Inulin Properties. Food Hydrocoll. 2009, 23, 922–927. [Google Scholar] [CrossRef]
- Robert, P.; García, P.; Reyes, N.; Chávez, J.; Santos, J. Acetylated Starch and Inulin as Encapsulating Agents of Gallic Acid and Their Release Behaviour in a Hydrophilic System. Food Chem. 2012, 134, 1–8. [Google Scholar] [CrossRef]
- García, P.; Vergara, C.; Robert, P. Release Kinetic in Yogurt from Gallic Acid Microparticles with Chemically Modified Inulin. J. Food Sci. 2015, 80, C2147–C2152. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-Drying Microencapsulation of Polyphenol Bioactives: A Comparative Study Using Different Natural Fibre Polymers as Encapsulants. Food Bioprocess Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Atiq, O.; Ricci, E.; Baschetti, M.G.; De Angelis, M.G. Modelling Solubility in Semi-Crystalline Polymers: A Critical Comparative Review. Fluid Phase Equilibria 2022, 556, 113412. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Julien-David, D.; Zhao, M.; Villain-Gambier, M.; Trébouet, D. Stability and Preservation of Phenolic Compounds and Related Antioxidant Capacity from Agro-Food Matrix: Effect of pH and Atmosphere. Food Biosci. 2024, 57, 103586. [Google Scholar] [CrossRef]
- Silva, G.S.; Gomes, M.H.G.; de Carvalho, L.M.; Abreu, T.L.; dos Santos Lima, M.; Madruga, M.S.; Kurozawa, L.E.; Bezerra, T.K.A. Microencapsulation of Organic Coffee Husk Polyphenols: Effects on Release, Bioaccessibility, and Antioxidant Capacity of Phenolics in a Simulated Gastrointestinal Tract. Food Chem. 2024, 434, 137435. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.D.L.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia Siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the Limits for Ellagic Acid Bioavailability: A Crossover Pharmacokinetic Study in Healthy Volunteers after Consumption of Pomegranate Extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Li, S.; Lei, D.; Zhu, Z.; Cai, J.; Manzoli, M.; Jicsinszky, L.; Grillo, G.; Cravotto, G. Complexation of Maltodextrin-Based Inulin and Green Tea Polyphenols via Different Ultrasonic Pretreatment. Ultrason. Sonochem. 2021, 74, 105568. [Google Scholar] [CrossRef]
