Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. The Plant Materials, Microorganisms, and Culture Conditions
2.2. Physico–Chemical and Biochemical Characterization
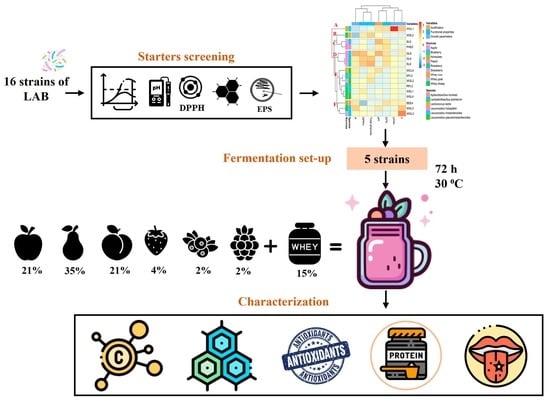
2.3. Starters Screening
2.4. Fermentation of Whey Milk-Based Fruit Smoothies (WFS)
2.5. Determination of Ascorbic Acid
2.6. Identification and Quantification of Free Phenolic Compounds
2.7. Identification and Quantification of Anthocyanins
2.8. Antioxidant In Vitro Assays
2.9. Amino Acids Profile
2.10. In Vitro Protein Digestibility (IVPD) and PDCAAS
2.11. Color, Viscosity, and Sensory Analysis
2.12. Statistical Analysis
3. Results
3.1. Physico–Chemical Characterization of Raw Ingredients
3.2. Starters Screening
3.3. Fermentation of Whey Milk-Based Fruit Smoothies
3.4. Analysis of Sugars, Organic Acids, and Vitamin C
3.5. Free Phenolic Compounds
3.6. Antioxidant Activity
3.7. Amino Acids Profile, Protein Digestibility, and PDCAAS
3.8. Color, Texture, and Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostertag, F.; Schmidt, C.M.; Berensmeier, S.; Hinrichs, J. Development and validation of an RP-HPLC DAD method for the simultaneous quantification of minor and major whey proteins. Food Chem. 2021, 342, 128176. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Minervini, G.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 2011, 28, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Leahu, A.; Ghinea, C.; Ropciuc, S.; Oroian, M.A.; Damian, C. Polyphenol-rich smoothies: Sensory and chemical characterization. Stud. Univ. Vasile Goldis Arad. Ser. Stiintele Vietii 2019, 29, 37–45. [Google Scholar]
- Stübler, A.S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of pilot-scale processing (thermal, PEF, HPP) on the stability and bioaccessibility of polyphenols and proteins in mixed protein-and polyphenol-rich juice systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res. Int. 2015, 76, 761–768. [Google Scholar] [CrossRef]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Preheated milk proteins improve the stability of grape skin anthocyanins extracts. Food Chem. 2016, 210, 221–227. [Google Scholar] [CrossRef]
- He, W.; Mu, H.; Liu, Z.; Lu, M.; Hang, F.; Chen, J.; He, Z. Effect of preheat treatment of milk proteins on their interactions with cyanidin-3-O-glucoside. Food Res. Int. 2018, 107, 394–405. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Dairy-based functional beverages. Milk-Based Beverages 2019, 9, 67–93. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people. J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Smith, B. Effects of high hydrostatic pressure on the quality and functionality of protein isolates, concentrates, and hydrolysates derived from pulse legumes: A review. Trends Food Sci. Technol. 2021, 107, 466–479. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Lievore, P.; Simões, D.R.; Silva, K.M.; Drunkler, N.L.; Barana, A.C.; Nogueira, A.; Demiate, I.M. Chemical characterisation and application of acid whey in fermented milk. J. Food Sci. Technol. 2015, 52, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Skryplonek, K.; Jasińska, M. Fermented probiotic beverages based on acid whey. Acta Sci. Pol. Technol. Aliment. 2015, 14, 397–405. [Google Scholar] [CrossRef]
