Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Supercritical Fluid Extraction
2.3. Response Surface Methodology
2.4. Chemicals and Reagents
2.5. Quantification of Essential Oils and Waxes
2.6. Phytochemical Screening of Secondary Metabolites by 96-Well Plate Spectrophotometric Assays
2.7. UHPLC-HRMS Analysis
2.8. GC-MS Analysis
2.9. Cytotoxicity
2.10. Phototoxicity
2.11. Antimicrobial Activity
2.12. Proliferation and Viability of Melanocytes
2.13. Quantification of Melanin Content
3. Results
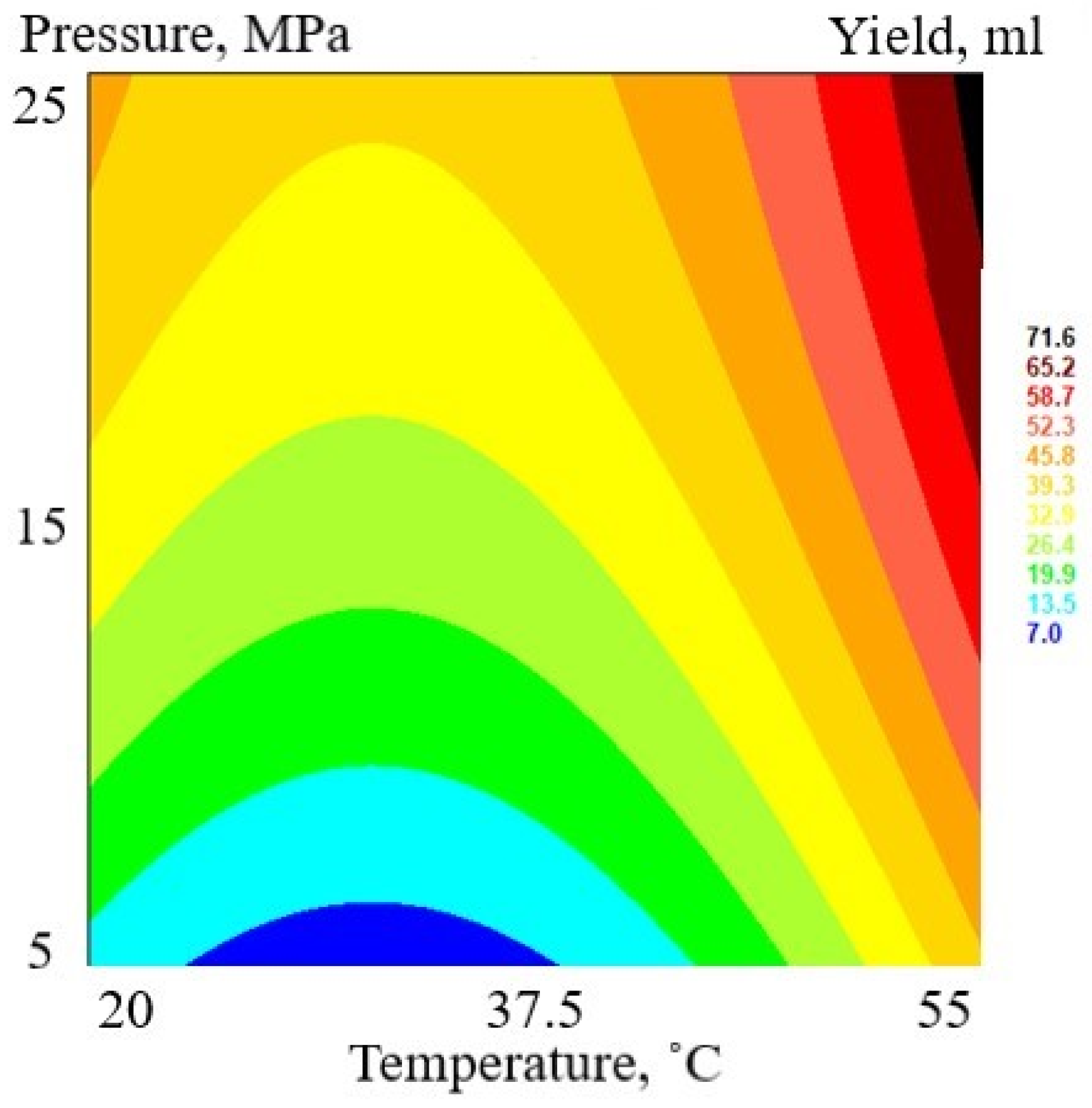
3.1. Supercritical Fluid Extraction
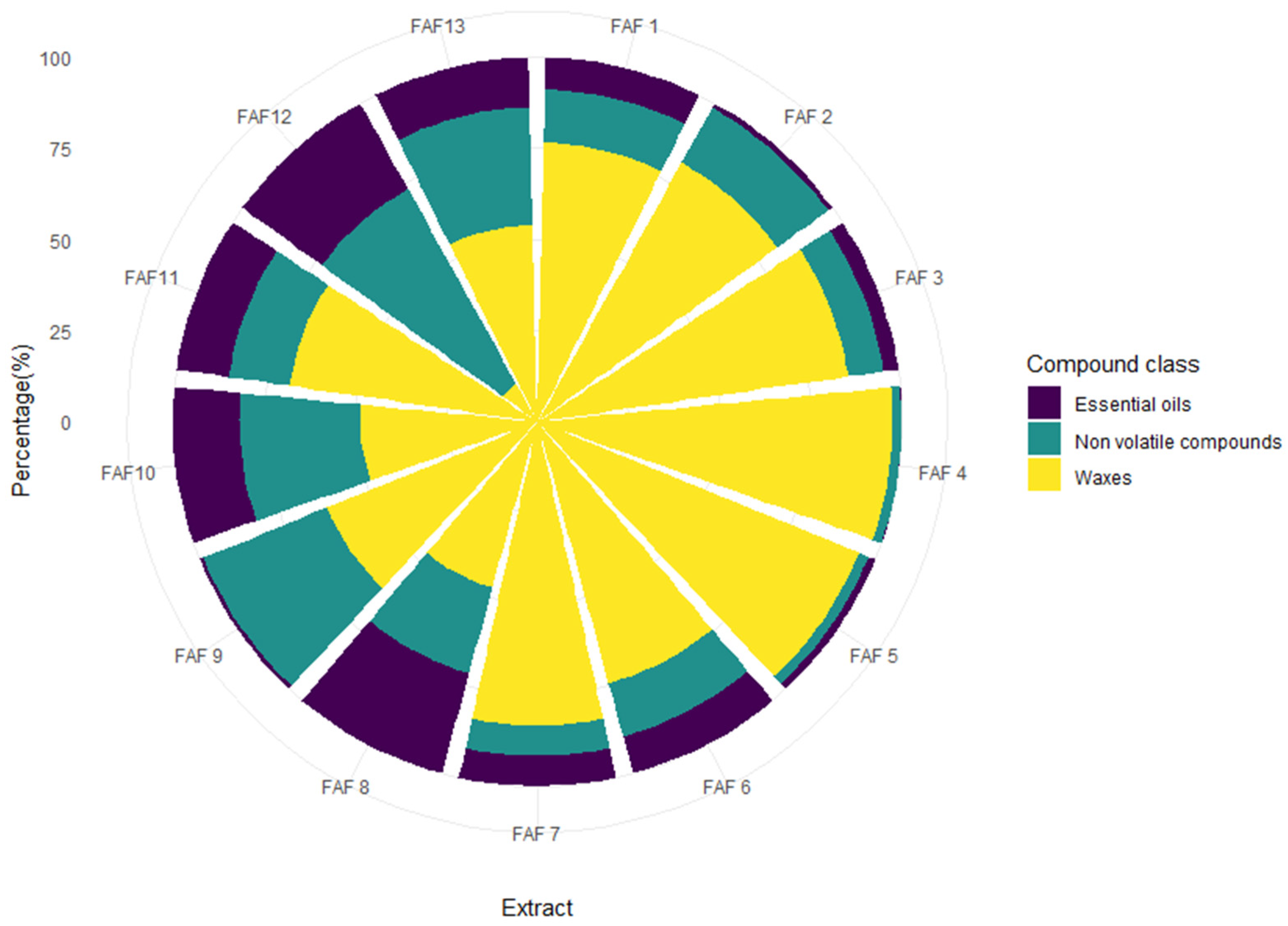
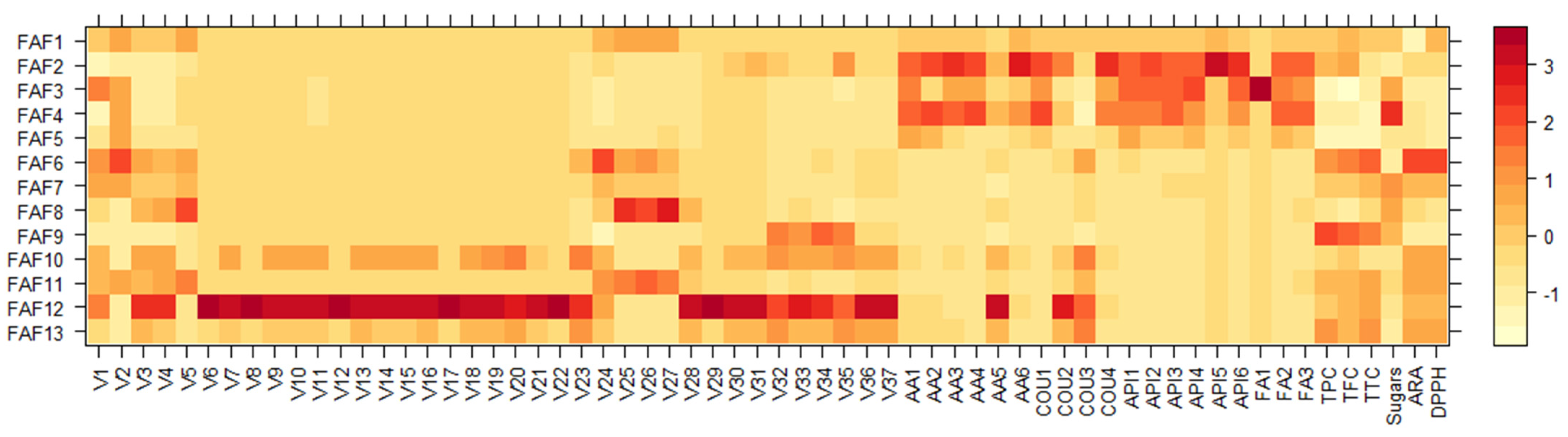
3.2. Phytochemical Screening of Matricaria chamomilla White Ray Floret Supercritical Fluid Extracts
3.3. Quantitative Analysis of the Volatile Components in Matricaria chamomilla White Ray Floret Supercritical Fluid Extracts
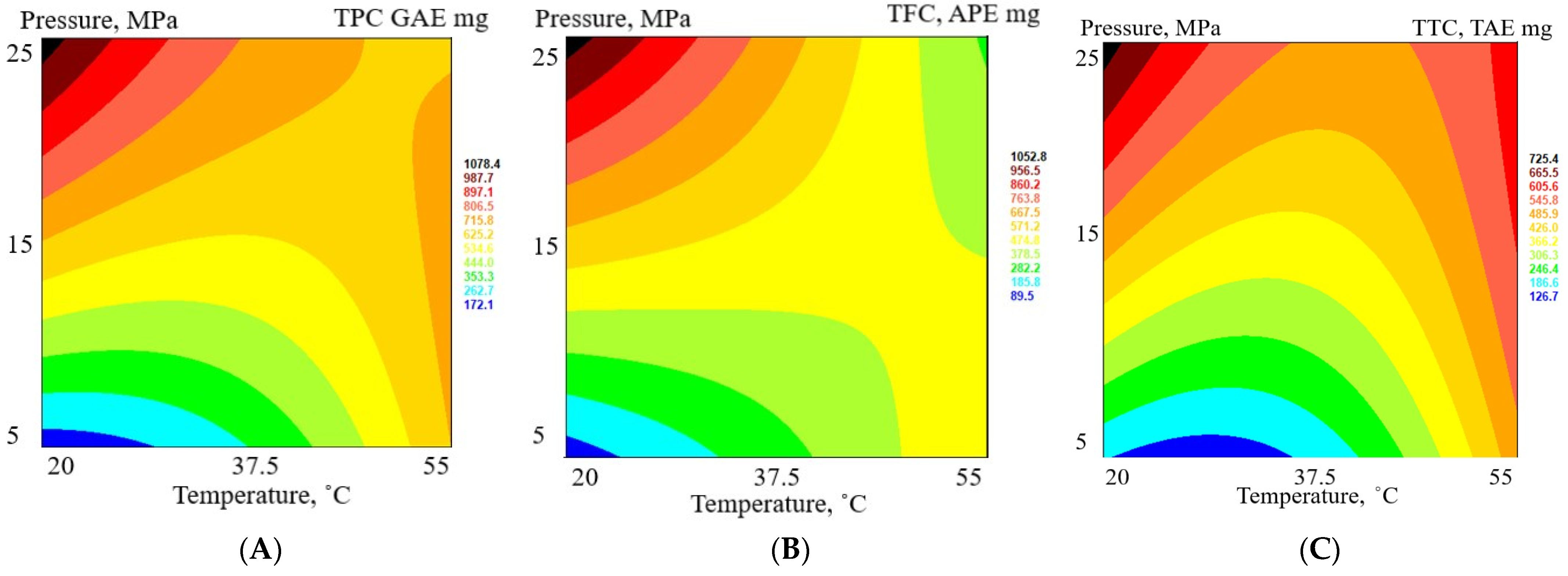
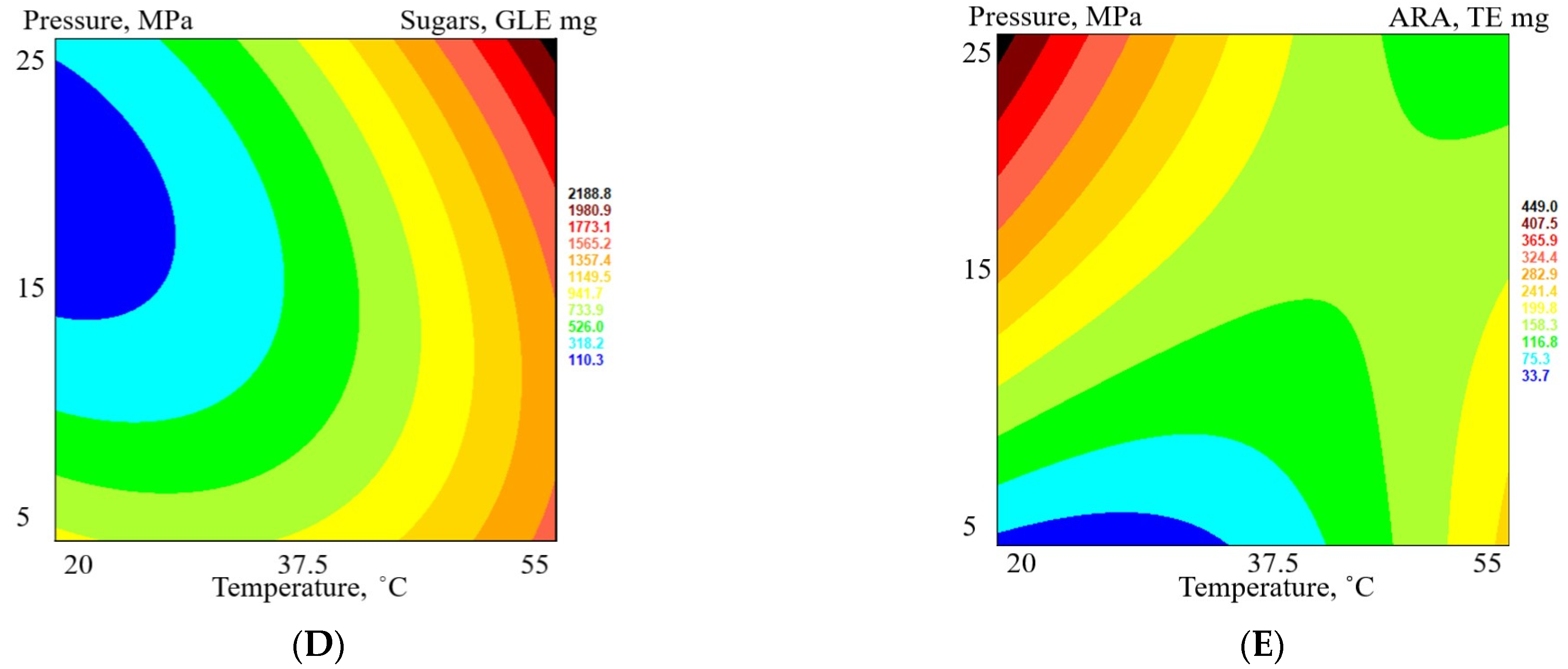
3.4. Quantitative Analysis of the Nonvolatile Components in Matricaria chamomilla White Ray Florets Supercritical Fluid Extracts
3.5. Antimicrobial Activity
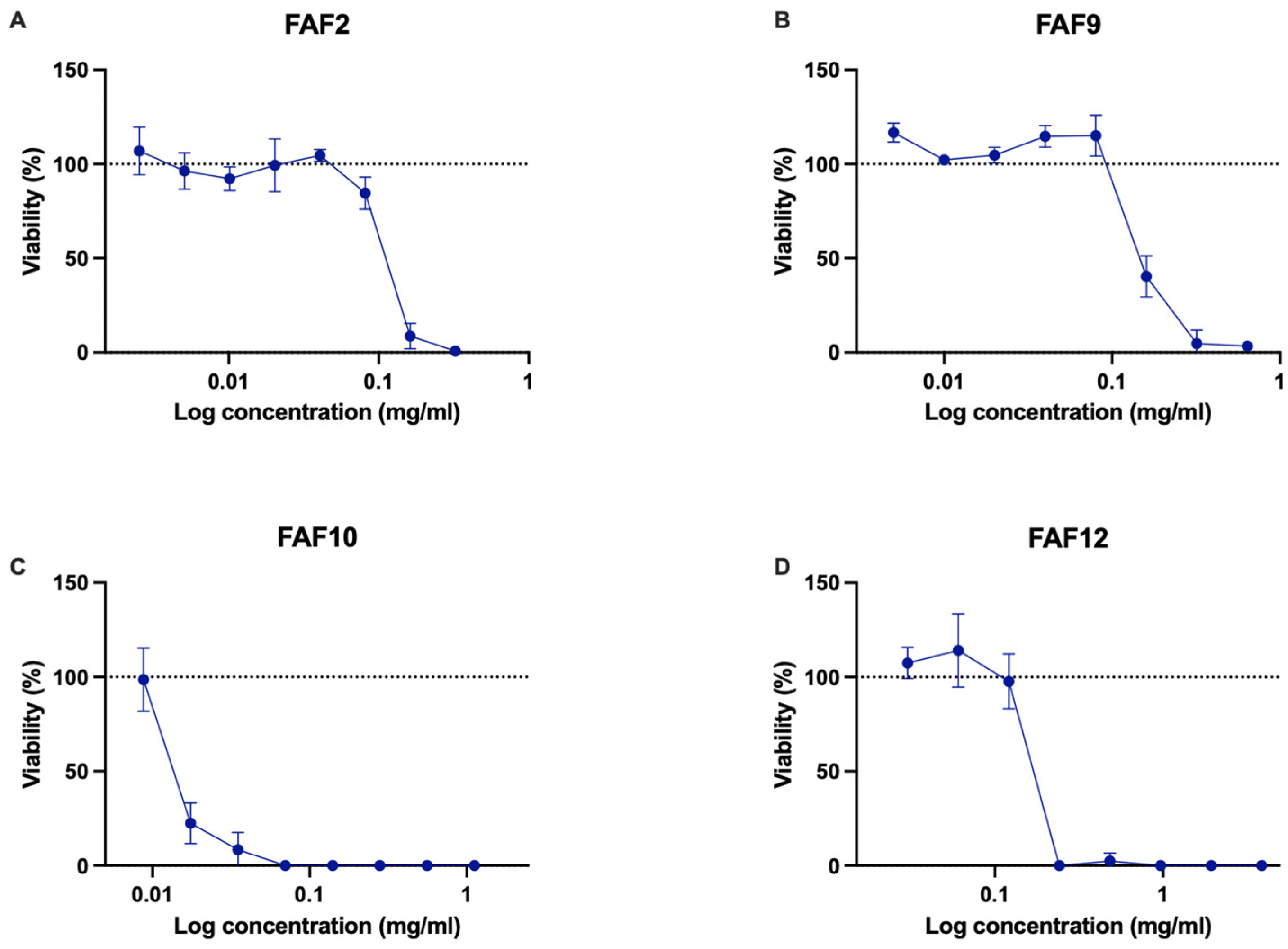
3.6. Cytotoxicity and Phototoxicity
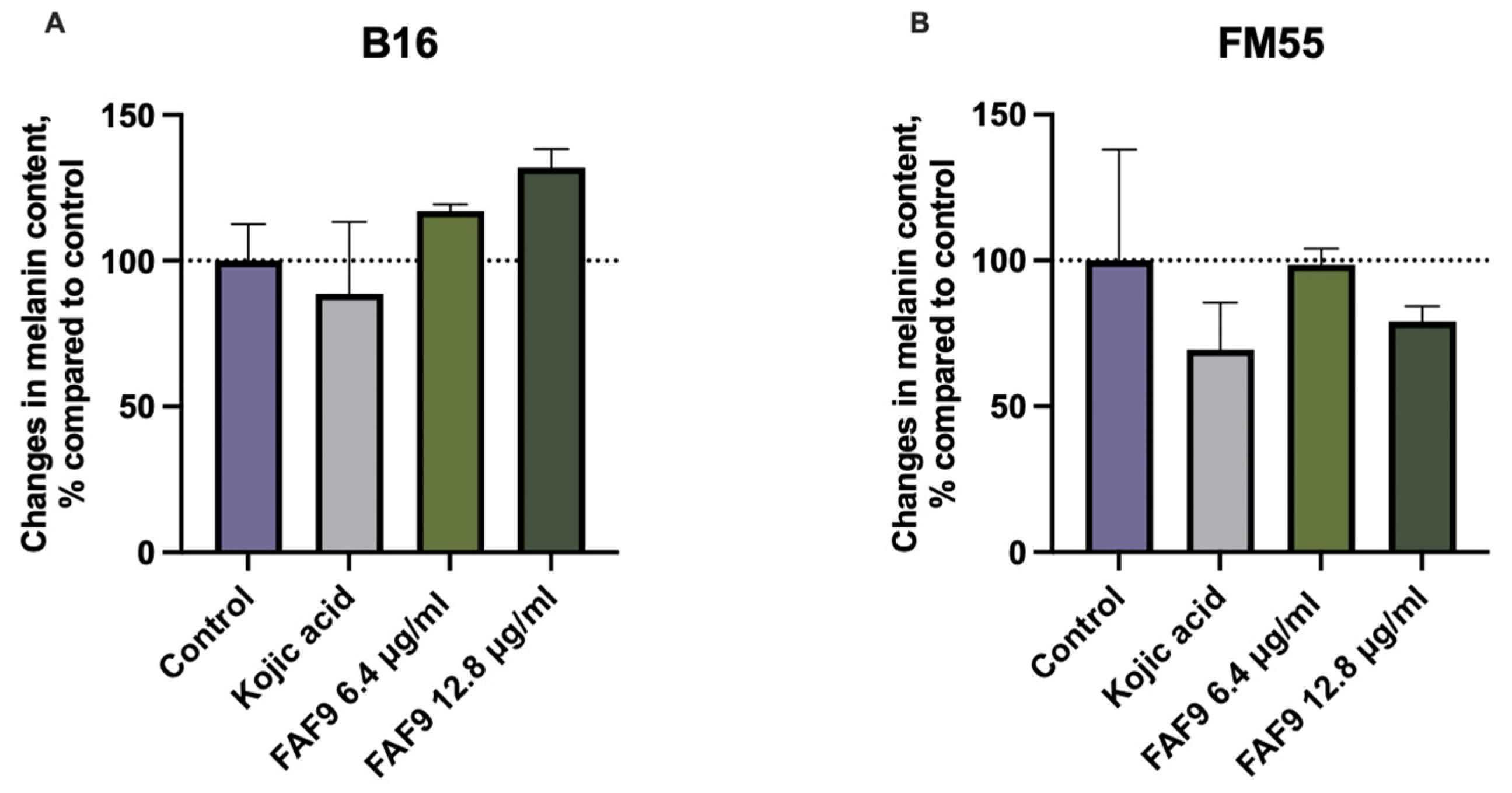
3.7. Effect on Melanocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M. Chamomile (Matricaria Chamomilla L.): An Overview. Phcog. Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A Herbal Medicine of the Past with a Bright Future (Review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and Cancer Chemoprevention: Progress, Potential and Promise (Review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Antiproliferative and Apoptotic Effects of Chamomile Extract in Various Human Cancer Cells. J. Agric. Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef]
- Merfort, I.; Heilmann, J.; Hagedorn-Leweke, U.; Lippold, B.C. In Vivo Skin Penetration Studies of Camomile Flavones. Pharmazie 1994, 49, 509–511. [Google Scholar]
- Lee, S.-H.; Heo, Y.; Kim, Y.-C. Effect of German Chamomile Oil Application on Alleviating Atopic Dermatitis-like Immune Alterations in Mice. J. Vet. Sci. 2010, 11, 35. [Google Scholar] [CrossRef]
- Li, G.; Wu, H.; Sun, L.; Cheng, K.; Lv, Z.; Chen, K.; Qian, F.; Li, Y. (-)-α-Bisabolol Alleviates Atopic Dermatitis by Inhibiting MAPK and NF-ΚB Signaling in Mast Cell. Molecules 2022, 27, 3985. [Google Scholar] [CrossRef]
- Avonto, C.; Rua, D.; Lasonkar, P.B.; Chittiboyina, A.G.; Khan, I.A. Identification of a Compound Isolated from German Chamomile (Matricaria chamomilla) with Dermal Sensitization Potential. Toxicol. Appl. Pharmacol. 2017, 318, 16–22. [Google Scholar] [CrossRef]