- Ortenzi, M.A.; Antenucci, S.; Marzorati, S.; Panzella, L.; Molino, S.; Rufián-Henares, J.Á.; Napolitano, A.; Verotta, L. Pectin-Based Formulations for Controlled Release of an Ellagic Acid Salt with High Solubility Profile in Physiological Media. Molecules 2021, 26, 433. [Google Scholar] [CrossRef]
- Luo, X.; Tian, M.; Cheng, Y.; Ji, C.; Hu, S.; Liu, H.; Lu, J.; Ren, J. Effects of Simulated In Vitro Gastrointestinal Digestion on Antioxidant Activities and Potential Bioaccessibility of Phenolic Compounds from K. coccinea Fruits. Front. Nutr. 2022, 9, 1024651. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, D.; Cao, C.; Kong, B.; Sun, F.; Xia, X.; Liu, Q. Changes of in Vitro Digestion Rate and Antioxidant Activity of Digestion Products of Ethanol-Modified Whey Protein Isolates. Food Hydrocoll. 2022, 131, 107756. [Google Scholar] [CrossRef]
- Marković, Z.; Milenković, D.; Đorović, J.; Dimitrić Marković, J.M.; Lučić, B.; Amić, D. A DFT and PM6 Study of Free Radical Scavenging Activity of Ellagic Acid. Monatsh. Chem. 2013, 144, 803–812. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
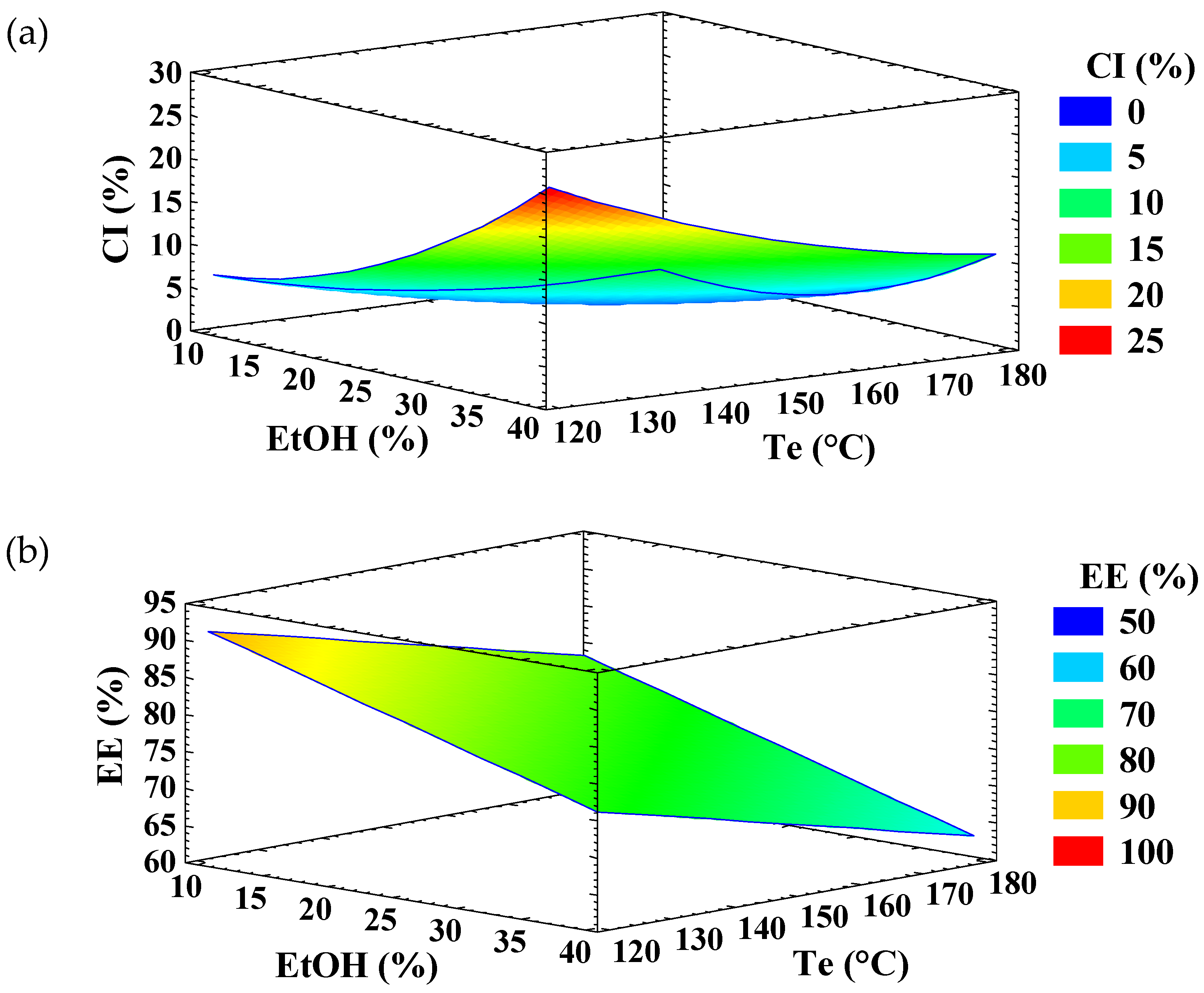
| EtOH (%) | Te (°C) | CI (%) | EE (%) |
|---|---|---|---|
| 10 | 120 | 5.8 ± 0.1 | 86.0 ± 0.2 |
| 40 | 120 | 24.6 ± 1.8 | 81.4 ± 0.5 |
| 10 | 180 | 1.0 ± 0.1 | 77.6 ± 1.2 |
| 40 | 180 | 11.3 ± 0.2 | 57.6 ± 1.9 |
| 7 | 150 | 1.8 ± 0.3 | 87.5 ± 1.0 |
| 43 | 150 | 19.7 ± 0.5 | 63.4 ± 2.3 |
| 25 | 114 | 15.3 ± 0.8 | 82.1 ± 0.7 |
| 25 | 186 | 1.4 ± 0.1 | 73.2 ± 0.5 |
| 25 | 150 | 4.8 ± 0.1 | 77.8 ± 0.0 |
| 25 | 150 | 4.4 ± 0.4 | 83.0 ± 2.3 |
| 25 | 150 | 4.7 ± 0.6 | 80.9 ± 0.4 |
| 25 | 150 | 5.4 ± 0.3 | 76.7 ± 1.3 |
| ANOVA crystallinity index | |||
| Effect | Estimate | p-value | |
| Intercept | 4.9 | ||
| EtOH | 14.65 | 0.0000 * | |
| Te | −10.08 | 0.0001 * | |
| EtOH2 | 7.62 | 0.0003 * | |
| EtOH × Te | −4.25 | 0.002 * | |
| Te2 | 4.34 | 0.0017 * | |
| Lack-of-fit | 0.0927 | ||
| R2 (%) | 99.5 | ||
| R2 adjusted for degrees of freedom (%) | 99 | ||
| ANOVA encapsulation efficiency | |||
| Effect | Estimate | p-value | |
| Intercept | 78.85 | ||
| EtOH | −15.52 | 0.0058 * | |
| Te | −12.4 | 0.0109 * | |
| EtOH2 | 0.1426 | ||
| EtOH x Te | 0.0755 | ||
| Te2 | 0.4363 | ||
| Lack-of-fit | 0.1894 | ||
| R2 (%) | 79.1 | ||