- Flinois, J.C.; Dando, R.; Padilla-Zakour, O.I. Effects of replacing buttermilk with yogurt acid whey in ranch dressing. J. Dairy Sci. 2019, 102, 7874–7883. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Da Ros, A.; Filannino, P.; Vincentini, O.; Gobbetti, M.; Di Cagno, R. Biotechnological re-cycling of apple by-products: A reservoir model to produce a dietary supplement fortified with biogenic phenolic compounds. Food Chem. 2021, 336, 127616. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Kanwal, S.; Filannino, P.; Albiac, M.A.; Gobbetti, M.; Di Cagno, R. Effect of sequential or ternary starters-assisted fermentation on the phenolic and glucosinolate profiles of sauerkraut in comparison with spontaneous fermentation. Food Res. Int. 2022, 156, 111116. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Chandra-Hioe, M.V.; Wong, C.H.; Arcot, J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Nutritional and functional effects of the lactic acid bacteria fermentation on gelatinized legume flours. Int. J. Food Microbiol. 2020, 316, 108426. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Polo, A.; Filannino, P.; Cantatore, V.; Gobbetti, M.; Di Cagno, R. Biofilm formation as an extra gear for Apilactobacillus kunkeei to counter the threat of agrochemicals in honeybee crop. Microb. Biotechnol. 2022, 15, 2160–2175. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, O.; Lemos Junior, W.J.; Borruso, L.; Campanaro, S.; De Angelis, M.; Vogel, R.F.; Gobbetti, M. How multiple farming conditions correlate with the composition of the raw cow’s milk lactic microbiome. Environ. Microbiol. 2021, 23, 1702–1716. [Google Scholar] [CrossRef]
- Filannino, P.; Tlais, A.Z.; Morozova, K.; Cavoski, I.; Scampicchio, M.; Gobbetti, M.; Di Cagno, R. Lactic acid fermentation enriches the profile of biogenic fatty acid derivatives of avocado fruit (Persea americana Mill.). Food Chem. 2020, 317, 126384. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K.J.A.E.M. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Yu, L. Free radical scavenging properties of conjugated linoleic acids. J. Agric. Food Chem. 2001, 49, 3452–3456. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Di Cagno, R. Lactic acid fermentation of cactus cladodes (Opuntia ficus-indica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Mangiafico, S.S.; Bell, K.; Hetzell, N. Fecal Coliform Concentrations in the Upper Cohansey River Watershed Predicted by Air Temperature, Discharge, and Land Use. J. Ext. 2016, 54, 14. [Google Scholar]
- Bonilla-Aldana, D.K.; Dhama, K.; Rodriguez-Morales, A.J. Revisiting the one health approach in the context of COVID-19: A look into the ecology of this emerging disease. Adv. Anim. Vet. Sci. 2020, 8, 234–237. [Google Scholar] [CrossRef]
- Lee, S.H.; Moore, L.V.; Park, S.; Harris, D.M.; Blanck, H.M. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of cheese whey bioactive proteins and peptides in the development of antimicrobial edible film composite: A review of recent trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jood, S.; Khetarpaul, N. Effect of germination and probiotic fermentation on nutrient profile of pearl millet based food blends. Br. Food J. 2011, 113, 470–481. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Kumar, A.; Sharma, N. Reduction in phytic acid content and enhancement of antioxidant properties of nutricereals by processing for developing a fermented baby food. J. Food Sci. Technol. 2015, 52, 3219–3234. [Google Scholar] [CrossRef]