- Paulsen, E.; Otkjaer, A.; Andersen, K.E. The Coumarin Herniarin as a Sensitizer in German Chamomile [Chamomilla Recutita (L.) Rauschert, Compositae]. Contact Dermat. 2010, 62, 338–342. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Zou, J.; Jia, Y.; Wang, Y.; Li, J.; Wang, C.; Sun, J.; Guo, D.; Wang, F.; et al. The Mechanism Action of German Chamomile (Matricaria recutita L.) in the Treatment of Eczema: Based on Dose–Effect Weight Coefficient Network Pharmacology. Front. Pharmacol. 2021, 12, 706836. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and Anticancer Activities of Chamomile (Matricaria Recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare L.) and Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Fırat, Z.; Demirci, F.; Demirci, B. Antioxidant Activity of Chamomile Essential Oil and Main Components. Nat. Volatiles Essent. Oils 2018, 5, 11–16. [Google Scholar]
- Lu, J.; Xie, D.; He, L.; Wang, Y.; Zheng, Z. Isolation, Purification, Properties, Structure and Antioxidant Activity of Chamomile Polysaccharides. Food Ferment. Ind. 2021, 47, 72–78. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Bijak, M.; Saluk, J.; Ponczek, M.B.; Zbikowska, H.M.; Nowak, P.; Tsirigotis-Maniecka, M.; Pawlaczyk, I. Radical Scavenging and Antioxidant Effects of Matricaria chamomilla Polyphenolic–Polysaccharide Conjugates. Int. J. Biol. Macromol. 2015, 72, 1152–1158. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in Dermatology: An Evidence-Based Review. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Aćimović, M.; Sknepnek, A.; Miletić, D.; Mrkonjić, Ž.; Kljakić, A.C.; Jerković, J.; Mišan, A.; Pojić, M.; Stupar, A.; et al. Sustainable Raw Materials for Efficient Valorization and Recovery of Bioactive Compounds. Ind. Crops Prod. 2023, 193, 116167. [Google Scholar] [CrossRef]
- Lahmass, I.; Lamkami, T.; Delporte, C.; Sikdar, S.; Van Antwerpen, P.; Saalaoui, E.; Megalizzi, V. The Waste of Saffron Crop, a Cheap Source of Bioactive Compounds. J. Funct. Foods 2017, 35, 341–351. [Google Scholar] [CrossRef]
- Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules 2022, 27, 1613. [Google Scholar] [CrossRef]
- Slavov, A.; Yantcheva, N.; Vasileva, I. Chamomile Wastes (Matricaria chamomilla): New Source of Polysaccharides. Waste Biomass Valor. 2019, 10, 2583–2594. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Manivel, P.; Kumar, J. Valorization of Java Citronella (Cymbopogon Winterianus Jowitt) Distillation Waste as a Potential Source of Phenolics/Antioxidant: Influence of Extraction Solvents. J. Food Sci. Technol. 2021, 58, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Žutić, I.; Radman, S.; Pleša, M.; Brnčić, M.; Barba, F.J.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M.; Domínguez, R.; et al. Effect of Different Green Extraction Methods and Solvents on Bioactive Components of Chamomile (Matricaria chamomilla L.) Flowers. Molecules 2020, 25, 810. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and Biological Activity of Chamomile Extracts Obtained by Different Techniques: Perspective of Using Superheated Water for Isolation of Biologically Active Compounds. Ind. Crops Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Nakurte, I.; Berga, M.; Pastare, L.; Kienkas, L.; Senkovs, M.; Boroduskis, M.; Ramata-Stunda, A. Valorization of Bioactive Compounds from By-Products of Matricaria Recutita White Ray Florets. Plants 2023, 12, 396. [Google Scholar] [CrossRef]