| R2 adjusted for degrees of freedom (%) | 74.5 | ||
| EA-InSc | EA-InA | GA-InSc | GA-InA | |
|---|---|---|---|---|
| Drying conditions | ||||
| Ta (°C) | 20 | 20 | 20 | 20 |
| Te (°C) | 114 | 148 | 114 | 148 |
| Ethanol (%) | 36.5 | 6.8 | 36.5 | 6.8 |
| Microparticle characterization | ||||
| Total GA (mg/g) | - | - | 9.6 ± 0.0 a | 9.5 ± 0.1 a |
| Total EA (mg/g) | 9.7 ± 0.1 a | 9.6 ± 0.1 a | - | - |
| Yield | 94.5 ± 0.5 aA | 91.7 ± 0.9 bA | 93.5 ± 0.5 aA | 91.6 ± 0.6 bA |
| CI (%) | 23.5 ± 0.5 aA | 2.1 ± 0.1 bA | 20.0 ± 0.02 aB | 1.7 ± 0.01 bB |
| EE (%) | 82.6 ± 0.7 aB | 83.4 ± 0.1 aB | 98.9 ± 0.02 bA | 99.2 ± 0.04 bA |
| D4,3 (µm) | 3.8 ± 0.4 aA | 3.3 ± 0.3 aA | 3.6 ± 0.2 aA | 3.2 ± 0.4 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcanqui, Y.; Quintriqueo-Cid, A.; Romero-Hasler, P.; Giménez, B.; Soto-Bustamante, E.; Robert, P. Effect of Microparticle Crystallinity and Food Matrix on the Release Profile and Antioxidant Activity of Encapsulated Gallic and Ellagic Acids During Simulated In Vitro Intestinal Digestion. Antioxidants 2025, 14, 1211. https://doi.org/10.3390/antiox14101211
Vilcanqui Y, Quintriqueo-Cid A, Romero-Hasler P, Giménez B, Soto-Bustamante E, Robert P. Effect of Microparticle Crystallinity and Food Matrix on the Release Profile and Antioxidant Activity of Encapsulated Gallic and Ellagic Acids During Simulated In Vitro Intestinal Digestion. Antioxidants. 2025; 14(10):1211. https://doi.org/10.3390/antiox14101211
Chicago/Turabian StyleVilcanqui, Yesica, Alejandra Quintriqueo-Cid, Patricio Romero-Hasler, Begoña Giménez, Eduardo Soto-Bustamante, and Paz Robert. 2025. "Effect of Microparticle Crystallinity and Food Matrix on the Release Profile and Antioxidant Activity of Encapsulated Gallic and Ellagic Acids During Simulated In Vitro Intestinal Digestion" Antioxidants 14, no. 10: 1211. https://doi.org/10.3390/antiox14101211
APA StyleVilcanqui, Y., Quintriqueo-Cid, A., Romero-Hasler, P., Giménez, B., Soto-Bustamante, E., & Robert, P. (2025). Effect of Microparticle Crystallinity and Food Matrix on the Release Profile and Antioxidant Activity of Encapsulated Gallic and Ellagic Acids During Simulated In Vitro Intestinal Digestion. Antioxidants, 14(10), 1211. https://doi.org/10.3390/antiox14101211