- Labuschagne, I.L.; Van Niekerk, E.; Lombard, M.J. Acidified infant formula explained. S. Afr. Fam. Pract. 2013, 55, 354–356. [Google Scholar] [CrossRef]
- Verón, H.E.; Di Risio, H.D.; Isla, M.I.; Torres, S. Isolation and selection of potential probiotic lactic acid bacteria from Opuntia ficus-indica fruits that grow in Northwest Argentina. LWT 2017, 84, 231–240. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, J.; Wang, M.; Lu, M.; Chen, G.; Gao, F.; Yi, M. Effects of Lactococcus lactis subsp. lactis JCM5805 on colonization dynamics of gut microbiota and regulation of immunity in early ontogenetic stages of tilapia. Fish Shellfish. Immunol. 2019, 86, 53–63. [Google Scholar] [CrossRef]
- van Hijum, S.A.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Zaunmüller, T.; Eichert, M.; Richter, H.; Unden, G. Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Appl. Microbiol. Biotechnol. 2006, 72, 421–429. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Zhang, Y.; Chen, W. Changes of quality in the fruits of Prunus mume during deacidification by fermentation with Lactobacillus fermentium. J. Food Sci. 2015, 80, M405–M410. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Peñas, E.; Frías, J.; Ciska, E.; Honke, J.; Piskula, M.K.; Vidal-Valverde, C. Influence of fermentation conditions on glucosinolates, ascorbigen, and ascorbic acid content in white cabbage (Brassica oleracea var. capitata cv. Taler) cultivated in different seasons. J. Food Sci. 2009, 74, C62–C67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Xiao, X. Fermentation affects the antioxidant activity of Plant-Based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Curiel, J.A.; Pinto, D.; Marzani, B.; Filannino, P.; Farris, G.A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb. Cell Factories 2015, 14, 67. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Concomitant ingestion of lactic acid bacteria and black tea synergistically enhances flavonoid bioavailability and attenuates D-galactose-induced oxidative stress in mice via modulating glutathione antioxidant system. J. Nutr. Biochem. 2016, 38, 116–124. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Filannino, P.; Azzi, L.; Cavoski, I.; Vincentini, O.; Rizzello, C.G.; Gobbetti, M.; Di Cagno, R. Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juice through lactic acid fermentation. Int. J. Food Microbiol. 2013, 163, 184–192. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Lemos Junior, W.J.F.; Filannino, P.; Campanaro, S.; Gobbetti, M.; Di Cagno, R. How microbiome composition correlates with biochemical changes during sauerkraut fermentation: A focus on neglected bacterial players and functionalities. Microbiol. Spectr. 2022, 10, e00168-22. [Google Scholar] [CrossRef]