- OECD. Guidance document using cytotoxicity tests to estimate starting doses for acute oral systemic toxicity tests. In Series on Testing and Assesment; OECD: Paris, France, 2010. [Google Scholar]
- OECD. Test No. 432: In vitro 3T3 NRU phototoxicity test. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD: Paris, France, 2019; ISBN 978-92-64-07116-2. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Babacan, Ü.; Kaba, A.; Akçakale, F.; CengïZ, M.F.; Akinci, E. Optimization of Some Parametric Values of MTT for the Determination of Human Melanoma (SK-Mel-30) Cell Viability. Int. J. Life Sci. Biotechnol. 2021, 5, 9–20. [Google Scholar] [CrossRef]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Melanin Synthesis of B16 Mouse Melanoma Cells by 1 Alpha, 25-Dihydroxyvitamin D3 and Retinoic Acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar] [PubMed]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial Attributes of Apigenin, Isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 175851. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.-R.; Lee, D.G. Apigenin Promotes Antibacterial Activity via Regulation of Nitric Oxide and Superoxide Anion Production. J. Basic Microbiol. 2020, 60, 862–872. [Google Scholar]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago Campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of Various Techniques for the Extraction of Umbelliferone and Herniarin in Matricaria chamomilla Processing Fractions. Chem. Cent. J. 2017, 11, 78. [Google Scholar] [CrossRef]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical Fluid Extraction of Chamomile Flower Heads: Comparison with Conventional Extraction, Kinetics and Scale-Up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Scalia, S.; Giuffreda, L.; Pallado, P. Analytical and Preparative Supercritical Fluid Extraction of Chamomile Flowers and Its Comparison with Conventional Methods. J. Pharm. Biomed. Anal. 1999, 21, 549–558. [Google Scholar] [CrossRef]
- Povh, N.P.; Marques, M.O.M.; Meireles, M.A.A. Supercritical CO2 Extraction of Essential Oil and Oleoresin from Chamomile (Chamomilla recutita [L.] Rauschert). J. Supercrit. Fluids 2001, 21, 245–256. [Google Scholar] [CrossRef]
- Al-Suod, H.; Ratiu, I.; Krakowska-Sieprawska, A.; Lahuta, L.; Górecki, R.; Buszewski, B. Supercritical Fluid Extraction in Isolation of Cyclitols and Sugars from Chamomile Flowers. J. Sep. Sci. 2019, 42, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Senatore, F. Supercritical Carbon Dioxide Extraction of Chamomile Essential Oil and Its Analysis by Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 1994, 42, 154–158. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Seweryn, A.; Małysa, A.; Zięba, M.; Lipińska, J. The Effect of Chamomile Extract Obtained in Supercritical Carbon Dioxide Conditions on Physicochemical and Usable Properties of Pharmaceutical Ointments. Pharm. Dev. Technol. 2018, 23, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria Recutita. Int. J. Food Prop. 2015, 18, 1784–1792. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and Therapy with Essential Oils Having Antiviral, Anti-Inflammatory, and Immunomodulatory Properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents, Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In-Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Melo-Guerrero, M.M.; Ortiz-Jurado, D.E.; Hurtado-Benavides, A.M. Comparación de La Composición y de La Actividad Antioxidante Del Aceite Esencial de Manzanilla (Matricaria chamomilla L.) Obtenido Mediante Extracción Con Fluidos Supercríticos y Otras Técnicas Verdes. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2020, 44, 845–856. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D. A-(-)-Bisabolol Reduces Pro-Inflammatory Cytokine Production and Ameliorates Skin Inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Azizi, M.; Rezaee, R.; Madarshahi, F.S.; Mehmandoust, M.; Karimi, G.; Asili, J. Cytotoxic Activity of Cis-(E)- and Trans-(Z)-Spiroethers Isolated from Various Arnebia Species. South Afr. J. Bot. 2021, 142, 114–123. [Google Scholar] [CrossRef]
- Kaiser, C.S.; Römpp, H.; Schmidt, P.C. Supercritical Carbon Dioxide Extraction of Chamomile Flowers: Extraction Efficiency, Stability, and in-Line Inclusion of Chamomile–Carbon Dioxide Extract Inβ-Cyclodextrin. Phytochem. Anal. 2004, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of Essential Oil from Different Plants and Herbs by Supercritical Fluid Extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Ruiz-Rodriguez, A.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Kinetic Study of the Supercritical CO2 Extraction of Different Plants from Lamiaceae Family. J. Supercrit. Fluids 2012, 64, 1–8. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Baldino, L.; Reverchon, E. Fractional Separation and Characterization of Cuticular Waxes Extracted from Vegetable Matter Using Supercritical CO2. Separations 2022, 9, 80. [Google Scholar] [CrossRef]
- Zengin, G.; Mollica, A.; Arsenijević, J.; Pavlić, B.; Zeković, Z.; Sinan, K.I.; Yan, L.; Cvetanović Kljakić, A.; Ražić, S. A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity. Separations 2022, 10, 18. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Lima-Junior, R.C.P.; Cordeiro, W.M.; Vieira-Júnior, G.M.; Chaves, M.H.; Almeida, F.R.C.; Silva, R.M.; Santos, F.A.; Rao, V.S.N. Pentacyclic Triterpenoids, α,β-Amyrins, Suppress the Scratching Behavior in a Mouse Model of Pruritus. Pharmacol. Biochem. Behav. 2004, 78, 719–725. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-Silva, C.; Malheiros, Â.; Muller, L.A.; Cani, G.S.; Santos, A.R.S.; Yunes, R.A.; Calixto, J.B. Antinociceptive Properties of Mixture of α-Amyrin and β-Amyrin Triterpenes: Evidence for Participation of Protein Kinase C and Protein Kinase A Pathways. J. Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef]