- Hollands, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennett, R.N.; Kroon, P.A. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Maya, I.J.; Campos-Terán, J.; Hernández-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Budziak-Wieczorek, I.; Maciołek, U. Synthesis and Characterization of a (−)-Epicatechin and Barbituric Acid Cocrystal: Single-Crystal X-ray Diffraction and Vibrational Spectroscopic Studies. ACS Omega 2021, 6, 8199–8209. [Google Scholar] [CrossRef]
- Daliri, H.; Ahmadi, R.; Pezeshki, A.; Hamishehkar, H.; Mohammadi, M.; Beyrami, H.; Ghorbani, M. Quinoa bioactive protein hydrolysate produced by pancreatin enzyme-functional and antioxidant properties. LWT 2021, 150, 111853. [Google Scholar] [CrossRef]
- Guo, M. Whey Protein Production, Chemistry, Functionality, and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 251–260. ISBN 9781119256038. [Google Scholar]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Comp. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Alba, K.; Offiah, V.; Laws, A.P.; Falade, K.O.; Kontogiorgos, V. Baobab polysaccharides from fruits and leaves. Food Hydrocoll. 2020, 106, 105874. [Google Scholar] [CrossRef]
- Almeida, C.C.; Monteiro, M.L.G.; da Costa-Lima, B.R.C.; Alvares, T.S.; Conte-Junior, C.A. In vitro digestibility of commercial whey protein supplements. LWT 2015, 61, 7–11. [Google Scholar] [CrossRef]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Bertrand-Harb, C.; Ivanova, I.V.; Dalgalarrondo, M.; Haertllé, T. Evolution of β-lactoglobulin and α-lactalbumin content during yoghurt fermentation. Int. Dairy J. 2003, 13, 39–45. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H.; Park, B.J.; Kim, S.H.; Oh, D.H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef]
- Faruque, S.; Tong, J.; Lacmanovic, V.; Agbonghae, C.; Minaya, D.M.; Czaja, K. The dose makes the poison: Sugar and obesity in the United States—A review. Polish J. Food Nutr. Sci. 2019, 69, 219. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Weiland, S.; Hickmann, T.; Lederer, M.; Marquardt, J.; Schwindenhammer, S. The 2030 agenda for sustainable development: Transformative change through the sustainable development goals? Politics Gov. 2021, 9, 90–95. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT 2008, 41, 1876–1883. [Google Scholar] [CrossRef]



| Fruits | pH | TTA (mL of 0.1 M NaOH) | Sugars (mg g−1 DW) | |||
|---|---|---|---|---|---|---|
| pH | TTA | Fructose | Glucose | Mannitol | Sucrose | |
| Apple | 4.07 ± 0.1 | 4.95 ± 0.2 | 604.69 ± 4.1 | 179.64 ± 29.2 | 11.89 ± 1.3 | 6.03 ± 0.9 |
| Strawberry | 3.41 ± 0.0 | 13.9 ± 0.3 | 221.88 ± 28.5 | 301.88 ± 13.6 | 0.00 | 0.00 |
| Pear | 5.09 ± 0.0 | 4.2 ± 0.1 | 457.59 ± 37.2 | 88.07 ± 16.2 | 149.38 ± 11.6 | 2.97 ± 0.9 |
| Peach | 3.79 ± 0.0 | 5.65 ± 0.1 | 222.10 ± 5.0 | 312.37 ± 13.6 | 0.00 | 20.67 ± 2.1 |
| Raspberry | 2.97 ± 0.0 | 43.15 ± 0.4 | 159.63 ± 6.8 | 175.78 ± 2.5 | 0.00 | 0.00 |
| Blueberry | 3.08 ± 0.0 | 18.55 ± 0.2 | 177.58 ± 22.8 | 191.00 ± 23.0 | 0.00 | 0.00 |
| Samples | Fructose | Glucose | Mannitol | Sucrose | Lactose | Lactic Acid | Acetic Acid | Ascorbic Acid |
|---|---|---|---|---|---|---|---|---|
| Raw_WFS | 403.39 ± 8.76 a | 188.39 ± 5.62 a | 51.72 ± 13.07 b | 10.00 ± 1.69 a | 65.52 ± 3.07 | 4.10 ± 0.25 d | 0.21 ± 0.05 c | 1.30 ± 0.06 a |