- Agra, L.C.; Ferro, J.N.S.; Barbosa, F.T.; Barreto, E. Triterpenes with Healing Activity: A Systematic Review. J. Dermatol. Treat. 2015, 26, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, M.; Baumann, D.; Adler, S. Supercritical Carbon Dioxide Extraction of Selected Medicinal Plants—Effects of High Pressure and Added Ethanol on Yield of Extracted Substances. Phytochem. Anal. 2004, 15, 46–54. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Md Saleh, N. Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia Pumila. Separations 2022, 9, 385. [Google Scholar] [CrossRef]
- Švehlíková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, Identification and Stability of Acylated Derivatives of Apigenin 7-O-Glucoside from Chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Chamomile Tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yue, R.-F.; Jin, Z.; He, L.-M.; Shen, R.; Du, D.; Tang, Y.-Z. Efficiency Comparison of Apigenin-7-O-Glucoside and Trolox in Antioxidative Stress and Anti-Inflammatory Properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.-S.; Oh, H.; Kim, Y.-S.; Jang, S.I. Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and Its Dermatological Applications: A Comprehensive Review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Repčák, M.; Imrich, J.; Franeková, M. Umbelliferone, a Stress Metabolite of Chamomilla recutita (L.) Rauschert. J. Plant Physiol. 2001, 158, 1085–1087. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Bernacik, K.; Typek, R. Umbelliferone Instability during an Analysis Involving Its Extraction Process. Monatsh. Chem. 2018, 149, 1327–1340. [Google Scholar] [CrossRef]
- Redaelli, C.; Formentini, L.; Santaniello, E. HPLC Determination of Coumarins in Matricaria chamomilla. Planta Med. 1981, 43, 412–413. [Google Scholar] [CrossRef]
- Jerković, I.; Molnar, M.; Vidović, S.; Vladić, J.; Jokić, S. Supercritical CO 2 Extraction of Lavandula angustifolia Mill. Flowers: Optimisation of Oxygenated Monoterpenes, Coumarin and Herniarin Content: Optimization of Supercritical CO 2 Extraction of Lavandula angustifolia. Phytochem. Anal. 2017, 28, 558–566. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Seoane-Rivero, R.; Ruiz-Bilbao, E.; Navarro, R.; Laza, J.M.; Cuevas, J.M.; Artetxe, B.; Gutiérrez-Zorrilla, J.M.; Vilas-Vilela, J.L.; Marcos-Fernandez, Á. Structural Characterization of Mono and Dihydroxylated Umbelliferone Derivatives. Molecules 2020, 25, 3497. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, T.; Wang, P.; Sun, T.; Xu, Y. Ameliorative Effects of Total Coumarins from the Fructus of Cnidium monnieri (L.) Cuss. on 2,4-dinitrochlorobenzene-induced Atopic Dermatitis in Rats. Phytother. Res. 2021, 35, 3310–3324. [Google Scholar] [CrossRef]
- Roh, E.-J. Inhibitory Effects of Coumarin Derivatives on Tyrosinase. Molecules 2021, 26, 2346. [Google Scholar] [CrossRef]
- Guillon, C.; Jan, Y.-H.; Heck, D.E.; Mariano, T.M.; Rapp, R.D.; Jetter, M.; Kardos, K.; Whittemore, M.; Akyea, E.; Jabin, I.; et al. Phototoxicity of 7-Oxycoumarins with Keratinocytes in Culture. Bioorganic Chem. 2019, 89, 103014. [Google Scholar] [CrossRef]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Leu, Y.-L.; Hung, Y.-Y.; Fang, J.-Y. Coumarin Derivatives, but Not Coumarin Itself, Cause Skin Irritation via Topical Delivery. Toxicol. Lett. 2014, 226, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ku, B.; Kim, D.; Choi, E.-M. Umbelliferone Stimulated Melanogenesis and Increased Glutathione Level in B16F10 Cells. Toxicol. Environ. Health Sci. 2017, 9, 152–160. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, Chemistry and Bioactivities Review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative Analysis of Phenolic Composition of Six Commercially Available Chamomile (Matricaria chamomilla L.) Extracts: Potential Biological Implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vincieri, F.F.; Prucher, D. Characterization OfMatricaria Recutita L. Flower Extracts by HPLC-MS and HPLC-DAD Analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. LC-PDA-ESI/MS Identification of the Phenolic Components of Three Compositae Spices: Chamomile, Tarragon, and Mexican Arnica. Nat. Prod. Commun. 2012, 7, 749–752. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Song, H.; Tian, S. Extraction of Ferulic Acid from Angelica Sinensis with Supercritical CO2. Nat. Prod. Res. 2006, 20, 835–841. [Google Scholar] [CrossRef]
- Faehnrich, B.; Franz, C.; Nemaz, P.; Kaul, H.-P. Medicinal Plants and Their Secondary Metabolites—State of the Art and Trends in Breeding, Analytics and Use in Feed Supplementation-with Special Focus on German Chamomile. J. Appl. Bot. Food Qual. 2021, 94, 61–74. [Google Scholar] [CrossRef]
- Turghun, C.; Bakri, M.; Liu, G.-Y.; Bobakulov, K.; Aisa, H.A. Phenolic Glycosides from Nitraria sibirica Leaves and Their in Vitro Biological Activities. Nat. Prod. Res. 2021, 35, 1388–1392. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Duarte, F.I.C.; Converti, A.; de Lima, Á.A.N. Ferulic Acid Activity in Topical Formulations: Technological and Scientific Prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zduńska-Pęciak, K.; Dębowska, R.; Kołodziejczak, A.; Rotsztejn, H. Ferulic Acid—A Novel Topical Agent in Reducing Signs of Photoaging. Dermatol. Ther. 2022, 35, e15543. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A Review of the Current Evidence of Fruit Phenolic Compounds as Potential Antimicrobials against Pathogenic Bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]



| Variable | Factor Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Pressure (MPa) | 5 | 15 | 25 |
| Temperature (°C) | 20 | 37.5 | 55 |
| Carried Test | Procedure | Wavelength | Standard Concentration Range |
|---|---|---|---|