| Unstarted_ WFS | 375.68 ± 8.23 ab | 174.78 ± 8.81 ab | 79.20 ± 1.41 b | 0 ± 0 b | 58.58 ± 1.99 | 11.82 ± 0.61 cd | 2.31 ± 0.20 b | 1.06 ± 0.07 b |
| SL8_WFS | 394.00 ± 4.24 a | 191.84 ± 2.64 a | 74.38 ± 0.99 b | 0 ± 0 b | 60.92 ± 1.49 | 46.65 ± 0.04 b | 0.78 ± 0.07 c | 1.39 ± 0.21 a |
| BpL2_WFS | 349.29 ± 7.69 bc | 175.93 ± 5.01 ab | 62.91 ± 1.31 b | 0 ± 0 b | 69.37 ± 1.67 | 75.34 ± 2.84 a | 0.39 ± 0.05 c | 1.36 ± 0.07 a |
| PHE5_WFS | 326.56 ± 7.96 c | 165.12 ± 3.23 ab | 148.00 ± 2.45 a | 0 ± 0 b | 66.91 ± 3.10 | 20.39 ± 2.29 c | 3.06 ± 0.43 ab | 1.28 ± 0.02 a |
| WSL2_WFS | 320.13 ± 4.9 c | 149.86 ± 0.59 b | 128.22 ± 1.87 a | 0 ± 0 b | 66.01 ± 0.37 | 24.65 ± 2.25 d | 0.81± 0.10 c | 1.20 ± 0.05 a |
| BEE4_WFS | 329.85 ± 9.89 c | 152.46 ± 3.65 b | 151.42 ± 3.62 a | 0 ± 0 b | 60.76 ± 0.69 | 40.99 ± 1.76 b | 3.62 ± 0.22 a | 1.21 ± 0.03 a |
| Compounds | Raw_WFS | Unstarted_WFS | SL8_WFS | BpL2_WFS | PHE5_WFS | WSL2_WFS | BEE4_WFS |
|---|---|---|---|---|---|---|---|
| Gallic acid | 0.31 ± 0.00 bc | 0.35 ± 0.01 b | 0.31 ± 0.01 c | 0.24 ± 0.00 d | 0.27 ± 0.01 cd | 0.45 ± 0.01 a | 0.43 ± 0.01 a |
| 3- hydroxybenzoic acid | 13.31 ± 1.31 ab | 19.54 ± 3.97 ab | 8.64 ± 0.68 b | 12.56 ± 2.50 ab | 23.72 ± 2.84 a | 15.87 ± 0.50 ab | 18.83 ± 0.53 ab |
| Chlorogenic acid | 62.94 ± 0.06 ab | 69.36 ± 6.02 a | 44.97 ± 0.69 b | 63.38 ± 1.51 ab | 66.00 ± 6.10 ab | 68.94 ± 3.98 ab | 72.06 ± 6.02 a |
| p-coumaric acid | 9.38 ± 0.20 | 9.82 ± 0.26 | 9.24 ± 0.02 | 9.21 ± 0.17 | 9.44 ± 0.07 | 9.73 ± 0.09 | 9.7 ± 0.26 |
| Hydrocaffeic acid | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0.99 ± 0.45 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Phloridzin | 11.23 ± 3.24 | 10.24 ± 4.00 | 11.10 ± 2.10 | 12.52 ± 2.77 | 10.61 ± 2.05 | 9.65 ± 3.92 | 11.97 ± 3.26 |
| Isorhamnetin | 5.59 ± 1.16 | 5.98 ± 0.11 | 4.62 ± 0.03 | 6.25 ± 0.42 | 4.49 ± 0.02 | 6.29 ± 0.46 | 6.25 ± 0.20 |
| Naringenin | 1.46 ± 0.45 | 1.73 ± 0.11 | 1.18 ± 0.03 | 1.82 ± 0.13 | 1.47 ± 0.06 | 1.74 ± 0.02 | 1.84 ± 0.01 |
| Phloretin | 6.14 ± 0.04 | 6.06 ± 0.01 | 6.03 ± 0.00 | 6.15 ± 0.04 | 6.05 ± 0.01 | 6.09 ± 0.02 | 6.07 ± 0.01 |
| Quercetin | 0.92 ± 0.22 ab | 0.23 ± 0.03 bc | 0.80 ± 0.09 ab | 1.059 ± 0.14 a | 0 ± 0 b | 0.96 ± 0.14 a | 0.94 ± 0.08 ab |
| Isoquercetin | 3.59 ± 0.13 | 2.55 ± 0.92 | 3.83 ± 0.17 | 4.23 ± 0.34 | 3.31 ± 0.63 | 3.03 ± 0.94 | 2.52 ± 0.17 |
| Epicatechin | 25.04 ± 0.49 b | 24.19 ± 1.00 b | 11.62 ± 0.94 c | 6.70 ± 0.76 d | 33.51 ± 0.47 a | 26.77 ± 0.24 b | 25.41 ± 0.64 b |
| Procyanidin B2 | 25.45 ± 0.51 ab | 23.96 ± 0.20 ab | 19.85 ± 0.23 b | 26.35 ± 3.70 ab | 31.72 ± 0.03 a | 31.54 ± 3.95 a | 25.62 ± 0.39 ab |
| Ellagic acid | 8.70 ± 0.78 ab | 7.91 ± 0.93 ab | 3.32 ± 0.04 b | 7.74 ± 1.38 ab | 5.44 ± 0.10 ab | 9.79 ± 1.06 a | 8.69 ± 1.47 ab |
| Vanillin | 6.13 ± 0.12 | 6.22 ± 0.01 | 6.06 ± 0.00 | 6.20 ± 0.09 | 6.21 ± 0.08 | 6.13 ± 0.02 | 6.17 ± 0.04 |
| Amino Acids | FAO * (mg/g Protein) | Raw_WFS | Unstarted_WFS | SL8_WFS | BpL2_WFS | PHE5_WFS | WSL2_WFS | BEE4_WFS |
|---|---|---|---|---|---|---|---|---|
| Thr | 34 | 76.5 | 69.6 | 67.7 | 71.9 | 70.6 | 54.8 | 74.8 |
| Val | 35 | 23.8 | 19.7 | 17.6 | 21.4 | 16.5 | 16.3 | 18 |
| Cys + Met | 25 | 137.7 | 150.9 | 130.8 | 160.7 | 114.6 | 100.8 | 117.7 |
| Ile | 28 | 12.9 | 13.9 | 15.8 | 16.8 | 9.8 | 8.7 | 9.9 |
| Leu | 66 | 47.4 | 38.2 | 44.1 | 55.5 | 41.6 | 37 | 45.3 |
| Tyr + Phe | 63 | 77.6 | 56.8 | 60.2 | 85.2 | 65.6 | 78.2 | 77.6 |
| Lys | 58 | 52.2 | 44.4 | 39.2 | 37.9 | 44.7 | 49.2 | 56 |
| His | 19 | 24.8 | 20.6 | 17.7 | 24.8 | 19.5 | 18.4 | 20.2 |
| Trp | 11 | 79.8 | 70 | 70.9 | 77.1 | 70.7 | 69 | 69.8 |
| Protein (g/100 g DW) | 0.62 ± 0.03 | 0.7 ± 0.02 | 0.71 ± 0.05 | 0.68 ± 0.03 | 0.67 ± 0.01 | 0.73 ± 0.01 | 0.75 ± 0.1 | |
| Protein digestibility (%) | 82.6 ± 0 c | 82.85 ± 0.02 c | 84.22 ± 0.01 b | 87.35 ± 0 a | 81.75 ± 0.04 c | 79.5 ± 0.03 d | 79.85 ± 0.02 d | |
| First limiting Amino acid | Ile | Ile | Val | Ile | Ile | Ile | Ile | |
| Amino acids score | 0.46 | 0.5 | 0.5 | 0.6 | 0.35 | 0.31 | 0.35 | |
| PDCAAS | 0.38 | 0.41 | 0.42 | 0.52 | 0.29 | 0.25 | 0.28 |
| Samples | Color indices | Texture | Sensory Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Viscosity | Appearance | Texture | Aroma | Flavor | Acidity | Sweetness | Astringency | |
| Raw_WFS | 31.99 ± 0.55 c | 21.79 ± 0.03 b | 12.63 ± 0.23 d | 94.31 ± 3.62 | 7.2 ± 0.74 | 6.8 ± 0.97 | 7.2 ± 1.46 a | 7.2 ± 1.46 a | 4.2 ± 2.31 ab | 5.4 ± 1.85 | 3.4 ± 2.93 |
| Unstarted_WFS | 39.63 ± 0.63 a | 16.35 ± 0.34 d | 22.04 ± 0.54 a | 80.94 ± 6.5 | 5.2 ± 1.93 | 6.4 ± 1.35 | 6.2 ± 0.74 ab | 5.8 ± 0.97 ab | 4.8 ± 1.46 ab | 5.2 ± 1.16 | 3.2 ± 2.71 |
| SL8_WFS | 35.65 ± 0.35 b | 19.5 ± 0.50 c | 17.38 ± 0.63 c | 75.95 ± 6.01 | 6.4 ± 1.02 | 6.4 ± 1.01 | 7.2 ± 1.32 a | 6.4 ± 1.01 ab | 5.8 ± 2.13 ab | 5.4 ± 1.01 | 4.0 ± 3.34 |
| BpL2_WFS | 32.19 ± 0.19 c | 24.25 ± 0.75 a | 14.4 ± 0.60 d | 93.25 ± 1.42 | 7.2 ± 0.74 | 6.2 ± 1.60 | 6.8 ± 1.46 a | 5.0 ± 1.67 ab | 7.4 ± 0.48 a | 5.4 ± 1.01 | 6.0 ± 3.16 |
| PHE5_WFS | 37.36 ± 0.83 ab | 14.83 ± 0.18 d | 18.12 ± 0.38 bc | 73.85 ± 7.61 | 5.2 ± 2.40 | 6.0 ± 1.41 | 5.2 ± 1.16 ab | 5.2 ± 2.31 ab | 3.0 ± 2.19 b | 5.0 ± 2.09 | 2.8 ± 2.48 |
| WSL2_WFS | 39.20 ± 0.60 a | 15.96 ± 0.04 d | 21.14 ± 0.41 a | 80.98 ± 6.47 | 4.6 ± 1.85 | 5.8 ± 1.16 | 3.4 ± 1.62 b | 3.4 ± 1.35 b | 3.4 ± 2.41 ab | 4.6 ± 0.80 | 4.2 ± 2.40 |
| BEE4_WFS | 38.65 ± 0.25 a | 18.55 ± 0.15 c | 20.36 ± 0.36 ab | 70.51 ± 3.40 | 5.8 ± 0.97 | 6.4 ± 0.80 | 5.6 ± 1.01 ab | 6.0 ± 0.89 ab | 5.0 ± 1.26 ab | 5.6 ± 1.01 | 4.8 ± 2.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlais, A.Z.A.; Trossolo, E.; Tonini, S.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity. Antioxidants 2023, 12, 1091. https://doi.org/10.3390/antiox12051091
Tlais AZA, Trossolo E, Tonini S, Filannino P, Gobbetti M, Di Cagno R. Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity. Antioxidants. 2023; 12(5):1091. https://doi.org/10.3390/antiox12051091
Chicago/Turabian StyleTlais, Ali Zein Alabiden, Elisabetta Trossolo, Stefano Tonini, Pasquale Filannino, Marco Gobbetti, and Raffaella Di Cagno. 2023. "Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity" Antioxidants 12, no. 5: 1091. https://doi.org/10.3390/antiox12051091
APA StyleTlais, A. Z. A., Trossolo, E., Tonini, S., Filannino, P., Gobbetti, M., & Di Cagno, R. (2023). Fermented Whey Ewe’s Milk-Based Fruit Smoothies: Bio-Recycling and Enrichment of Phenolic Compounds and Improvement of Protein Digestibility and Antioxidant Activity. Antioxidants, 12(5), 1091. https://doi.org/10.3390/antiox12051091