| Total phenolic content (TPC) | A total of 25 µL of extract was mixed with 75 µL of H2O and 25 µL of Folin–Ciocalteu reagent (1:10) for 6 min. Then, 100 µL of a 7% Na2CO3 solution was added; the plate was shaken for 30 s and left in a dark place at room temperature for 90 min. | 765 nm | 0.025–0.20 mg mL−1 gallic acid solutions |
| Total flavonoid content (TFC) | A total of 20 µL of extract was mixed with 15 µL of 5% NaNO2 solution for 5 min. Then, 15 µL of a 10% AlCl3 solution was added, and the reaction time was 6 min. Then, 100 µL of a 1 M NaOH solution was added. The plate was shaken for 30 s before being placed in a dark, room-temperature environment for 15 min. | 510 nm | 0.025–0.2 mg mL−1 apigenin solutions |
| Total tannin content (TTC) | A total of 50 µL of extract was mixed with 50 µL H2O and 50 µL Folin–Ciocalteu reagent (1:1 dilution); reaction time was 5 min. Then, 100 µL of a 35% Na2CO3 solution was added, the plate was shaken for 30 s, and it was left in a dark place at room temperature for 30 min. | 700 nm | 0.01–0.15 mg mL−1 tannic acid solutions |
| Total sugar content (Sugars) | A total of 50 µL of extract was mixed with 150 µL of H2SO4 and 30 µL of 5% phenol reagent before being heated in an oven at 90 °C for 5 min. After heating, the plates were cooled. | 490 nm | 0.045–0.90 mg mL−1 glucose solutions |
| Antiradical activity/DPPH radical scavenging (ARA/DPPH) | A total of 20 µL of extract was mixed with 180 µL of 150 µM DPPH reagent. The plate was kept in the dark at room temperature for 60 min. | 517 nm | 0.018–0.22 µg mL−1 trolox solutions |
| Compound | Class | Purity | Mass, mg | Volume, mL | Stock Solution, mg mL−1 | Calibration Range |
|---|---|---|---|---|---|---|
| Leucine | Amino acids | 99% | 9.8 | 10 mL water | 0.98 | 1–50 μg mL−1 R2 = 0.996 |
| Ferulic acid | Phenolic acids | 98% | 10.1 | 10 mL methanol | 1.01 | 1–100 μg mL−1 R2 = 0.9999 |
| Apigenin | Flavonoids | 99.08% | 9.0 | 100 mL ethanol | 0.09 | 100–100,000 ng mL−1 R2 = 0.9997 |
| Coumarin | Hydroxycoumarins | 98% | 9.8 | 10 mL methanol | 0.98 | 100–100,000 ng mL−1 R2 = 0.997 |
| Run | Sample | Temperature (°C) | Pressure (MPa) | Yield | ||
|---|---|---|---|---|---|---|
| C | U | C | U | mL 100 g−1 DW | ||
| 1 | FAF1 | 0 | 37.5 | 0 | 15 | 5.62 |
| 8 | FAF2 | 0 | 37.5 | 1 | 25 | 5.62 |
| 6 | FAF3 | 1 | 55 | 1 | 25 | 14.98 |
| 13 | FAF4 | 0 | 37.5 | −1 | 5 | 3.75 |
| 7 | FAF5 | 1 | 55 | 0 | 15 | 11.24 |
| 3 | FAF6 | −1 | 20 | 1 | 25 | 9.36 |
| 12 | FAF7 | 1 | 55 | −1 | 5 | 7.49 |
| 4 | FAF8 | −1 | 20 | −1 | 5 | 1.87 |
| 2 | FAF9 | 0 | 37.5 | 0 | 15 | 4.68 |
| 10 | FAF10 | 0 | 37.5 | 0 | 15 | 3.75 |
| 11 | FAF11 | −1 | 20 | 0 | 15 | 5.62 |
| 5 | FAF12 | 0 | 37.5 | 0 | 15 | 7.49 |
| 9 | FAF13 | 0 | 37.5 | 0 | 15 | 5.62 |
| Extract | TPC a, GAE mg mL−1 | TFC b, APE mg mL−1 | TTC c, TAE mg mL−1 | Sugars d, GLE mg mL−1 | ARA e, TE mg mL−1 | DPPH f Quenched, % |
|---|---|---|---|---|---|---|
| FAF1 | 18.7 ± 1.8 | 16.2 ± 1.1 | 12.6 ± 1.8 | 21.7 ± 1.1 | 0.57 ± 0.07 | 31.6 ± 1.3 |
| FAF2 | 19.3 ± 1.2 | 19.1 ± 0.5 | 10.2 ± 1.7 | 5.8 ± 0.4 | 0.38 ± 0.08 | 21.6 ± 0.9 |
| FAF3 | 9.5 ± 1.5 | 4.3 ± 0.6 | 9.3 ± 1.7 | 36.9 ± 2.2 | 0.17 ± 0.05 | 10.6 ± 0.5 |
| FAF4 | 11.5 ± 1.5 | 8.7 ± 1.9 | 8.1 ± 1.0 | 63.3 ± 1.1 | 0.21 ± 0.06 | 13.1 ± 0.4 |
| FAF5 | 9.7 ± 1.1 | 5.1 ± 0.4 | 7.7 ± 1.5 | 12.7 ± 1.2 | 0.24 ± 0.02 | 14.5 ± 0.6 |
| FAF6 | 24.2 ± 2.0 | 23.1 ± 0.2 | 16.9 ± 1.1 | 3.7 ± 0.6 | 1.04 ± 0.07 | 55.5 ± 1.4 |
| FAF7 | 17.6 ± 2.4 | 14.1 ± 2.1 | 12.7 ± 2.1 | 42.7 ± 1.6 | 0.57 ± 0.09 | 31.5 ± 0.8 |
| FAF8 | 13.8 ± 1.7 | 8.5 ± 1.8 | 10.8 ± 2.3 | 34.8 ± 1.9 | 0.31 ± 0.05 | 18.0 ± 0.3 |
| FAF9 | 30.6 ± 0.3 | 25.3 ± 2.6 | 16.5 ± 3.0 | 29.0 ± 1.6 | 0.16 ± 0.04 | 10.2 ± 0.2 |
| FAF10 | 15.6 ± 0.2 | 15.3 ± 0.9 | 10.1 ± 2.0 | 11.6 ± 2.0 | 0.66 ± 0.05 | 36.2 ± 0.7 |
| FAF11 | 19.0 ± 1.9 | 15.8 ± 1.0 | 13.8 ± 1.7 | 19.2 ± 1.2 | 0.67 ± 0.06 | 36.7 ± 0.6 |
| FAF12 | 18.0 ± 2.1 | 16.9 ± 2.3 | 13.9 ± 2.1 | 12.3 ± 2.0 | 0.57 ± 0.08 | 31.6 ± 0.4 |
| FAF13 | 23.3 ± 2.5 | 15.9 ± 1.2 | 14.9 ± 2.2 | 7.0 ± 0.7 | 0.61 ± 0.06 | 33.4 ± 1.2 |
| Extract | S. aureus | S. epidermidis | E. coli | P. aeruginosa | C. albicans | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| FAF2 | 0.051 | 0.203 | 0.051 | 0.051 | 0.813 | 0.813 | 0.203 | 0.406 | 0.203 | 0.813 |
| FAF6 | 0.097 | 0.388 | 0.388 | 0.388 | 0.775 | 0.775 | 0.194 | n | 0.194 | 0.194 |
| FAF7 | 0.045 | 0.181 | 0.181 | 0.181 | n | n | 0.091 | n | 0.091 | 0.362 |
| FAF9 | 0.20 | 0.40 | 0.10 | 0.10 | 1.60 | 1.60 | 0.40 | 0.80 | 0.20 | 0.20 |
| FAF10 | 0.088 | 0.088 | 0.044 | 0.088 | 1.40 | 1.40 | 1.40 | 1.40 | 0.175 | 1.40 |
| FAF12 | 0.076 | 0.151 | 0.076 | 0.078 | 4.844 | 4.844 | 2.422 | 4.844 | 0.303 | 2.422 |
| Solvent control (70% ETOH) | 25 | n | 6.25 | 25 | 12.5 | 12.5 | 12.5 | 25 | 12.5 | n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastare, L.; Berga, M.; Kienkas, L.; Boroduskis, M.; Ramata-Stunda, A.; Reihmane, D.; Senkovs, M.; Skudrins, G.; Nakurte, I. Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients. Antioxidants 2023, 12, 1092. https://doi.org/10.3390/antiox12051092
Pastare L, Berga M, Kienkas L, Boroduskis M, Ramata-Stunda A, Reihmane D, Senkovs M, Skudrins G, Nakurte I. Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients. Antioxidants. 2023; 12(5):1092. https://doi.org/10.3390/antiox12051092
Chicago/Turabian StylePastare, Laura, Marta Berga, Liene Kienkas, Martins Boroduskis, Anna Ramata-Stunda, Dace Reihmane, Maris Senkovs, Gundars Skudrins, and Ilva Nakurte. 2023. "Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients" Antioxidants 12, no. 5: 1092. https://doi.org/10.3390/antiox12051092
APA StylePastare, L., Berga, M., Kienkas, L., Boroduskis, M., Ramata-Stunda, A., Reihmane, D., Senkovs, M., Skudrins, G., & Nakurte, I. (2023). Exploring the Potential of Supercritical Fluid Extraction of Matricaria chamomilla White Ray Florets as a Source of Bioactive (Cosmetic) Ingredients. Antioxidants, 12(5), 1092. https://doi.org/10.3390/antiox12051092








